Abstract
Context:
Young women with anorexia nervosa (AN) have a normal vitamin D status. The bioavailability of vitamin D during malnutrition is unknown.
Objective:
The objective of the study was to examine the serum response to oral ergocalciferol in AN.
Design/Setting:
This was a prospective cohort study, conducted in 2007–2009 at a tertiary care center.
Patients/Interventions:
Twelve adolescents with AN (age 19.6 ± 2.0 yr, body mass index 16.5 ± 1.4 kg/m2) and 12 matched healthy controls (20.0 ± 2.4 yr, 22.7 ± 1.0 kg/m2) received one baseline 50,000 IU oral dose of ergocalciferol.
Main Outcomes:
Serum D2, D3, 25-hydroxyvitamin D, and 1,25-dihydroxyvitamin D, collected before ingestion, at 6 and 24 h and weekly for 4 wk, and body composition measures were measured.
Results:
The AN group was severely malnourished (77.2 ± 6.3% median body weight), whereas the control group was normal weighted (106.2 ± 6.2%). From a common baseline D2 (1.5 ± 1.6 nmol/liter, P =0.34) the groups diverged (time × group interaction P = 0.04), peaking at 70 ± 34 nmol/liter at 6 h in controls compared with 43 ± 28 nmol/liter in AN subjects (P = 0.008). The D2 trajectories converged at 24 h (57 nmol/liter, P = 0.98) and returned to near baseline at 1 wk. Baseline D3 was higher in AN subjects (12.1 ± 9.6 vs. 3.1 ± 2.3 nmol/liter, P < 0.001) and remained higher throughout. 25-Hydroxyvitamin D followed a common trajectory (time × group interaction P = 0.15), rising to 45 ± 10 nmol/liter at 24 h but returning to baseline by wk 3 (P = 0.36). Correlating vitamin D levels with fat measures (body mass index, body fat) produced similar findings.
Conclusions:
Despite severe malnutrition, young women with AN had a similar bioavailability of oral ergocalciferol as the healthy-weighted controls. Vitamin D dosing for patients suffering from malnutrition may not differ from that for normal-weighted adolescents.
Patients with anorexia nervosa (AN) are at increased risk for fracture and early osteoporosis. Despite poor nutritional intake, preliminary data suggest that young women with AN have a low prevalence of vitamin D deficiency. In a study of 50 adolescents with AN and 200 healthy control subjects who underwent vitamin D screening, Haagensen et al. (1) found that the prevalence of vitamin D deficiency [25-hydroxyvitamin D (25[OH]D) < 20 ng/ml] was 2% in adolescents with AN vs. 24% among controls. Although it is unknown why girls with AN have a decreased rate of vitamin D deficiency, it has been postulated that these malnourished young women, who possess minimal body fat, have decreased metabolic clearance and decreased uptake of vitamin D by adipose tissue.
Conversely, Wortsman et al. (2) reported that obese adults had lower baseline 25(OH)D concentrations and higher PTH levels than normal-weighted adults as well as lower peak blood concentrations of vitamin D2 after ingestion of a single oral dose of ergocalciferol. The authors hypothesized that the metabolic clearance of vitamin D increased in obesity because of enhanced uptake by adipose tissue, resulting in vitamin D insufficiency. This relationship between serum vitamin D concentrations and adiposity has been supported by other investigations (3, 4). However, to our knowledge, no studies have evaluated the relationship between adiposity and vitamin D in adolescents with AN.
The current prospective, interventional study examined whether the bioavailability of vitamin D is altered in adolescents with AN compared with normal-weighted adolescents. We hypothesized that adolescents with AN would have higher peak blood concentrations of vitamin D2 as well as a slower rate of decline in serum 25(OH)D concentrations.
Subjects and Methods
Subjects
From 2007 to 2010, eligible patients were identified during visits to the adolescent medicine outpatient clinic or during a hospital admission to the Adolescent Medicine Service at Children's Hospital Boston. All eligible participants were female, aged 13–22 yr, at least 2 yr after menarche, Caucasian, and English speaking. All were free of concomitant diseases affecting bone health and taking no medications known to affect vitamin D metabolism. Twenty-four patients completed the study (12 AN and 12 controls). Patients with AN were amenorrheic and had a body mass index (BMI) 85% or less of median for age and gender (5). Control patients had spontaneous, regular menses (menstrual cycle lengths of <45 d); were normal weighted with a BMI between the 30th and 85th percentiles for age and gender; and had no history of an eating disorder. AN and control participants were matched on the basis of age and skin pigmentation, as measured by a validated scale (6, 7). Patients with AN and control subjects were recruited within 6 wk of each other to be sure that matching occurred within the same season. Study recruitment occurred only between October and March to account for the confounding of season. The Committee on Clinical Investigation of Children's Hospital Boston approved the study protocol. Informed consent was obtained from study participants or their parent/guardian.
Design
The study was designed as a prospective, experimental pilot study. Both groups received a single oral dose of vitamin D2 (ergocalciferol) 50,000 IU, provided as a capsule, at baseline after a minimum of 4 h of fasting. Serum samples were collected before ingestion of the ergocalciferol, at 6 and 24 h, and at 1, 2, 3, and 4 wk after ingestion. Participants were asked to refrain from excessive sunlight exposure without the use of sunscreen or any intake of vitamin D supplements for the duration of the 4-wk study.
Study assessments
The primary study outcome was serum vitamin D2 concentration, which was measured by HPLC (8) at baseline, 6 and 24 h after the ergocalciferol challenge, and at 1 wk. Serum vitamin D3 concentrations were measured by HPLC (8) at the same time points. Serum 25(OH)D was measured by liquid chromatography tandem mass spectrometry (9) at baseline, 24 h, and weekly for 4 wk after ergocalciferol administration. Serum 1,25-dihydroxyvitamin D [1,25(OH)2D] was measured by radioreceptor assay (10) at baseline and wk 4. Calcium, phosphorus, and albumin were analyzed in the Children's Hospital Boston Core Laboratory by Ektachem methodology (cholesterol oxidase; Vitros; Johnson & Johnson, New Brunswick, NJ) at each study visit. A baseline PTH was also obtained (chemiluminescent immunoassay; Beckman Coulter, Fullerton, CA).
Areal bone mineral density (BMD) of the total body, left total hip, and lumbar spine (L1-L4), as well as body composition, was measured at baseline by dual-energy x-ray absorptiometry (DXA; QDR 4500; Hologic, Inc., Bedford, MA). Participants responded to a structured interview regarding their past medical history. All subjects completed the Eating Attitudes Test (EAT-26), a short, self-administered questionnaire that evaluates body image and eating behaviors (11). In addition, participants completed two validated questionnaires outlining their recent nutritional intake and physical activity: the Youth/Adolescent Questionnaire, a detailed, semiquantitative food frequency questionnaire (12), and the Youth/Adolescent Activity Questionnaire, a measure of typical time spent, over the past year, in various activities and team sports (13).
Statistical analyses
The study was designed with 80% power to detect a difference in serum vitamin D2 concentrations of 14.8 ng/ml (37 nmol/liter) between groups at 24 h. Compared with the mean 24-h level in controls in the Wortsman study (150 nmol/liter or 60.1 ng/ml), this is a 25% difference. We considered anything smaller to be clinically insignificant.
Baseline comparison of all continuous measures between trial arms, including anthropometrics, vitamin D levels, serum chemistries, DXA, and questionnaire responses, was made by Student t test, corroborated by Wilcoxon two-sample test for variables with skewed distribution. Dichotomies were compared by Fisher exact test.
The time course of vitamin D2 concentration from baseline to 6 h, 24 h, and 1 wk was compared between groups by repeated-measures ANOVA, with an autoregressive covariance model to account for visit-to-visit correlation within subjects. Terms were added to the ANOVA model for study group (AN vs. control) and time × group interaction. Concentrations were log transformed for ANOVA to stabilize variance over the wide range of serum levels. For postbaseline time points, we report untransformed mean ± sd for each group and compare them using P values derived from contrasts constructed from the fitted ANOVA parameters. Repeated-measures ANOVA was similarly performed for the D3, 25(OH)D, and 1,25(OH)2D measurements and for serum albumin, calcium, and phosphorus, extending to the end of the 4-wk study. Associations between body composition and the peak concentrations of vitamin D2, D3, and 25(OH)D were assessed using Pearson correlations. SAS software (version 9.2; Cary, NC) was used for all computations. P < 0.05 was considered a statistically significant difference.
Results
Subject age was similar between groups (AN group mean ± sd 19.6 ± 2.0 yr vs. controls 20.1 ± 2.3 yr, P = 0.63; Table 1). The two groups differed on expected baseline characteristics including weight, BMI, and body composition (Table 1). Subjects with AN were severely malnourished (BMI 16.5 ± 1.4 kg/m2; 77.2 ± 6.3% median body weight) and had amenorrhea of median duration of 6 months, range 1–60 months. In contrast, the control group was normal weighted (BMI 22.7 ± 1.0 kg/m2; 106.2 ± 6.2% median body weight). At baseline, vitamin D insufficiency [25(OH)D <30 ng/ml or <75 nmol/liter] was present in three controls (25%) and two girls with AN (17%). Skeletal health was slightly compromised at the lumbar spine in girls with AN (lumbar spine BMD Z-score −1.18 ± 1.42) but normal in the control group (lumbar spine BMD Z-score 0.22 ± 0.74; Table 1). Hip BMD Z-scores were within the normal range in both groups.
Table 1.
Baseline characteristics of adolescents with AN (n = 12) and normal-weighted control adolescent girls (n = 12)a
| Characteristic | AN | Control | P |
|---|---|---|---|
| Demographic data | |||
| Age (yr) | 19.6 ± 2.0 | 20.0 ± 2.4 | 0.63 |
| Weight (kg) | 45.7 ± 5.1 | 61.3 ± 1.8 | <0.0001 |
| Height (cm) | 166 ± 4 | 165 ± 5 | 0.33 |
| BMI (kg/m2) | 16.5 ± 1.4 | 22.7 ± 1.0 | <0.0001 |
| Median body weight (%) | 77.2 ± 6.3 | 106.2 ± 6.2 | <0.0001 |
| DXA data | |||
| Lean mass (kg) | 36.8 ± 3.6 | 41.7 ± 2.4 | 0.001 |
| Fat mass (kg) | 7.4 ± 2.9 | 17.8 ± 2.4 | <0.0001 |
| Body fat percentage (%) | 15.8 ± 5.4 | 28.9 ± 3.8 | <0.0001 |
| Total body BMD Z-score | −0.58 ± 1.35 | 0.17 ± 0.74 | 0.11 |
| Hip BMD Z-score | −0.87 ± 1.31 | 0.40 ± 0.95 | 0.01 |
| Lumbar spine BMD Z-score | −1.18 ± 1.42 | 0.22 ± 0.74 | 0.008 |
| Questionnaire data | |||
| EAT scores | 35 ± 22 | 3 ± 4 | 0.0003 |
| Activity hours (h/wk) | 2.8 ± 5.0 | 5.2 ± 4.0 | 0.22b |
| Caloric intake (kcal/d) | 1829 ± 599 | 1897 ± 560 | 0.77 |
| Total fat intake (g/d) | 50 ± 18 | 58 ± 21 | 0.29 |
| Calcium intake (mg/d) | 1239 ± 582 | 1178 ± 341 | 0.76 |
| Vitamin D intake (IU/d) | 318 ± 203 | 252 ± 171 | 0.40 |
Mean ± sd. P value from Student t test comparing AN with control mean, corroborated by Wilcoxon test in cases of skewed distribution.
Median 0 h for AN, 4.5 h for control; P = 0.06 by Wilcoxon test.
On average, the sample of girls with AN reported significantly disordered eating behaviors (EAT-26 score 35 ± 22) compared with the control group (EAT-26 score 3 ± 4, P = 0.0003). Although the mean reported hours of exercise per week did not differ significantly between the two groups (Table 1), seven AN subjects (58%) reported no exercise at all compared with only two controls (17%, P = 0.09). The median exercise was accordingly 0 for AN vs. 4.5 h/wk for controls (P = 0.06 by Wilcoxon test). Reported caloric consumption was comparable in adolescents with AN (1829 ± 599 kcal/d) and normal-weighted subjects (1897 ± 560 kcal/d, P = 0.77), as were calcium and vitamin D intake before the study.
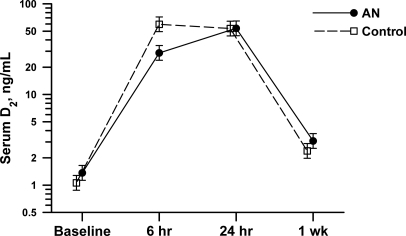
Baseline serum vitamin D2 concentrations did not differ between groups [AN subjects: 1.9 ± 2.2 ng/ml (4.7 ± 5.5 nmol/liter) vs. control subjects: 1.1 ± 0.3 ng/ml (2.7 ± 0.7 nmol/liter), P = 0.20), nor did PTH (Table 2). After the oral administration of ergocalciferol, there was a marked increase in the serum vitamin D2 concentrations (Fig. 1). The trajectories of change for vitamin D2 in the two groups diverged from baseline (time × group interaction P = 0.04), with normal-weighted subjects reaching a higher vitamin D2 peak at 6 h [70 ± 34 ng/ml (175 ± 85 nmol/liter)] than subjects with AN [43 ± 28 ng/ml (107 ± 70 nmol/liter); P = 0.008]. The vitamin D2 levels in the two groups converged at 24 h [controls: 58 ± 22 ng/ml (145 ± 55 nmol/liter) vs. AN: 55 ± 14 ng/ml (137 ± 35 nmol/liter), P = 0.98] and returned to near baseline at 1 wk [controls: 2.9 ± 1.7 ng/ml (7.2 ± 4.2 nmol/liter) vs. AN: 3.4 ± 1.6 ng/ml (8.5 ± 4.0 nmol/liter), P = 0.34]. Vitamin D3 concentrations were 3- to 4-fold higher in AN subjects at baseline [12.1 ± 9.6 ng/ml (30.2 ± 24.0 nmol/liter)] than normal-weighted subjects [3.1 ± 2.3 ng/ml (7.7 ± 5.7 nmol/liter), P = 0.004] and remained so throughout the study, despite a significant rise among controls at 1 wk [+1.4 ± 1.7 ng/ml (+3.5 ± 4.2 nmol/liter), P = 0.01].
Table 2.
Serum vitamin D concentrations, electrolyte levels, and PTH measurements at baseline in adolescents with AN (n = 12) and healthy control subjects (n = 12)a
| Measurement | AN | Control | P |
|---|---|---|---|
| Vitamin D2 (ng/ml) | 1.9 ± 2.2 | 1.1 ± 0.3 | 0.20 |
| Vitamin D3 (ng/ml) | 12.1 ± 9.6 | 3.1 ± 2.3 | 0.004 |
| 25(OH)D (ng/ml) | 41 ± 11 | 38 ± 11 | 0.48 |
| 1,25(OH)2D (pg/ml) | 32 ± 21 | 55 ± 16 | 0.006 |
| PTH (pg/ml) | 33 ± 10 | 29 ± 12 | 0.36 |
| Calcium (mg/dl) | 9.6 ± 0.4 | 9.6 ± 0.3 | 0.83 |
| Albumin (mg/dl) | 4.5 ± 0.4 | 4.2 ± 0.3 | 0.09 |
| Phosphorus (mg/dl) | 3.8 ± 0.3 | 3.8 ± 0.4 | 0.70 |
Mean ± sd. P value from Student t test comparing AN with control mean, corroborated by Wilcoxon test in cases of skewed distribution.
Fig. 1.
Mean serum vitamin D2 (ergocalciferol) concentrations in the control (●) and anorexic (■) groups 0–24 h after oral intake of vitamin D2 (50,000 IU, 1.25 mg). Vitamin D2 rose rapidly to 6 h after intake and then declined slightly thereafter. Normal-weighted subjects had higher peak concentrations of vitamin D2 than did subjects with AN (P = 0.04 for time × group interaction). The error bars indicate ±1 se on the log scale from repeated-measures ANOVA.
Serum 25(OH)D followed similar changes in AN and control subjects over the 4-wk study period (time × group interaction P = 0.15), starting at 25(OH)D = 39 ± 11 ng/ml (97 ± 24 nmol/liter) in the combined sample before ergocalciferol ingestion (P = 0.0.48 for group differences at baseline; Table 2), rising to a peak level of 45 ± 10 ng/ml (112 ± 25 nmol/liter) at 24 h and returning to baseline by wk 3. Serum 1,25(OH)2D was lower in subjects with AN at baseline [32 ± 21 pg/ml (77 ± 50 pmol/liter) vs. 55 ± 16 pg/ml (132 ± 38 pmol/liter), P = 0.006] and did not change significantly at 4 wk in either group (P > 0.40). Serum calcium, phosphorus, and albumin started within the normal range (Table 2) and remained similar in both groups throughout the 4-wk trial.
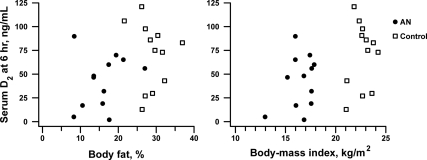
In the combined sample, there was an inverse correlation between body fat and vitamin D3 concentrations at every measurement time from baseline (r = −0.57, P = 0.004) until 1 wk after ingestion, the last measurement point for D3 (r = −0.60, P = 0.02). Conversely, peak (6 h) measures of plasma vitamin D2 were positively associated with BMI (r = 0.47, P = 0.02) and body fat percentage (r = 0.38, P = 0.07; Fig. 2). However, no significant correlations between vitamin D2 and measures of adiposity were seen at other time points. Serum 25(OH)D was not correlated with body fat measures at baseline or any point over the 4-wk study.
Fig. 2.
Correlation between body fat percentage or BMI and serum vitamin D2 concentrations in adolescents with AN (●) or normal-weighted adolescents (■) at 6 h after an oral dose of ergocalciferol (50,000 IU). The correlation coefficients were r = 0.38 for fat percentage (P = 0.07) and r = 0.47 for BMI (P = 0.02).
Discussion
In this study, we sought to determine why adolescents with AN were less prone to vitamin D insufficiency than healthy-weighted adolescents. We hypothesized that teens with AN would have increased peak concentrations of vitamin D2 and a slower rate of decline in vitamin D levels compared with normal-weighted girls after an oral challenge of ergocalciferol due to their significantly lower body fat. Contrary to our hypothesis, young women with AN had a similar response to a single dose of oral ergocalciferol compared with healthy-weighted control subjects despite their severe malnutrition. The control group exhibited higher peak concentrations of vitamin D. There was no difference between groups in the rate of decline of vitamin D concentrations after the oral challenge. Although speculative and not examined in this study, the gastroparesis known to be present in these patients (14) may have led to slowed absorption in the face of a slower gastrointestinal transit time.
Patients with AN, who are severely malnourished, would be expected to have vitamin D deficiency, leading to secondary hyperparathyroidism, increased bone resorption, and loss of BMD. Hadigan et al. (15) found that only 33–43% of adult women with AN met the recommended daily allowance for vitamin D. However, data from our group have shown that patients with AN have relatively normal or elevated concentrations of vitamin D compared with normative standards or healthy control subjects (1, 16).
The reason for the decreased risk of vitamin D deficiency in young women with AN is intriguing and unknown. It is possible that these young women, who significantly restrict their caloric intake because of fear of weight gain, are more likely to consume a noncaloric nutritional source (e.g. a multivitamin) than a normal-weighted adolescent. In our previous study, significantly more girls with AN reported vitamin D supplementation (86%) than the full control group (14%) or a Caucasian-only subgroup (27%) (1). Interestingly, in this study, subjects with AN self-reported similar total caloric intake to the control subjects despite profoundly abnormal attitudes toward eating and weight (EAT scores). Participants in this study with AN were undergoing treatment for their eating disorder at the time of study recruitment, which may explain the discrepancy between self-reported caloric intake and disordered eating behaviors. In addition, patients with AN tend to overestimate their food intake as well as underreport their physical activity and exercise, highlighting a limitation of self-report in this population. In addition, our study sample was small; future studies should include a larger number of patients studied for time periods longer than the current study.
It has also been hypothesized that these malnourished young women, who possess minimal body fat, have decreased metabolic clearance and decreased uptake of vitamin D by adipose tissue. This hypothesis is supported by studies conducted in obese subjects with excess adipose tissue. Wortsman et al. (2) demonstrated that obese individuals had lower baseline 25(OH)D levels and higher PTH levels as compared with normal-weight, healthy control subjects. Twenty-four hours after ingestion of an oral dose of ergocalciferol, there was a marked increase in serum vitamin D2 concentrations, with a significant effect of both time and group. Peak blood concentrations of vitamin D2 were inversely correlated with body weight. The authors hypothesized that the orally supplied vitamin D2 was possibly sequestered in the increased body fat of the obese subjects after drug administration, resulting in decreased bioavailability (2). In our malnourished adolescents with AN, who possessed minimal body fat, we did not find comparable results. We anticipated that there would be a significant inverse correlation between peak measures of plasma vitamin D2 and body fat percentage after ergocalciferol administration. However, we found a positive correlation between peak vitamin D2 and body fat and/or BMI. This finding makes an interesting contrast with the low levels of vitamin D seen in obese persons, which are commonly attributed to the sequestering of vitamin D in large adipose stores. The low levels of 6-h plasma vitamin D2 that we observed in our subjects with AN, who have a profound deficit in body fat, must be due to a different mechanism. Lastly, our protocol did not include measurements of vitamin D binding protein, a variable that could have affected circulating vitamin D levels and one that is modulated by hormonal concentrations such as estradiol in an adolescent female.
Our findings have implications for defining the baseline vitamin D requirements in young women with AN, a fat-soluble vitamin that may play an important role in the prevention of skeletal deficits in this population. Based on our data, it appears that vitamin D dosing for patients suffering from malnutrition, irrespective of etiology, may not differ from that for normal-weighted adolescents. These data have important implications for the care of adolescents around supplementation recommendations, especially given that eating disorders are so common among youth.
Acknowledgments
We acknowledge the excellent technical assistance of Jamie Nydegger, Yailka Cardenas, Maria Balestrino, and Julie Ringelheim; the skilled nursing care of the Clinical and Translational Study Unit; and our patients and their families, who made all of this research possible.
This work was supported by the Clinical and Translational Study Unit of Children's Hospital Boston, National Institute of Child Health and Human Development Grant R01 HD043869 and Grant K23 HD060066, the Harvard Clinical and Translational Science Center (Harvard Catalyst Grant UL1 RR 025758), the McCarthy Family Foundation, a Children's Hospital Boston Pilot Research Grant, and the Children's Hospital Boston Career Development Fund.
Disclosure Summary: The authors have no conflicts of interest to disclose.
Footnotes
- AN
- Anorexia nervosa
- BMD
- bone mineral density
- BMI
- body mass index
- DXA
- dual-energy x-ray absorptiometry
- EAT-26
- Eating Attitudes Test
- 25(OH)D
- 25-hydroxyvitamin D.
References
- 1. Haagensen AL, Feldman HA, Ringelheim J, Gordon CM. 2008. Low prevalence of vitamin D deficiency among adolescents with anorexia nervosa. Osteoporos Int 19:289–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. 2000. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr 72:690–693 [DOI] [PubMed] [Google Scholar]
- 3. Parikh SJ, Edelman M, Uwaifo GI, Freedman RJ, Semega-Janneh M, Reynolds J, Yanovski JA. 2004. The relationship between obesity and serum 1,25-dihydroxy vitamin D concentrations in healthy adults. J Clin Endocrinol Metab 89:1196–1199 [DOI] [PubMed] [Google Scholar]
- 4. Arunabh S, Pollack S, Yeh J, Aloia JF. 2003. Body fat content and 25-hydroxyvitamin D levels in healthy women. J Clin Endocrinol Metab 88:157–161 [DOI] [PubMed] [Google Scholar]
- 5. Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, Mei Z, Curtin LR, Roche AF, Johnson CL. 2000. CDC growth charts: United States. Adv Data 1–27 [PubMed] [Google Scholar]
- 6. Jimbow K, Fitzpatrick B, Wick MM. 1991. Biochemistry and physiology of melanin pigmentation. In: Goldsmith LA. ed. Physiology, biochemistry, and molecular biology of the skin. New York: Oxford University Press [Google Scholar]
- 7. Pathak MA, Jimbow K, Szabo G, Fitzpatrick TB. 1976. Sunlight and melanin pigmentation. In: Goldsmith K. ed. Photochemical and photobiological reviews. New York: Plenum Press [Google Scholar]
- 8. Chen TC, Turner AK, Holick MF. 1990. A method for the determination of the circulating concentration of vitamin D. J Nutr Biochem 1:272–276 [DOI] [PubMed] [Google Scholar]
- 9. Holick MF. 2005. 25-OH-vitamin D assays. J Clin Endocrinol Metab 90:3128–3129 [DOI] [PubMed] [Google Scholar]
- 10. Chen TC, Turner AK, Holick MF. 1990. A method for the determination of the circulating concentration of 1,25-dihydroxyvitamin D. J Nutr Biochem 1:320–327 [DOI] [PubMed] [Google Scholar]
- 11. Garner DM, Olmsted MP, Bohr Y, Garfinkel PE. 1982. The eating attitudes test: psychometric features and clinical correlates. Psychol Med 12:871–878 [DOI] [PubMed] [Google Scholar]
- 12. Rockett HR, Wolf AM, Colditz GA. 1995. Development and reproducibility of a food frequency questionnaire to assess diets of older children and adolescents. J Am Diet Assoc 95:336–340 [DOI] [PubMed] [Google Scholar]
- 13. Berkey CS, Rockett HR, Field AE, Gillman MW, Frazier AL, Camargo CA, Jr, Colditz GA. 2000. Activity, dietary intake, and weight changes in a longitudinal study of preadolescent and adolescent boys and girls. Pediatrics 105:E56. [DOI] [PubMed] [Google Scholar]
- 14. De Caprio C, Pasanisi F, Contaldo F. 2000. Gastrointestinal complications in a patient with eating disorders. Eat Weight Disord 5:228–230 [DOI] [PubMed] [Google Scholar]
- 15. Hadigan CM, Anderson EJ, Miller KK, Hubbard JL, Herzog DB, Klibanski A, Grinspoon SK. 2000. Assessment of macronutrient and micronutrient intake in women with anorexia nervosa. Int J Eat Disord 28:284–292 [DOI] [PubMed] [Google Scholar]
- 16. Rigotti NA, Nussbaum SR, Herzog DB, Neer RM. 1984. Osteoporosis in women with anorexia nervosa. N Engl J Med 311:1601–1606 [DOI] [PubMed] [Google Scholar]