Abstract
Context:
The co-occurrence of insulin resistance (IR) and hypertension is a heritable condition leading to cardiovascular complications. Caveolin-1 (CAV1), a gene previously associated with metabolic dysfunction in animal and cellular models, may be a marker for these conditions in humans.
Objective:
The objective of the study was to examine the relationship between CAV1 variants and IR in two hypertensive cohorts and to corroborate the findings in a CAV1 knockout mouse.
Design, Setting, and Participants:
A candidate gene association study was conducted in two hypertensive cohorts: 1) Caucasian and 2) Hispanic. Multivariate associations between individual variants and insulin-resistant phenotypes were analyzed, accounting for age, gender, body mass index, and sibling relatedness. Intraperitoneal glucose tolerance tests were conducted in wild-type and CAV1 knockout mice.
Results:
In the Caucasian hypertensive cohort, minor allele carriers of two CAV1 single-nucleotide polymorphisms (rs926198, rs3807989) had significantly higher fasting insulin levels (P = 0.005, P = 0.007), increased homeostatic assessment model for insulin resistance (HOMA-IR) (P =0.005, P = 0.008), and decreased M value during hyperinsulinemic, euglycemic clamp procedure (P = 0.004, P = 0.05) than major allele homozygotes. Findings were replicated in the Hispanic hypertensive cohort cohort for fasting insulin levels (P = 0.005, P = 0.02) and HOMA-IR (P = 0.008 and P = 0.02). Meta-analysis demonstrated significant associations of both single-nucleotide polymorphisms with fasting insulin levels (P = 0.00008, P = 0.0004) and HOMA-IR (P = 0.0001, P = 0.0004). As compared with wild type, CAV1 knockout mice displayed higher blood pressure levels and higher fasting glucose, insulin, and HOMA-IR levels and an exaggerated glycemic response to a glucose challenge.
Conclusion:
Variations in the CAV1 gene are associated with IR and hypertension. CAV1 gene polymorphisms may be a biomarker for IR and hypertension, enabling earlier detection and improved treatment strategies.
The co-occurrence of insulin resistance (IR) and hypertension is a heritable condition that leads to long-term health complications (1, 2). Identifying genomic markers for this complex disease is important to aid development of effective prevention and treatment strategies. Unfortunately, genome-wide association studies have shown varied success when applied to complex diseases, limiting the ability to identify such genomic markers. Alternatively, the intermediate phenotype/candidate gene approach may prove successful, especially when dealing with a heterogeneous condition such as hypertension.
This study used this alternate approach to evaluate the relationship of a candidate gene to the IR intermediate phenotype of hypertension. The candidate gene is caveolin-1 (CAV1), implicated in insulin signaling and vascular function in both animal and cell culture studies (3–6). Thus, the purpose of this study was to test the hypotheses that variants in the CAV1 gene are associated with IR in two hypertensive cohorts in humans and that loss of CAV1 in mice leads to IR and hypertension.
Materials and Methods
Participants
A detailed description of the study methods can be found in the Supplementary Appendix, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org. Protocols for subject recruitment and data collection for the Caucasian hypertensive (HyperPATH) and the Hispanic hypertensive (HTN-IR) cohorts were described previously (1, 2). The analyses presented herein were restricted to participants who had the CAV1 genotype and primary phenotype data: 324 Caucasian hypertensive (HyperPATH-HTN) and 143 Caucasian normotensive participants (HyperPATH-NTN) from the HyperPATH cohort and 192 Mexican-American hypertensive participants from the replication cohort (HTN-IR). Participants' baseline characteristics did not differ greatly between cohorts (Supplemental Table 1). Fifteen participants were randomly selected from the HyperPATH-HTN cohort for the hyperinsulinemic euglycemic clamp protocol. Both protocols were approved by the institutional review boards of each site. Informed consent was obtained before enrollment.
Primary outcome measurement
The primary phenotype examined was fasting insulin. Secondary phenotypes, homeostatic assessment model for insulin resistance (HOMA-IR), M-value (glucose infusion rate to maintain euglycemia during a hyperinsulinemic clamp), and fasting glucose, were examined to clarify the mechanism underlying the primary association.
Genotyping
DNA extraction and genotyping were conducted as previously described (7). Six single-nucleotide polymorphisms (SNP) covering a 36.6-kb region of the CAV1 gene were analyzed in the HyperPATH cohort (rs926198, rs1543293, rs3807989, rs3757732, rs1022436, rs1049337; Supplemental Table 2). Two SNP, rs926198 and rs11773845 (a proxy for rs3807989; D′ = 1, r2 = 1, in HapMap Mexican-Americans) were examined in the replication cohort.
Animal protocol and measurements
All animal procedures were performed as previously described (5) or are detailed in the Supplemental Appendix. Five 12-wk-old, male CAV1 knockout (KO) and an equal number of genetically matched wild-type (WT) mice (stock no. 004585 and 101045, The Jackson Laboratory, Bar Harbor, ME) were studied. All experimental procedures followed the guidelines of and were approved by the Institutional Animal Care and Use Committee at Harvard Medical School.
Statistical analysis
Statistical analyses using the HyperPATH cohort were performed using SAS 9.1 (SAS Institute, Cary, NC). The natural log of fasting insulin and HOMA-IR were used to meet normality assumptions. Hardy-Weinberg equilibrium testing was performed using a χ2 test. Pairwise linkage (D′ and r2) was estimated using Haploview. A mixed-effect linear regression (PROC MIXED) was performed for fasting insulin, fasting glucose, and HOMA-IR, accounting for relatedness and adjusted for age, gender, body mass index, and study site. Point estimates represent least square means, and error bars represent the 95% confidence intervals from the regression model. Differences in M values by genotype were tested using an unpaired t test. For the primary phenotype, P = 0.008 was considered statistically significant to account for testing of six SNP. A P = 0.05 was considered statistically significant for all secondary analyses.
In the HTN-IR cohort, we evaluated association using a robust variance estimation approach, using the generalized estimating equation (8) to test hypothesized associations between phenotypes and genetic variants and account for familial correlations present in the data. The PROC GENMOD procedure in SAS was used for the analysis using the generalized estimating equation model. Family was taken as the cluster factor. Age, gender, and body mass index were specified as covariates. A P = 0.05 was considered statistically significant for the replication analyses.
The metaanalysis was conducted with a weighted z-score method using METAL (9). This approach accounts for the direction of association relative to a chosen reference allele and the sample size of each cohort. Details of this approach are described in the Supplementary Appendix.
For the animal studies, data were analyzed by Student's t tests using GraphPad Prism (San Diego, CA). Comparisons between groups were made with one-way ANOVA followed by Tukey's post hoc test.
Results
CAV1 SNPs associated with fasting insulin in HyperPATH HTN cohort
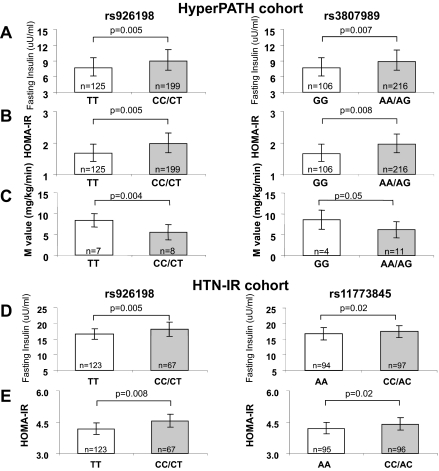
In the HyperPATH-HTN cohort, minor allele frequencies were greater than 10% for the six SNPs analyzed (Supplemental Table 2). Using a dominant model [homozygous major allele (TT) vs. minor allele carriers (CT/CC)], minor allele carriers of rs926198 and rs3807989 were found to have significantly higher fasting insulin levels than major allele homozygotes [milliunits per milliliter; mean estimate (95% confidence interval), rs926198: TT = 7.69 (6.15–9.58), CT/CC = 9.00 (7.23–11.13), P = 0.005 and rs3807989: GG = 7.63 (6.10–9.55), AA/AG = 8.90 (7.18–11.03), P = 0.007] (Fig. 1). No associations were seen for either SNP with fasting insulin levels in the HyperPATH-NTN cohort (data not shown).
Fig. 1.
CAV1 genotypes associate with fasting insulin (A and D), HOMA-IR (B and E), and M value (C) in the HyperPATH HTN (A–C) and HTN-IR cohorts (D and E). Point estimates (least square means), 95% confidence interval, and P values (F test, two sided) were obtained from a mixed model (A and B) or a general linear regression (D and E). Mean, sd, and P values were obtained from t test (C). The metaanalysis yielded the following P values: P = 0.00008 (rs926198) and P = 0.0004 (rs3807989) for fasting insulin and P = 0.0001 (rs926198) and P = 0.0004 (rs11773845) for HOMA-IR.
Replication of primary phenotype in Hispanic HTN-IR cohort
To replicate the findings, we assessed the association of rs926198 and rs11773845 with fasting insulin levels in HTN-IR. Again, minor allele carriers of both SNPs were significantly associated with increased fasting insulin levels (milliunits per milliliter; mean estimate (95% confidence interval), rs926198: TT = 16.65 (14.97–18.33) CT/CC = 18.09 (15.81–20.38) P = 0.005, rs11773845: AA = 16.76 (14.85–18.68) AC/CC = 17.48 (15.58–19.38) P = 0.02 (Fig. 1).
Mechanistic studies: secondary phenotypes
Because fasting hyperinsulinemia is a hallmark of IR, we analyzed whether measurements of IR (HOMA-IR, clamp derived M value) differed by CAV1 genotype in HyperPATH-HTN. Minor allele carriers for both SNPs had significantly higher HOMA-IR values than major allele homozygotes (Fig. 1) (mean estimate (95% confidence interval), rs926198: TT = 1.67 (1.42–1.69), CT/CC = 1.98 (1.69–2.31) P = 0.005; rs3807989: GG = 1.66 (1.42–1.69), AG/AA = 1.96 (1.68–2.28) P = 0.008). Fasting plasma glucose measurements did not significantly differ by genotype for either SNPs, suggesting that, in the CAV1 minor allele carriers, normal glucose levels were maintained at the expense of higher insulin levels.
Of the 15 participants in the HyperPATH-HTN cohort who underwent the euglycemic hyperinsulinemic clamp, minor allele carriers of both SNP had significantly lower M values than major allele homozygotes (milligram per kilogram per minute, mean ± sd; rs926198: TT = 8.35 ± 1.56, CT/CC = 5.48 ± 1.83, P = 0.004; rs3807989: GG = 8.62 ± 2.27, AG/AA = 6.17 ± 1.95, P = 0.05); consistent with IR in these individuals (Fig. 1).
Replication
Significant associations between the CAV1 SNPs and HOMA-IR were also found in the Hispanic HTN-IR cohort (Fig. 1) (rs926198, P = 0.008; rs11773845, P = 0.02). In the HTN-IR cohort, these SNP manifested trends for reduced M values that did not reach statistical significance.
Meta-analysis
A meta-analysis of the two cohorts found significant associations of both SNP with increased fasting insulin and increased HOMA-IR measurements using a dominant model [fasting insulin: rs926198 (P = 0.00008), rs3807989 (P = 0.0004); HOMA-IR: rs926198 (P = 0.0001), rs3807989 (P = 0.0004)].
Animal studies
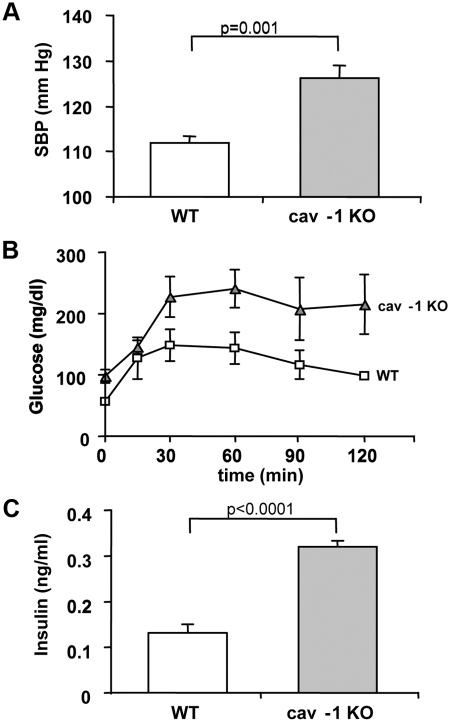
To assess the importance of CAV1 on the metabolic phenotype, we used a CAV1 KO animal model. These results provide evidence to suggest whether the minor allele is associated with a loss or gain of function. As previously shown, CAV1 KO mice displayed higher systolic blood pressure levels, compared with WT (Fig. 2A). An ip glucose tolerance test revealed significantly higher glucose responses in the CAV1 KO vs. WT (Fig. 2B), consistent with an IR phenotype. Indeed, fasting insulin levels were also significantly greater in the CAV1 KO compared with WT (Fig. 2C).
Fig. 2.
CAV1 KO mice have increased systolic blood pressure (SBP) levels (A), an exaggerated response to a glucose tolerance test (B), and greater fasting insulin (C). Experiments were carried out as described in Supplemental Materials.
Discussion
This study documents that variants in the CAV1 gene are associated with features of IR: elevated fasting insulin levels and increased HOMA-IR in two hypertensive cohorts of different ethnicity. Gene association studies of IR and hypertension have demonstrated inconsistent results (10). The successful outcome of the present study likely resides in several factors. First, we used a candidate gene-intermediate phenotype approach. Second, known environmental factors, including drug therapy, were controlled and/or eliminated, thereby reducing their confounding effects. Third, the primary findings were confirmed in a second population. And finally, the findings were verified in an animal model. Thus, these studies identify CAV1 as a genetic marker for metabolic dysfunction and provide insight into a potential mechanism underlying the interindividual variability of the coaggregation of IR and hypertension in humans.
Family studies indicate that the cooccurrence of IR and hypertension is heritable (1, 2). We and others provided evidence of linkage of blood pressure, vascular phenotypes, and IR with the 7q31 locus and its proximity (2, 11). Among the genes located in this region is the CAV1 gene (chromosome 7q31.1) (12).
CAV1 is a known regulator of insulin signaling and insulin receptor stability. Specifically, CAV1 binds directly to the insulin receptor in adipocytes (13) and disruption of this complex by ganglioside GM3 (14) causes altered insulin signaling. Furthermore, depletion of CAV1 results in a 90% decrease in adipocyte insulin receptor levels in CAV1 KO mice (15). Although the role of CAV1 in insulin-mediated glucose uptake is less clear (15), CAV1 has also been shown to be involved in glucose transporter-4 translocation to the plasma membrane in both adipocytes (16) and muscle cells (17). It is possible that alterations in the CAV1 gene are affecting one or both of these processes, leading to the hyperinsulinemic state seen in our data.
Although research in humans is more limited, three studies support a potential relationship between IR and CAV1: 1) CAV1 mRNA levels are greater in fat from obese than lean participants (18); 2) mutations in the CAV1 gene have been linked to lipodystrophy, a disease of abnormal fat distribution and severe IR (19); and 3) genetic variants within the CAV1 docking domain of the insulin receptor gene cause severe IR (13, 20). Together these studies suggest that CAV1 is involved in metabolic regulation and support our findings that CAV1 is a marker for IR in hypertensive humans.
This study has several limitations. First, although the mouse model suggests that the IR-hypertension phenotype is associated with reduced CAV1 protein levels, we did not assess this possibility in humans. Second, the causal alleles at the CAV1 locus remain unknown. The two SNP identified are located in introns of the CAV1 gene. However, both SNP are in strong linkage disequilibrium (D′ > 0.9) with CAV1 gene promoter variants (e.g. rs2215448), suggesting that this SNP may be a marker for altered CAV1 gene transcription. Third, the two cohorts described are small by genome-wide association studies standards. However, our results suggest that using a more homogeneous intermediate phenotype identified by studying individuals in a carefully controlled environment allows the use of smaller sample sizes to identify genotype/phenotype associations.
In summary, variants of the CAV1 gene are associated with hyperinsulinemia and IR in humans with hypertension. Our animal data suggest that the mechanism underlying this finding may be decreased CAV1 levels leading to alterations in glucose metabolism. These findings have important clinical implications. First, they identify a genetic marker that might aid in identifying individuals at risk for metabolic disease. Second, this study identifies a novel pathway that contributes to IR in humans. New therapies targeting this pathway may provide individualized treatment to patients identified to have a defect in the CAV1 gene.
Acknowledgments
We gratefully acknowledge the support of the dietary, nursing, administrative, and laboratory staff of the human research centers in which these studies were performed.
This work was supported by National Institutes of Health Grants P50HL055000 (HyperPATH cohort), P50-HL55005 (HTN-IR cohort), UL1 RR025758 (Harvard Clinical and Translational Science Center), and U54LM008748; General Clinical Research Center Grants M01-RR02635 (Brigham and Women's Hospital), M01-RR000425 (Cedars-Sinai Medical Center), M01-RR000043 (University of Southern California), T32HL007609, HL104032, HL47651, HL59424, HL67974, DK79888, K23 HL084236 (to J.S.W.), KL2 RR025757 (to L.H.P.), and F31 NR011108 (to P.C.U.). Further support came from the American Heart Association Scientist Development Grant 0735609T (to L.H.P.), the Cedars-Sinai Board of Governors' Chair in Medical Genetics (to J.I.R.), and the Winnick Clinical Scholars Award (to M.O.G.).
Disclosure Summary: The authors have no disclosures.
Footnotes
- CAV1
- Caveolin-1
- HOMA-IR
- homeostatic assessment model for insulin resistance
- IR
- insulin resistance
- KO
- knockout
- SNP
- single-nucleotide polymorphism
- WT
- wild type.
References
- 1. Raji A, Williams GH, Jeunemaitre X, Hopkins PN, Hunt SC, Hollenberg NK, Seely EW. 2001. Insulin resistance in hypertensives: effect of salt sensitivity, renin status and sodium intake. J Hypertens 19:99–105 [DOI] [PubMed] [Google Scholar]
- 2. Xiang AH, Azen SP, Raffel LJ, Tan S, Cheng LS, Diaz J, Toscano E, Henderson PC, Hodis HN, Hsueh WA, Rotter JI, Buchanan TA. 2001. Evidence for joint genetic control of insulin sensitivity and systolic blood pressure in Hispanic families with a hypertensive proband. Circulation 103:78–83 [DOI] [PubMed] [Google Scholar]
- 3. Hnasko R, Lisanti MP. 2003. The biology of caveolae: lessons from caveolin knockout mice and implications for human disease. Mol Interv 3:445–464 [DOI] [PubMed] [Google Scholar]
- 4. Pojoga LH, Adamova Z, Kumar A, Stennett AK, Romero JR, Adler GK, Williams GH, Khalil RA. 2010. Sensitivity of NOS-dependent vascular relaxation pathway to mineralocorticoid receptor blockade in caveolin-1 deficient mice. Am J Physiol Heart Circ Physiol 298:H1776–H1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pojoga LH, Romero JR, Yao TM, Loutraris P, Ricchiuti V, Coutinho P, Guo C, Lapointe N, Stone JR, Adler GK, Williams GH. 2010. Caveolin-1 ablation reduces the adverse cardiovascular effects of N-omega-nitro-l-arginine methyl ester and angiotensin II. Endocrinology 151:1236–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pojoga LH, Yao TM, Sinha S, Ross RL, Lin JC, Raffetto JD, Adler GK, Williams GH, Khalil RA. 2008. Effect of dietary sodium on vasoconstriction and eNOS-mediated vascular relaxation in caveolin-1-deficient mice. Am J Physiol Heart Circ Physiol 294:H1258–H1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Underwood PC, Sun B, Williams JS, Pojoga LH, Chamarthi B, Lasky-Su J, Raby BA, Hopkins PN, Jeunemaitre X, Brown NJ, Adler GK, Williams GH. 2010. The relationship between peroxisome proliferator-activated receptor-γ and renin: a human genetics study. J Clin Endocrinol Metab 95:E75–E79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zeger SL, Liang KY. 1986. Longitudinal data analysis for discrete and continuous outcomes. Biometrics 42:121–130 [PubMed] [Google Scholar]
- 9. Willer CJ, Li Y, Abecasis GR. 2010. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics 26:2190–2191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lusis AJ, Attie AD, Reue K. 2008. Metabolic syndrome: from epidemiology to systems biology. Nat Rev Genet 9:819–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hunt SC, Ellison RC, Atwood LD, Pankow JS, Province MA, Leppert MF. 2002. Genome scans for blood pressure and hypertension: the National Heart, Lung, and Blood Institute Family Heart Study. Hypertension 40:1–6 [DOI] [PubMed] [Google Scholar]
- 12. Williams TM, Lisanti MP. 2004. The caveolin genes: from cell biology to medicine. Ann Med 36:584–595 [DOI] [PubMed] [Google Scholar]
- 13. Cohen AW, Razani B, Wang XB, Combs TP, Williams TM, Scherer PE, Lisanti MP. 2003. Caveolin-1-deficient mice show insulin resistance and defective insulin receptor protein expression in adipose tissue. Am J Physiol Cell Physiol 285:C222–C235 [DOI] [PubMed] [Google Scholar]
- 14. Kabayama K, Sato T, Saito K, Loberto N, Prinetti A, Sonnino S, Kinjo M, Igarashi Y, Inokuchi J. 2007. Dissociation of the insulin receptor and caveolin-1 complex by ganglioside GM3 in the state of insulin resistance. Proc Natl Acad Sci USA 104:13678–13683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cohen AW, Combs TP, Scherer PE, Lisanti MP. 2003. Role of caveolin and caveolae in insulin signaling and diabetes. Am J Physiol Endocrinol Metab 285:E1151–E1160 [DOI] [PubMed] [Google Scholar]
- 16. Karlsson M, Thorn H, Parpal S, Strålfors P, Gustavsson J. 2002. Insulin induces translocation of glucose transporter GLUT4 to plasma membrane caveolae in adipocytes. FASEB J 16:249–251 [DOI] [PubMed] [Google Scholar]
- 17. Oh YS, Cho KA, Ryu SJ, Khil LY, Jun HS, Yoon JW, Park SC. 2006. Regulation of insulin response in skeletal muscle cell by caveolin status. J Cell Biochem 99:747–758 [DOI] [PubMed] [Google Scholar]
- 18. Catalan V, Gomez-Ambrosi J, Rodriguez A, Silva C, Rotellar F, Gil MJ, Cienfuegos JA, Salvador J, Fruhbeck G. 2008. Expression of caveolin-1 in human adipose tissue is upregulated in obesity and obesity-associated type 2 diabetes mellitus and related to inflammation. Clin Endocrinol (Oxf) 68:213–219 [DOI] [PubMed] [Google Scholar]
- 19. Cao H, Alston L, Ruschman J, Hegele RA. 2008. Heterozygous CAV1 frameshift mutations (MIM 601047) in patients with atypical partial lipodystrophy and hypertriglyceridemia. Lipids Health Dis 7:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Iwanishi M, Haruta T, Takata Y, Ishibashi O, Sasaoka T, Egawa K, Imamura T, Naitou K, Itazu T, Kobayashi M. 1993. A mutation (Trp1193–>Leu1193) in the tyrosine kinase domain of the insulin receptor associated with type A syndrome of insulin resistance. Diabetologia 36:414–422 [DOI] [PubMed] [Google Scholar]