Abstract
Context:
Leptin affects neurogenesis, neuronal growth, and viability. We previously reported that leptin supplementation increased gray matter (GM) concentration in the anterior cingulate gyrus (ACG), cerebellum, and inferior parietal lobule, areas that are also involved in food intake.
Objective:
The aim of this study was to report the changes in brain structure at different states of leptin supplementation.
Design:
We conducted a nonrandomized trial.
Setting and Patients:
We studied three adults with congenital leptin deficiency due to a mutation in the leptin gene.
Intervention:
Patients received treatment with recombinant methionyl human leptin, with annual 11- to 36-d periods of treatment withholding followed by treatment restoration over 3 yr.
Main Outcome Measures:
GM concentration (by voxel-based morphometry analysis of magnetic resonance scans) was correlated with body mass index (BMI) and leptin supplementation.
Results:
Annually withholding leptin supplementation for several weeks increased BMI and reversed the original effects of leptin in the cerebellum and ACG. The changes in the ACG were consistent with an indirect effect of leptin mediated through increased BMI. In the cerebellum, where leptin receptors are most dense, GM changes appeared to be direct effects of leptin. Leptin restoration did not lead to recovery of GM in the short term but did lead to an unexpected GM increase in the posterior half of the left thalamus, particularly the pulvinar nucleus.
Conclusion:
These findings provide the first in vivo evidence of remarkably plastic, reversible, and regionally specific effects of leptin on human brain morphology. They suggest that leptin may have therapeutic value in modulating plasticity-dependent brain functions.
Because almost two thirds of adults in the United States are overweight or obese, discovery of the adipocyte-synthesized satiety hormone leptin and its effects to modulate body weight in mice generated great interest (1). We and others have shown that daily leptin supplementation in obese adults and children who were leptin-deficient due to rare mutations in the leptin gene normalized eating behavior, body weight, and endocrine function (2–4). Those effects are determined by leptin's effects in the hypothalamus and other brain structures. Leptin-deficient rodents exhibit smaller brains than their counterparts without leptin deficiency, and leptin administration to deficient animals results in increased brain size (5).
Obesity and higher body fat content have been associated with structural deficits throughout the brain (6–10), but the contribution of leptin to these associations remains unclear. In leptin-deficient humans, leptin supplementation leads to functional and structural changes in the brain (11–13). In a cohort of three leptin-deficient Turkish adults, 6 months of supplementation increased gray matter (GM) concentration in the medial cerebellum, anterior cingulate gyrus [ACG; in Brodmann area (BA) 32], and inferior parietal lobule (IPL; in BA 40), and these effects were sustained after 18 months of supplementation (13). We suggested that this effect reflected an increase in either the number or the size of neurons composing the GM of the three affected brain regions. In keeping with this view, a variety of studies, primarily in vitro, have shown that leptin is a pleiotropic hormone that, while regulating food intake through a primarily hypothalamic mechanism, also promotes neurogenesis, neural growth, and neural survival in brain areas other than the hypothalamus (14, 15). These findings suggest that the effects of leptin on behavior may be mediated in part by plastic structure-function brain remodeling.
To verify and extend our observation of regional leptin-induced alteration of brain structure in vivo, we tested the reversibility of these effects in the same congenitally leptin-deficient Turkish adults by briefly (11–36 d) withholding leptin treatment and assessed their relationship to changes in body fat.
Materials and Methods
Patients
Three leptin-deficient adult patients participated in this study. Leptin supplementation was undertaken with recombinant methionyl human leptin (r-metHuLeptin; Amylin Pharmaceuticals, Inc., San Diego, CA). Treatment was started in 2001, and the initial doses were 0.02 to 0.04 mg/kg given sc once a day at 1800 h, designed to achieve a normal leptin concentration based on a body fat of 30% in females and 20% in males. Subsequently, doses were decreased as patients lost weight to avoid excessively rapid weight loss. Because body weights have been stable since 2004, patients have been on the same dose since that year and throughout this study (patient A: 30-yr-old male, 0.15 mg/d; patient B: 39-yr-old female, 0.2 mg/d; patient C: 44-yr-old female, 2.5 mg/d). During the fourth year of treatment (2005), doses of leptin were equal to 2.33 μg/kg · d for patient A, 3.40 μg/kg · d for patient B, and 33.69 μg/kg · d for patient C. During the fifth year of treatment (2006), doses were 2.30 μg/kg · d for patient A, 3.12 μg/kg · d for patient B, and 34.12 μg/kg · d for patient C. In the sixth year of treatment (2007), patient A was receiving 2.33 μg/kg · d, patient B was receiving 3.19 μg/kg · d, and patient C was receiving 23.07 μg/kg · d. Higher doses in patient C were required to normalize metabolic and endocrine parameters because this patient suffers from common obesity and therefore has leptin resistance.
Clinical response to the initial leptin supplementation has been extensively described elsewhere (4, 16–18). In brief, leptin replacement led to resolution of hyperphagia and to massive weight loss, mostly through reduction of fat mass. The mean body mass index (BMI) decreased from 51.2 ± 2.5 kg/m2 at baseline to 36.5 ± 2.3 kg/m2 after 6 months; BMI was 28.9 ± 3.2 kg/m2 after 12 months of treatment and 26.9 ± 2.1 kg/m2 after 18 months of treatment. The weight losses of patients A, B, and C after 18 months of treatment were 76.2, 47.5, and 60.0 kg, respectively, corresponding to 53.8, 43.5, and 44.5% of their body weight, respectively. After the initial loss, weight stabilized. With weight loss, patient C, who was diabetic, became normoglycemic. Important metabolic changes, such as decreases in serum free fatty acids, triglycerides, total cholesterol, insulin, and insulin resistance were also observed. The patients had hypogonadotropic hypogonadism but became eugonadal after treatment. The circadian rhythms of cortisol and TSH also normalized.
The study protocol was approved by the Food and Drug Administration and by the University of California-Los Angeles Institutional Review Board. Informed written consent was given by all subjects.
Data acquisition
Between the fourth and the sixth years of treatment, patients underwent T1-weighted MPRAGE magnetic resonance imaging whole-brain images using a Siemens 1.5 Tesla scanner (repetition time = 1900 msec, echo time = 4.38 msec, field of view = 256, matrix = 256 × 256, 160 sagittal 1-mm slices). The images were acquired at three times during each annual visit: after more than 10 months of uninterrupted leptin supplementation (LEPTIN+); after 11–36 d without leptin (LEPTIN−) (2005 mean = 18 d, 2006 = 33 d, 2007 = 35 d off leptin); and 11–22 d after leptin was restored (LEPTIN+Brief) (2005 mean = 14 d, 2006 = 15 d, 2007 = 20 d on leptin). Table 1 presents the number of days of ongoing leptin supplementation at the time of each structural scan, with the associated weight and BMI of each patient.
Table 1.
Weight, BMI, and duration of leptin supplementation at each structural scan
| Patient | Leptin condition | 2005 |
2006 |
2007 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Weight (kg) | BMI (kg/m2) | Leptin days | Weight (kg) | BMI (kg/m2) | Leptin days | Weight (kg) | BMI (kg/m2) | Leptin days | ||
| Male, age 30 yr (2005) | + | 63.56 | 23.1 | >300 | 65.70 | 23.8 | >300 | 65.83 | 23.9 | >300 |
| − | 67.30 | 24.4 | −11 | 72.70 | 26.4 | −33 | 71.28 | 25.9 | −34 | |
| Brief | 65.80 | 23.9 | 11 | 71.60 | 26.0 | 15 | 72.64 | 26.4 | 21 | |
| Female, age 39 yr (2005) | + | 60.00 | 25.6 | >300 | 64.40 | 27.5 | >300 | 64.47 | 27.5 | >300 |
| − | 61.40 | 26.2 | −20 | 70.00 | 29.9 | −33 | 68.10 | 29.1 | −34 | |
| Brief | 60.10 | 25.7 | 12 | 66.90 | 28.6 | 15 | 65.83 | 28.1 | 16 | |
| Female, age 44 yr (2005) | + | 72.30 | 30.9 | >300 | 76.70 | 32.8 | >300 | 81.72 | 34.9 | >300 |
| − | 77.30 | 33.0 | −23 | 81.80 | 34.9 | −33 | 86.26 | 36.8 | −36 | |
| Brief | 78.20 | 33.4 | 18 | 83.80 | 35.8 | 15 | 85.81 | 36.7 | 22 | |
Statistical analysis
Structural changes were assessed with voxel-based morphometry (VBM) within the SPM2 software package (Welcome Department of Imaging Neuroscience, London, UK; http://www.fil.ion.ucl.ac.uk/spm/software/spm2/). VBM is an objective technique for investigating regional changes in brain structure by contrasting segmented GM images from high-resolution structural magnetic-resonance scans to an atlas-based stereotactic space and applying voxelwise statistics in the context of Gaussian random fields (19, 20); each voxel represents the average of itself and its neighbors, allowing the detection of small differences in volume. We used a VBM toolbox optimized for longitudinal analysis (two-step intra-subject registration, correction of bias between subsequent scans) to assess changes in GM tissue concentration over the three conditions (http://dbm.neuro.uni-jena.de/vbm/vbm2-for-spm2/longitudinal-data/).
All images were segmented to a study-specific template to control for scanner bias and then transformed into 1-mm isotropic voxels in the standard coordinate system developed at the Montreal Neurological Institute (MNI space), where positive values of the x, y, and z coordinates approximately represent millimeters to the right, anterior, and superior directions, relative to the midpoint of the anterior commissure. One-tailed statistical parametric maps were constructed of voxels where t>2.82 (P < 0.005), when comparing leptin supplementation conditions.
We hypothesized that short-term removal of leptin supplementation would reverse increases in GM (i.e. reduce GM concentration) and that restoration of supplementation would restore them in the three structures where the initial 18 months of leptin supplementation increased GM in our previous study (13). The hypotheses were tested using a spatial-extent criterion for predicted local effects by evaluating the size of the cluster of contiguous suprathreshold voxels closest to each of the three previously reported effects. These locations were in the anterior cingulate cortex (−6, 32, 22), the cerebellum (−10, −46, −46), and the IPL (−46, −46, 48). In Statistical Parametric Mapping result tables, the appropriate results are listed in the cluster column labeled “uncorrected P value” to designate that the P value reflects the probability of obtaining this large a cluster in the closest cluster to an a priori hypothesized location. The probability, therefore, should not be corrected for multiple location comparisons. We also required the nearest cluster to be within the anatomical structure of interest—the cerebellum, ACG, or IPL—to be considered as supportive evidence. Results were adjusted for local differences in smoothness of the underlying t-image, as required for valid VBM spatial extent inference (21).
As a supplemental source of evidence, we also assessed the magnitude of GM change in each a priori hypothesized exact location (effect-size criterion). Because a single voxel and cluster were tested for each hypothesized effect, no multiple-comparison correction is needed for assessment of the reversibility of the GM effect at an individual location. A Bonferroni multiple-comparison correction (0.05/3 = 0.017) was made, however, for the superordinate hypothesis that the total effects of the initial 18 months of leptin supplementation on brain structure were reversed by withholding leptin for 1 month. Unexpected GM changes in other structures were assessed using the criterion of P < 0.05 for cluster size after multiple-comparisons correction for whole-brain search volume.
To explore the relative contributions of direct effects of leptin and indirect effects mediated by changes in body mass and fat content, an otherwise identical analysis modeled two additional factors as covariates in addition to the three leptin conditions. The first factor was the BMI at the time of each scan. The second factor was the number of days of continuous leptin supplementation at the time of each scan. This procedure allowed comparison of the extent to which regional GM changes across all conditions were explained by changes in body mass after removal of effects of the duration of leptin supplementation and effects of the duration of leptin supplementation after removal of effects of body mass. A value of 300 d was used for the first scan each year when leptin supplementation had been ongoing for at least 10 months. Negative numbers quantified the number of days since leptin had been stopped during the second scan of each year.
Results
Effects of withholding leptin supplementation
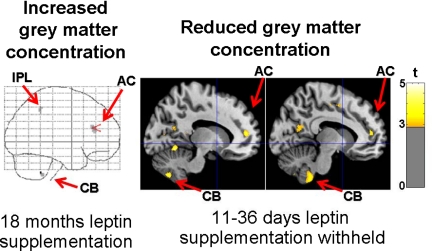
There was no evidence that withholding leptin affected GM concentration in structures outside of the three structures (ACG, cerebellum, IPL) where the original 18 months of supplementation increased GM (13). There were, however, substantial clusters of voxels with decreased GM in the left ACG (857 voxels, t = 4.26 at x, y, z coordinates −12, 44, 14), and cerebellum (931 voxels, t = 4.25 at −7, −50, −47), as well as a small cluster in the IPL (36 voxels, t = 3.06 at −40, −44, 41) (Fig. 1).
Fig. 1.
Statistical parametric maps indicate effects of leptin supplementation and withholding of supplementation on brain structure. Areas where 18 months of leptin supplementation previously increased GM concentration are shown at left, superimposed on the “glass-brain” [Adapted with permission from J. A. Matochik et al.: J Clin Endocrinol Metab 90:2851–2854, 2005 (13). © The Endocrine Society.] Colored voxels in the right panel depict decreases in GM concentration (P < 0.005 uncorrected) after withholding leptin for 11–36 d (mean, 28.6) in the current study, superimposed on sagittal slices 13 and 10 mm to the left of midline on a normalized T1 image. AC, Anterior cingulate; CB, cerebellum; IPL, inferior parietal lobule.
The size of the clusters closest to the locations of expected GM decrease were significant in the left anterior cingulate cortex (857 voxels; P = 0.013) and cerebellum (931 voxels; P = 0.011), but not within the IPL (36 voxels). The hypothesized cerebellar voxel itself (−10, −46, −46) also showed significant GM decrease by the effect-size criterion (t = 3.29; P = 0.002). In addition, there were seven other clusters where GM decreased within the left cerebellum. Together, they comprised 16.4% of the left cerebellum. In sum, there was evidence, after Bonferroni correction, that short-term removal of long-standing leptin supplementation reversed GM increases recorded during the early months of supplementation in the anterior cingulate cortex (spatial extent criterion) and cerebellum (spatial extent and effect-size criteria). GM concentration was reduced by about 2% at the exact location of hypothesized decrease within the cerebellum and a nearby location within the anterior cingulate cortex (Fig. 2).
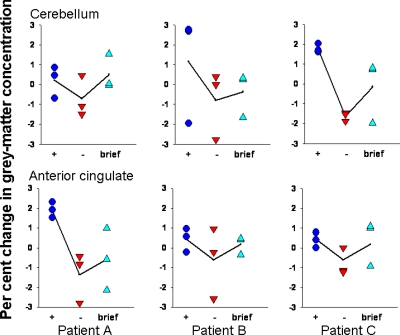
Fig. 2.
Graphs quantify GM change for all subjects and scans at the a priori hypothesized location in the cerebellum (−10, −46, −46) and near the a priori hypothesized location within the anterior cingulate (−12, 42, 14). +, More than 10 months of daily leptin; −, 11–36 d without leptin (mean, 28.6); brief, 11–22 d after daily leptin was restored (mean, 16.1).
Effects of restoring leptin supplementation
Figure 2 also suggests that GM decreases when leptin was withheld for 11–36 d may have shown some recovery of GM after leptin was restored for 11–22 d. However, the contrast LEPTIN+Brief greater than LEPTIN− indicated that recovery of GM in the three expected locations (13) did not attain significance. There were no significant areas of unexpected GM decrease after leptin was briefly restored, but there was an unexpected GM increase in the posterior half of the left thalamus, particularly the pulvinar nucleus (5179 voxels, spatial extent P < 0.005 corrected for whole-brain search volume).
Roles of changes in body mass and duration of leptin supplementation
Withholding leptin supplementation resulted in increased weight and BMI during the second structural scan of each year, in comparison to the first scan. The average increase in weight per day without leptin was 0.24 kg for patient A, 0.12 kg for patient B, and 0.17 kg for patient C. During the 11–22 d of resupplementation between structural scans 2 and 3, there were average decreases in weight of 0.05 kg/d by patient A and 0.15 kg/d by patient B. Patient C, who suffers from common obesity, continued to gain 0.05 kg/d during initial resupplementation, but in both 2005 and 2006 she lost that weight and some of the additional weight gained while leptin was withheld during the more than 10 months of leptin supplementation before the first scan of the next year (Table 1).
In the analysis that modeled both the number of days of contiguous leptin supplementation and the BMI at each scan, the contrast LEPTIN+ greater than LEPTIN− generated no voxels with t > 3.87 and no clusters that were significant for spatial extent. This indicates that the administration of leptin and weight loss jointly explained most or all effects of leptin therapy on regional GM. The results for the two covariates are presented in Table 2 and depicted in Fig. 3.
Table 2.
Relationship of regional GM to BMI and days of contiguous leptin supplementation
| Cluster size |
Peak voxel |
Structures | |||||
|---|---|---|---|---|---|---|---|
| P | k | t | Location | ||||
| Negative covariation with BMI after removing effect of contiguous leptin daysa | 0.003 | 6012 | 5.32 | −9 | 32 | 0 | L anterior cingulate (B24 and 32) |
| 0.011 | 4220 | 5.06 | −47 | −55 | 23 | L superior temporal G (B39) | |
| L IPL | |||||||
| 0.027 | 3298 | 4.95 | 23 | 48 | −5 | R middle frontal G (B11) | |
| R anterior cingulate (B32) | |||||||
| 0.041 | 2893 | 6.97 | 45 | −48 | −4 | R inferior temporal G (B19) | |
| Positive covariation with contiguous leptin days after removing effect of BMIa | 0.016 | 3828 | 5.22 | 33 | −15 | −20 | R hippocampus |
| 0.030 | 3188 | 6.28 | −29 | −57 | −42 | L cerebellum | |
P, Whole-brain corrected spatial extent (cluster size) probability; k, number of 1 × 1 × 1 mm voxels; t, Student's t-statistic; G, gyrus; L, left; R, right.
Clusters of contiguous voxels from a whole-brain analysis with voxel P < 0.005 (uncorrected).
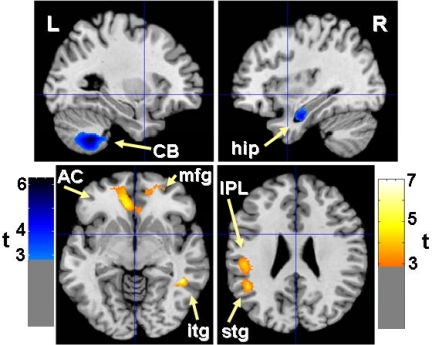
Fig. 3.
Statistical parametric maps indicate areas where GM concentration showed positive covariation with days of leptin supplementation (blue voxels) and negative covariation with BMI (yellow voxels). Results (uncorrected voxel, P < 0.005; and whole-brain corrected spatial extent, P < 0.05) are superimposed on a normalized T1 image for sagittal slices 29 mm to the left (upper left panel) and 34 mm to the right of midline (upper right panel), and axial slices 3 mm below (lower left panel) and 29 mm above the anterior commissure (lower right panel). AC, Anterior cingulate; CB, cerebellum; mfg, middle frontal gyrus; stg, superior temporal gyrus; itg, inferior temporal gyrus; hip, hippocampus; IPL, inferior parietal lobule.
By contrasting the power of the two factors (duration of leptin supplementation and weight loss) to explain covariation at the locations of expected changes in GM structure, we observed that the closest cluster to the cerebellar location where initial leptin supplementation increased GM (13) was larger for positive covariation with days of leptin (3188 voxels; P < 0.0005) than for negative covariation with BMI (not in cerebellum). When corrected for whole-brain volume, the cerebellar cluster associated with leptin supplementation remained significant for spatial extent (P = 0.030).
In the two other locations, the same covariation analysis showed that weight loss was more significantly associated with the changes in GM. The closest cluster to the ACG location where initial leptin supplementation increased GM (13) was larger for negative covariation with BMI (6012 voxels; P < 0.0005) than for positive covariation with days of leptin (no anterior cingulate voxels). This BMI cluster included almost all of the left ACG (BA 24 and 32) and small portions of the right ACG (BA 32) and the left middle and superior frontal gyri; it remained significant for spatial extent when corrected for whole-brain volume (P < 0.011). Similarly, the closest cluster to the IPL location where initial leptin supplementation increased GM was also significant for negative covariation with BMI (4220 voxels; P < 0.0005), but not for positive covariation with duration of leptin supplementation (nearest cluster was not in the parietal lobe). This BMI cluster extended from the left IPL to the superior temporal gyrus and also remained significant for spatial extent when corrected for whole-brain volume (P < 0.003).
Outside of the three left hemisphere structures where we predicted GM effects based on our previous paper, there was an unexpected positive covariation between duration of leptin supplementation and GM concentration in the right hippocampus, and there were negative covariations with BMI in two right hemisphere clusters. One extended from the anterior perigenual portion of the ACG (B32) to the middle frontal gyrus (B11); the other was in the inferior temporal gyrus (B19).
Discussion
Although the hypothalamus is considered the primary site of the anorexigenic effects of the hormone, leptin supplementation to genetically deficient human subjects increased GM concentration in other regions—the left ACG, the cerebellum, and the IPL (13). We now report that repeated withholding of supplementation in the same subjects for 11–36 d/yr (mean, 28.6) over 3 yr was associated with GM loss in the same regions, indicating reversible effects of leptin in brain regions outside the hypothalamus. Daily leptin restoration for 11–22 d (mean, 16.1) did not lead to significant recovery of GM after withholding leptin supplementation, but it did lead to an unexpected GM increase in the posterior half of the left thalamus, particularly the pulvinar nucleus.
Although we found stronger effects of leptin on GM in the left cerebral hemisphere, this asymmetry derives partially from assessing the reversibility of three initial effects of leptin on GM that were all left-sided. Half of the results in Table 2 were located in the right cerebral hemisphere, indicating no methodological problem detecting such effects when statistics are applied equally to both hemispheres. Asymmetry of the reported findings could be due to the small sample size mandated by the rarity of the genetic defect studied because any factor, including random noise, that makes measurement in either cerebral hemisphere slightly more efficient could produce asymmetry in a small sample. The reported asymmetries may also be real, possibly determined by genetic factors, because these subjects come from the same family. We feel it would be premature to interpret asymmetries at this time.
The regions where leptin led to increases of GM have been associated with regulation of hunger in human subjects and with body weight disorders (22). The ACG, an executive center for behavioral control, is selectively activated by unpleasant sensations, such as those that often accompany hunger. Glucose metabolism in this region is higher in patients with Alzheimer's disease who have comorbid obesity than in those who do not; and glucose metabolism only in the ACG is positively correlated with BMI (23). In anorexic patients who recover, but not those who do not recover or healthy control subjects, food cues, but not emotional stimuli, increase activity in the ACG and decrease activity in the IPL (24).
Abnormally low IPL activation, observed in other studies of anorexia, may be related to distorted body image (25). More generally, the IPL has been implicated in attention, sensory adaptation, and sensorimotor integration of visual with proprioceptive information. It receives a major projection from the cerebellum (26). Aside from being important for motor control, the cerebellum itself has been implicated in cognition and emotion. It directly detects blood-borne nutritional signals and is activated during food anticipation (27) and cue-induced cocaine craving (28).
Caution should be taken in interpreting the unexpected, unilateral thalamic effect of restoring leptin for 11–22 d. The thalamus does, however, regulate food-seeking through relay of taste. Activity elicited by food cues in the pulvinar nucleus is increased by ghrelin and is correlated with self-rated hunger (29).
Structural and functional characteristics of these regions have also been associated with leptin effects in leptin-normal subjects. In a sample of 198 elderly subjects assessed with magnetic resonance imaging, leptin levels were positively associated with larger total cerebral brain volume after controlling for age, sex, depression, body fat content, and other risk factors for dementia and vascular disease (30). Plasma leptin concentration was positively correlated with cerebellar GM volume in lean human subjects who were fasting (31) and in nonobese elderly subjects (32). In leptin-normal obese patients who experienced 10% weight loss, hunger accompanied activation (measured with functional magnetic resonance imaging) in the medial cerebellum and decreased activity in the ACG and IPL, whereas leptin administration reversed these regional effects of hunger on brain function (33). Our findings suggest that site-specific plasticity in these brain regions may contribute to the mechanism by which leptin affects functional responses to hunger and satiety.
Although withholding leptin reversed the effects of prior leptin supplementation on GM structure in the cerebellum and ACG, it remained unclear whether these were direct effects of leptin on brain structure or were mediated by leptin-induced changes in BMI. Therefore, our second analysis modeled both BMI and a graded measure of direct leptin influence, the number of days of contiguous leptin supplementation.
It is difficult to separate these correlated variables (Pearson product-moment correlation = −0.74 for patient A, −0.39 for patient B, and −0.56 for patient C), but the analysis partitioned leptin influences on GM across all the scans into those best explained indirectly by leptin-induced changes in BMI vs. direct effects of the duration of leptin supplementation. The results indicated that GM changes in the cerebellum were direct effects of leptin. In contrast, GM changes in the ACG were explained by effects on BMI. Although there was not a direct effect of leptin on GM in the left anterior parietal lobule in our initial analysis, BMI was negatively correlated with GM structure, suggesting that changes in BMI during the initial 18 months of leptin supplementation produced the left IPL GM increase reported in our 2005 paper.
We also noted an unexpected positive correlation between duration of leptin supplementation and GM structure in the right hippocampus and an unexpected negative correlation between BMI and GM structure in the right inferior temporal gyrus (Table 2). Although these unexpected findings should be considered exploratory and should be interpreted with caution, there is substantial evidence from previous studies that leptin levels are positively correlated with hippocampal structure and function (32).
Unexpectedly, brief leptin restoration did not lead to recovery of GM after withholding leptin supplementation. This may reflect the relatively short period of resupplementation (11–22 d; mean, 16.1) before brain scans, and it suggests that a more prolonged period of renewed leptin supplementation may be necessary before any structural changes are observed. The results graphed in Fig. 2 suggest that some degree of recovery occurred during the 10–11 months of leptin replacement after the period of restriction in 2005 and 2006. Future studies should include more frequent measurement to address the time course of GM recovery.
In this study, BMI was strongly related to GM structure in the midline frontal lobes. Previous proton magnetic resonance spectroscopy evidence suggested that increased BMI at midlife was associated with GM and white matter abnormalities primarily in the frontal lobes (9), and a tensor-based morphometry study of elderly subjects found an inverse association of BMI with GM volumes in the ventromedial frontal cortex, including the anterior cingulate (10).
In humans, circulating leptin crosses the blood-brain barrier and interacts with its receptors (34). They are most densely expressed in the cerebellum, where the density is twice that in the hypothalamus and where leptin exerts potent neuritogenic and antiapoptotic effects on specific neuronal populations (35). It is remarkable that this area, which has the greatest density of brain leptin receptors, exhibited the strongest evidence for direct reversible effects of leptin on brain structure in the current study. Because of its role in motor control, leptin-related changes in physical activity level may also promote structural change in the cerebellum. For another study, we recorded the physical activity level of these patients while on and off treatment for 7 consecutive days in 2007 (16). Patients A and B, but not patient C, became more active off leptin. Because patient C had the largest decrease in cerebellar GM off leptin (Fig. 2), increased physical activity may protect cerebellar structure.
It is now well-established that neurogenesis occurs in the adult human brain (36). There is both in vivo and in vitro evidence from adult mice that adult hippocampal neurogenesis is at least partially induced by leptin (37). Moreover, leptin has additional effects on the development of oligodendroglial cells (38), which may contribute to the structural changes determined by the adipokine.
It is possible that the changes in GM volume associated with leptin therapy are at least partially mediated by changes in other neurotrophic factors, rather than being direct effects of leptin. In our patients, leptin therapy decreased insulin levels, increased insulin sensitivity, and normalized the gonadotropic axis, with increases in testosterone and estrogens (39). Because insulin and sex hormones have neurotrophic effects, it is possible that leptin's effects on GM volume are mediated by increases in insulin sensitivity and sex hormones. However, leptin therapy also increased the mean levels of serum cortisol measured over 24 h and increased IGF-binding protein (IGFBP) 1 and IGFPB2 (4). If the current GM changes are attributed to indirect neurotrophic factors, the antineurogenic effects of cortisol and the inhibitory effects of IGFBP1 and IGFBP2 on IGF-I would contribute to decreases in GM volume.
In conclusion, these results extend our previous findings on the initial effects of leptin supplementation on brain structure (13) by demonstrating remarkable short-term plasticity of cerebral GM in response to leptin withdrawal and replacement, both directly and mediated by leptin-associated changes in BMI. Overall, these reversible nonhypothalamic effects of leptin supplementation contribute to recent evidence for greater short-term plasticity of brain structure-function relationships (40) and suggest that leptin may have therapeutic value in modulating plasticity-dependent brain functions. Additional studies need to confirm whether the effects of leptin on brain structure in humans are attributed to its neuritogenic actions.
Acknowledgments
During the course of this study, Amgen, Inc., graciously provided leptin. Amylin, Inc., now provides leptin to these patients. Neither Amgen, Inc., nor Amylin, Inc., contributed to the design, analysis, or writing of this study.
This work was supported in part by National Institutes of Health (NIH) Grants P20DA022539 and R01DA020726 (to E.D.L.); K24RR016996, R01DK058851, and U01GM061394 (to J.L.); K24RR017365 and R01DK063240 (to M.-L.W.); and the UCLA General Clinical Research Center (NIH Grant M01RR00865 to G. S. Levy). G.P.-F., M.-L.W., and J.L. were supported by The Australian National University institutional funds. E.D.L. was supported by endowments from the Katherine K. and Thomas P. Pike Chair in Addiction Studies and the Marjorie M. Greene Family Trust.
Disclosure Summary: E.D.L., J.L., and M.-L.W. received NIH grants listed above. S.C., T.D., J.M., H.K.E., G.P.-F., and S.M.B. have nothing to disclose.
Footnotes
- ACG
- Anterior cingulate gyrus
- BA
- Brodmann area
- BMI
- body mass index
- GM
- gray matter
- IGFBP
- IGF-binding protein
- IPL
- inferior parietal lobule
- VBM
- voxel-based morphometry.
References
- 1. Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F. 1995. Effects of the obese gene product on body weight regulation in ob/ob mice. Science 269:540–543 [DOI] [PubMed] [Google Scholar]
- 2. Ozata M, Ozdemir IC, Licinio J. 1999. Human leptin deficiency caused by a missense mutation: multiple endocrine defects, decreased sympathetic tone, and immune system dysfunction indicate new targets for leptin action, greater central than peripheral resistance to the effects of leptin, and spontaneous correction of leptin-mediated defects. J Clin Endocrinol Metab 84:3686–3695 [DOI] [PubMed] [Google Scholar]
- 3. Farooqi IS, O'Rahilly S. 2009. Leptin: a pivotal regulator of human energy homeostasis. Am J Clin Nutr 89:980S–984S [DOI] [PubMed] [Google Scholar]
- 4. Licinio J, Caglayan S, Ozata M, Yildiz BO, de Miranda PB, O'Kirwan F, Whitby R, Liang L, Cohen P, Bhasin S, Krauss RM, Veldhuis JD, Wagner AJ, DePaoli AM, McCann SM, Wong ML. 2004. Phenotypic effects of leptin replacement on morbid obesity, diabetes mellitus, hypogonadism, and behavior in leptin-deficient adults. Proc Natl Acad Sci USA 101:4531–4536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ahima RS, Bjorbaek C, Osei S, Flier JS. 1999. Regulation of neuronal and glial proteins by leptin: implications for brain development. Endocrinology 140:2755–2762 [DOI] [PubMed] [Google Scholar]
- 6. Gustafson D, Lissner L, Bengtsson C, Björkelund C, Skoog I. 2004. A 24-year follow-up of body mass index and cerebral atrophy. Neurology 63:1876–1881 [DOI] [PubMed] [Google Scholar]
- 7. Pannacciulli N, Del Parigi A, Chen K, Le DS, Reiman EM, Tataranni PA. 2006. Brain abnormalities in human obesity: a voxel-based morphometric study. Neuroimage 31:1419–1425 [DOI] [PubMed] [Google Scholar]
- 8. Taki Y, Kinomura S, Sato K, Inoue K, Goto R, Okada K, Uchida S, Kawashima R, Fukuda H. 2008. Relationship between body mass index and gray matter volume in 1,428 healthy individuals. Obesity (Silver Spring) 16:119–124 [DOI] [PubMed] [Google Scholar]
- 9. Gazdzinski S, Kornak J, Weiner MW, Meyerhoff DJ. 2008. Body mass index and magnetic resonance markers of brain integrity in adults. Ann Neurol 63:652–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Raji CA, Ho AJ, Parikshak NN, Becker JT, Lopez OL, Kuller LH, Hua X, Leow AD, Toga AW, Thompson PM. 2010. Brain structure and obesity. Hum Brain Mapp 31:353–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Baicy K, London ED, Monterosso J, Wong ML, Delibasi T, Sharma A, Licinio J. 2007. Leptin replacement alters brain response to food cues in genetically leptin-deficient adults. Proc Natl Acad Sci USA 104:18276–18279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Farooqi IS, Bullmore E, Keogh J, Gillard J, O'Rahilly S, Fletcher PC. 2007. Leptin regulates striatal regions and human eating behavior. Science 317:1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Matochik JA, London ED, Yildiz BO, Ozata M, Caglayan S, DePaoli AM, Wong ML, Licinio J. 2005. Effect of leptin replacement on brain structure in genetically leptin-deficient adults. J Clin Endocrinol Metab 90:2851–2854 [DOI] [PubMed] [Google Scholar]
- 14. Morrison CD. 2009. Leptin signaling in brain: a link between nutrition and cognition? Biochim Biophys Acta 1792:401–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Paz-Filho G, Wong ML, Licinio J. 2010. The procognitive effects of leptin in the brain and their clinical implications. Int J Clin Pract 64:1808–1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Paz-Filho G, Esposito K, Hurwitz B, Sharma A, Dong C, Andreev V, Delibasi T, Erol H, Ayala A, Wong ML, Licinio J. 2008. Changes in insulin sensitivity during leptin replacement therapy in leptin-deficient patients. Am J Physiol Endocrinol Metab 295:E1401–E1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Paz-Filho GJ, Andrews D, Esposito K, Erol HK, Delibasi T, Wong ML, Licinio J. 2009. Effects of leptin replacement on risk factors for cardiovascular disease in genetically leptin-deficient subjects. Horm Metab Res 41:164–167 [DOI] [PubMed] [Google Scholar]
- 18. Galgani JE, Greenway FL, Caglayan S, Wong ML, Licinio J, Ravussin E. 2010. Leptin replacement prevents weight loss-induced metabolic adaptation in congenital leptin-deficient patients. J Clin Endocrinol Metab 95:851–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ashburner J, Friston KJ. 2000. Voxel-based morphometry—the methods. Neuroimage 11:805–821 [DOI] [PubMed] [Google Scholar]
- 20. Good CD, Scahill RI, Fox NC, Ashburner J, Friston KJ, Chan D, Crum WR, Rossor MN, Frackowiak RS. 2002. Automatic differentiation of anatomical patterns in the human brain: validation with studies of degenerative dementias. Neuroimage 17:29–46 [DOI] [PubMed] [Google Scholar]
- 21. Moorhead TW, Job DE, Spencer MD, Whalley HC, Johnstone EC, Lawrie SM. 2005. Empirical comparison of maximal voxel and non-isotropic adjusted cluster extent results in a voxel-based morphometry study of comorbid learning disability with schizophrenia. Neuroimage 28:544–552 [DOI] [PubMed] [Google Scholar]
- 22. Tataranni PA, Gautier JF, Chen K, Uecker A, Bandy D, Salbe AD, Pratley RE, Lawson M, Reiman EM, Ravussin E. 1999. Neuroanatomical correlates of hunger and satiation in humans using positron emission tomography. Proc Natl Acad Sci USA 96:4569–4574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hu X, Okamura N, Arai H, Higuchi M, Maruyama M, Itoh M, Yamaguchi K, Sasaki H. 2002. Neuroanatomical correlates of low body weight in Alzheimer's disease: a PET study. Prog Neuropsychopharmacol Biol Psychiatry 26:1285–1289 [DOI] [PubMed] [Google Scholar]
- 24. Uher R, Brammer MJ, Murphy T, Campbell IC, Ng VW, Williams SC, Treasure J. 2003. Recovery and chronicity in anorexia nervosa: brain activity associated with differential outcomes. Biol Psychiatry 54:934–942 [DOI] [PubMed] [Google Scholar]
- 25. Van Vugt DA. 2010. Brain imaging studies of appetite in the context of obesity and the menstrual cycle. Hum Reprod Update 16:276–292 [DOI] [PubMed] [Google Scholar]
- 26. Clower DM, West RA, Lynch JC, Strick PL. 2001. The inferior parietal lobule is the target of output from the superior colliculus, hippocampus, and cerebellum. J Neurosci 21:6283–6291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mendoza J, Pévet P, Felder-Schmittbuhl MP, Bailly Y, Challet E. 2010. The cerebellum harbors a circadian oscillator involved in food anticipation. J Neurosci 30:1894–1904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, Phillips RL, Kimes AS, Margolin A. 1996. Activation of memory circuits during cue-elicited cocaine craving. Proc Natl Acad Sci USA 93:12040–12045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Malik S, McGlone F, Bedrossian D, Dagher A. 2008. Ghrelin modulates brain activity in areas that control appetitive behavior. Cell Metab 7:400–409 [DOI] [PubMed] [Google Scholar]
- 30. Lieb W, Beiser AS, Vasan RS, Tan ZS, Au R, Harris TB, Roubenoff R, Auerbach S, DeCarli C, Wolf PA, Seshadri S. 2009. Association of plasma leptin levels with incident Alzheimer disease and MRI measures of brain aging. JAMA 302:2565–2572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pannacciulli N, Le DS, Chen K, Reiman EM, Krakoff J. 2007. Relationships between plasma leptin concentrations and human brain structure: a voxel-based morphometric study. Neurosci Lett 412:248–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Narita K, Kosaka H, Okazawa H, Murata T, Wada Y. 2009. Relationship between plasma leptin level and brain structure in elderly: a voxel-based morphometric study. Biol Psychiatry 65:992–994 [DOI] [PubMed] [Google Scholar]
- 33. Rosenbaum M, Sy M, Pavlovich K, Leibel RL, Hirsch J. 2008. Leptin reverses weight loss-induced changes in regional neural activity responses to visual food stimuli. J Clin Invest 118:2583–2591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Burguera B, Couce ME, Long J, Lamsam J, Laakso K, Jensen MD, Parisi JE, Lloyd RV. 2000. The long form of the leptin receptor (OB-Rb) is widely expressed in the human brain. Neuroendocrinology 71:187–195 [DOI] [PubMed] [Google Scholar]
- 35. Oldreive CE, Harvey J, Doherty GH. 2008. Neurotrophic effects of leptin on cerebellar Purkinje but not granule neurons in vitro. Neurosci Lett 438:17–21 [DOI] [PubMed] [Google Scholar]
- 36. Gould E. 2007. How widespread is adult neurogenesis in mammals? Nat Rev Neurosci 8:481–488 [DOI] [PubMed] [Google Scholar]
- 37. Garza JC, Guo M, Zhang W, Lu XY. 2008. Leptin increases adult hippocampal neurogenesis in vivo and in vitro. J Biol Chem 283:18238–18247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Udagawa J, Nimura M, Otani H. 2006. Leptin affects oligodendroglial development in the mouse embryonic cerebral cortex. Neuro Endocrinol Lett 27:177–182 [PubMed] [Google Scholar]
- 39. Paz-Filho G, Mastronardi C, Delibasi T, Wong ML, Licinio J. 2010. Congenital leptin deficiency: diagnosis and effects of leptin replacement therapy. Arq Bras Endocrinol Metabol 54:690–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A. 2004. Neuroplasticity: changes in grey matter induced by training. Nature 427:311–312 [DOI] [PubMed] [Google Scholar]