Abstract
Context:
Obesity and diabetes are more common in African-Americans than whites. Because free fatty acids (FFA) participate in the development of these conditions, studying race differences in the regulation of FFA and glucose by insulin is essential.
Objective:
The objective of the study was to determine whether race differences exist in glucose and FFA response to insulin.
Design:
This was a cross-sectional study.
Setting:
The study was conducted at a clinical research center.
Participants:
Thirty-four premenopausal women (17 African-Americans, 17 whites) matched for age [36 ± 10 yr (mean ± sd)] and body mass index (30.0 ± 6.7 kg/m2).
Interventions:
Insulin-modified frequently sampled iv glucose tolerance tests were performed with data analyzed by separate minimal models for glucose and FFA.
Main Outcome Measures:
Glucose measures were insulin sensitivity index (SI) and acute insulin response to glucose (AIRg). FFA measures were FFA clearance rate (cf).
Results:
Body mass index was similar but fat mass was higher in African-Americans than whites (P < 0.01). Compared with whites, African-Americans had lower SI (3.71 ± 1.55 vs. 5.23 ± 2.74 [×10−4 min−1/(microunits per milliliter)] (P = 0.05) and higher AIRg (642 ± 379 vs. 263 ± 206 mU/liter−1 · min, P < 0.01). Adjusting for fat mass, African-Americans had higher FFA clearance, cf (0.13 ± 0.06 vs. 0.08 ± 0.05 min−1, P < 0.01). After adjusting for AIRg, the race difference in cf was no longer present (P = 0.51). For all women, the relationship between cf and AIRg was significant (r = 0.64, P < 0.01), but the relationship between cf and SI was not (r = −0.07, P = 0.71). The same pattern persisted when the two groups were studied separately.
Conclusion:
African-American women were more insulin resistant than white women, yet they had greater FFA clearance. Acutely higher insulin concentrations in African-American women accounted for higher FFA clearance.
African-American women have a higher prevalence of obesity and type 2 diabetes mellitus (T2DM) than white women (1–4). Yet African-American women have lower triglyceride (TG) levels, less visceral adipose tissue (VAT), and less hepatic fat than white women (5–7). Free fatty acids (FFA) are linked to TG levels. FFA liberated from VAT can be reesterified in the liver into TG and either stored or secreted into circulation in the form of very low-density lipoprotein particles. Race differences in the regulation of FFA by insulin may contribute to these three paradoxical findings in otherwise insulin-resistant African-American women.
We recently developed a quantitative measure of FFA response to insulin (8). By using the insulin-modified frequently sampled iv glucose tolerance test (IM-FSIGT) and expanding on the work of Bergman and colleagues (8, 9), we augmented the minimal model for glucose disposal to include changes in FFA concentration. To determine whether race differences in the ability of insulin to regulate glucose and FFA exist, we performed IM-FSIGT in African-American and white women. The question addressed in this study is whether there are quantifiable race differences in the regulation of glucose and FFA by insulin during the IM-FSIGT.
Materials and Methods
Premenopausal women (17 African-Americans, 17 whites) matched for age and body mass index (BMI) participated. Recruitment was by flyers, newspaper advertisements, and the National Institutes of Health web site. Only nondiabetic women with normal hemograms, liver, kidney, and thyroid function were enrolled. Participants were not taking medications known to affect either glucose or lipid metabolism. No women were taking oral contraceptives. Because the menstrual cycle does not impact FFA metabolism (10), studies were performed throughout the cycle. The Institutional Review Board of the National Institute of Diabetes and Digestive and Kidney Diseases approved the study. All subjects gave informed consent.
Protocol
At visit 1, a medical history and physical examination were performed and routine blood work obtained.
At visit 2, an IM-FSIGT was performed. Subjects arrived at the center at 0700 h after a 12-h fast. An iv catheter was placed in each antecubital vein. After baseline samples were obtained, dextrose (0.3 g/kg) was administered iv over 1 min. A bolus injection of insulin (0.03 U/kg) was given at 20 min. Blood samples were taken at −10, −5, −1, 1, 2, 3, 4, 5, 6, 7, 8, 10, 12, 14, 16, 19, 22, 23, 24, 25, 27, 30, 40, 50, 60, 70, 80, 90, 100, 120, 150, and 180 min.
At visit 3, waist (WC), hip, and thigh circumferences were measured. Abdominal computerized tomographic and dual-energy x-ray absorptiometry scans were performed for VAT area and body composition, respectively.
Insulin resistance and acute insulin response to glucose (AIRg)
The insulin sensitivity index (SI) was determined from the minimal model using glucose and insulin concentrations from the IM-FSIGT (MinMODMillenium) (11). AIRg was calculated as the area under the curve for insulin between 0 and 10 min for the insulin concentration above basal. The disposition index (DI), calculated as the product of AIRg and SI, was determined as a measure of β-cell function, specifically defined as the ability of the circulating insulin concentration to compensate for insulin resistance (12).
Anthropometrics
For waist, hip, and thigh circumferences nonstretchable tape was used. WC was measured at the iliac crest. Hip circumference was measured at the level of the maximum extension of the buttocks posteriorly in a horizontal plane. Thigh circumference was measured midway between the inguinal crease and proximal border of the patella.
VAT and sc adipose tissue (SAT)
VAT and SAT were measured at L2–3 using a HiSpeed Advantage CT/I scanner (GE Medical Systems, Milwaukee, WI) and analyzed on a SUN workstation with MEDx image analysis software package (Sensor System, Inc., Sterling, VA) (13).
Body composition
Percent fat and fat mass were determined with a Hologic QDR 4500A (Hologic, Inc., Bedford, MA) in the array mode (software version 5.71A).
Analytic measures
Insulin was measured by a solid-phase, two-site chemiluminescence immunometric assay on Immulite 2500 analyzers (Siemens Healthcare Diagnostics, Deerfield, IL). Glucose and TG were measured on Dimension Vista 1500 analyzers (Siemens) using standard automated methods. Between-run coefficients of variation (CV) for glucose ranged from 1.7 to 1.8%, and TG ranged from 2.1 to 3.3%. FFA were measured with a Wako HR Series NEFA-HR(2) kit (Wako Diagnostics, Wako Chemicals USA, Inc., Richmond, VA) and run on a COBAS FARA-II analyzer (Roche Diagnostics, Indianapolis, IN). Between-run CV for FFA ranged from 6.2 to 8.6%, within-run CV for FFA ranged from 1.5 to 3.8%. Analytical reliability of assays is monitored by College of American Pathology proficiency testing programs.
Glucose and FFA minimal models
The minimal model for glucose and FFA has the following form
| (1) |
| (2) |
| (3) |
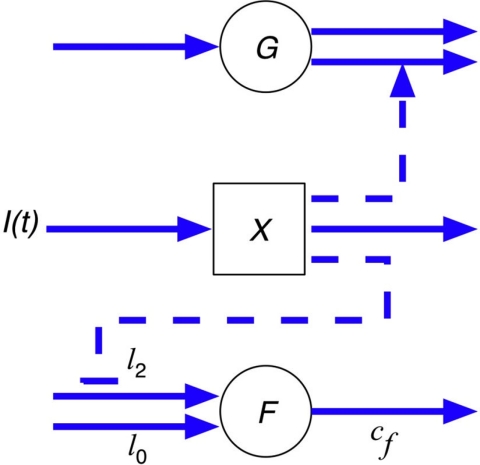
where G is the plasma glucose concentration, SG is glucose effectiveness, X is insulin action in a remote compartment, and I is the plasma insulin concentration. The FFA plasma concentration, denoted by F, has an appearance rate that is controlled by remote insulin (X) through a shifted power function, where X2 is the activation threshold for insulin's action on FFA appearance (X < X2), A is the exponent, l0 is the baseline nonsuppressible lipolysis rate, and l2 is the difference between maximum and nonsuppressible lipolysis rate. Therefore, l0 + l2 is the maximal rate of appearance, and cf is the clearance rate constant (8). Equations 1 and 2 are identical to the original minimal model for glucose disposal (9). Equation 3 models the FFA plasma kinetics where the rate of appearance of FFA through lipolysis is controlled by insulin acting through a delayed remote compartment (X), whereas FFA disappearance is through a first-order kinetic process parameterized by a clearance rate (cf). A schematic of the model is shown in Fig. 1.
Fig. 1.
Schematic for glucose and FFA minimal models. G, Plasma glucose concentration; X, insulin action in a remote compartment; F, free fatty acids; I, plasma insulin concentration; l0, baseline nonsuppressible lipolysis rate; l2, difference between maximum and nonsuppressible lipolysis rate.
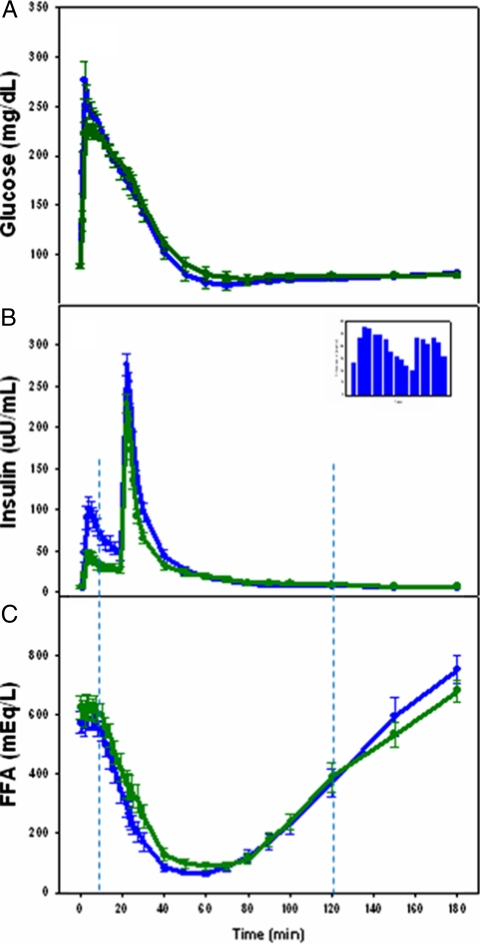
The glucose minimal model uses glucose and insulin values from the IM-FSIGT throughout the 0- to 180-min time course. However, during the IM-FSIGT, the FFA profile has four distinct phases (14). Phase 1 is the initial steady state before the impact of insulin on FFA is discernible and lasts about 10 min. Phase 2 is the decline in FFA and phase 3 is the rise of FFA. In phase 4, FFA levels return to baseline or overshoot baseline in studies lasting longer than 3 h. The FFA minimal model uses insulin and FFA from phases 2 and 3, i.e. 10–120 min (Fig. 2). The FFA model was fit to each subject with a maximum likelihood estimate for the parameters obtained (8).
Fig. 2.
Glucose, insulin, and FFA time course during the IM-FSIGT. African-American women are presented by blue lines and white women by green lines. A, Glucose concentrations (mean ± se). B, Insulin concentrations (mean ± se). Inset shows race difference in insulin concentration between 2 and 30 min (all P < 0.05). C, FFA concentrations (mean ± se). Vertical dashed lines demarcate the time period from which data are obtained for entry into the FFA minimal model (i.e. 10–120 min).
Statistical analyses
Unless stated otherwise, data presented as mean ± sd. P ≤ 0.05 defined significance. Student t tests were performed for comparison of unadjusted continuous variables with log transformation as needed to achieve normalization of data. Comparisons by race for continuous variables adjusted for BMI, fat mass, and AIRg were analyzed by analysis of covariance. The noncontinuous variables were compared by a χ2 test. Analyses were performed with STATA, version 11.0 (College Station, TX) and R, version 2.7.1 (http://www.R-project.org).
Results
BMI category distribution varied by race. The BMI category distribution for the African-American women was five normal-weight women, one overweight woman, and 11 obese women. For the white women, the BMI category distribution was seven normal-weight women, four overweight women, and six obese women. However, neither mean BMI nor mean age differed significantly by race (Table 1). Demographics including family history of diabetes, prevalence of smoking, exercise frequency, alcohol intake, and median household income also did not differ by race (Table 1). Similarly, nontraditional risk factors such as high-sensitivity C-reactive protein, fibrinogen, homocysteine, estradiol, and total testosterone did not vary by race (data not shown).
Table 1.
Demographic and metabolic characteristics
| Variable | African-Americans (n = 17) | Whites (n = 17) | P valuea |
|---|---|---|---|
| Age (yr) (mean ± sd) | 36 ± 9 | 37 ± 11 | 0.86 |
| Weight (kg) | 85.1 ± 21.0 | 79.8 ± 19.8 | 0.45 |
| Height (cm) | 163.8 ± 5.9 | 166.7 ± 7.4 | 0.20 |
| BMI (kg/m2) | 31.7 ± 7.5 | 28.4 ± 5.4 | 0.15 |
| WC (cm) | 96.5 ± 17.6 | 96.8 ± 17.0 | 0.94 |
| Hip circumference (cm) | 113.4 ± 16.1 | 109.3 ± 12.8 | 0.05b |
| WHR | 0.85 ± 0.08 | 0.88 ± 0.07 | 0.03b |
| Thigh circumference (cm) | 64.1 ± 10.1 | 55.0 ± 6.7 | <0.01 |
| Fat mass (kg) | 33.4 ± 14.8 | 30.7 ± 13.1 | <0.01b |
| Percent fat (%) | 37.1 ± 9.2 | 36.5 ± 8.0 | 0.02b |
| VAT (cm2) | 71.8 ± 58.2 | 93.3 ± 60.3 | <0.01b |
| SAT (cm2) | 295 ± 195 | 240 ± 147 | 0.21b |
| VAT to SAT ratio | 0.29 ± 0.23 | 0.41 ± 0.18 | 0.04b |
| TG (mg/dl) | 66 ± 47 | 106 ± 91 | 0.04b |
| HDL cholesterol (mg/dl) | 60 ± 17 | 51 ± 8 | 0.05b |
| Family history of DM | 41% | 41% | 0.99 |
| Smokers | 0% | 12% | 0.15 |
| Exercise intensityc | 40% | 18% | 0.08 |
| Alcohol intake (≤1 drink/wk) | 71% | 47% | 0.16 |
| Median household income ($/yr) | 55,000 | 55,000 | 0.70 |
HDL, High-density lipoprotein; DM, diabetes mellitus.
Continuous variables compared by unpaired t tests and discrete variables by χ2.
Adjusted for BMI by logistic regression.
Exercise defined as 30 min or more of vigorous activity three or more times per week.
Body fat content and distribution
Even though BMI did not differ, fat mass and percent fat were higher in African-American than white women (Table 1). VAT was lower in African-American than white women, but the WC was similar (Table 1). Waist to hip ratio (WHR) and VAT to SAT ratio were lower and thigh circumference higher in African-American than white women (Table 1). Based on these three measures, WHR, VAT to SAT ratio, and thigh circumference, African-American women have more peripheral fat and less central obesity than white women.
Glucose minimal model
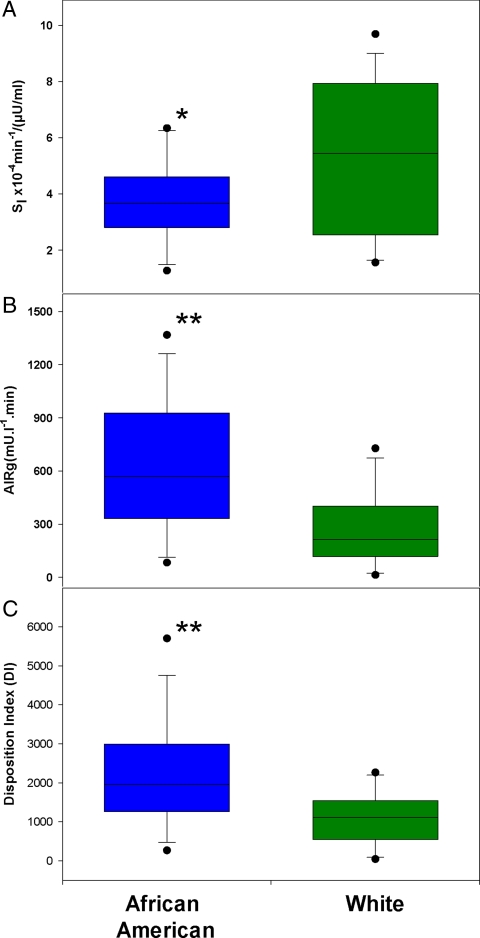
Compared with whites, African-Americans had lower SI (3.71 ± 1.55 vs. 5.23 ± 2.74 × 10−4 min−1/(μU/ml), P = 0.05), higher AIRg (642 ± 379 vs. 263 ± 206 mU/liter−1 · min, P < 0.01) and higher DI (2203 ± 1442 vs. 1108 ± 660, P < 0.01) (Fig. 3). The relationship between AIRg and SI in both African-American and white women was hyperbolic (Fig. 4). As observed graphically and by regression analyses, DI was higher in African-American than white women, and at every level of SI, AIRg was higher in African-American than white women (P < 0.01) (Fig. 4).
Fig. 3.
Box and whiskers plot for SI, AIRg, and DI. Data are presented by race. A, SI. B, AIRg. C, DI. Percentiles starting with lowest error bar are: 10th, 25th, median, 75th, and 90th. *, P < 0.05; **, P < 0.01.
Fig. 4.
Hyperbolic curve of the DI calculated as the product of AIRg and SI. African-American women are represented by blue lines and symbols and white women by green lines and symbols.
Glucose, insulin, and FFA time course
Fasting glucose did not differ by race (88 ± 8 vs. 88 ± 8, P = 0.98). But glucose levels were higher in African-American than white women from 1 to 5 min (all P < 0.05) (Fig. 2A). Fasting insulin concentrations were not different by race (6.48 ± 7.43 vs. 6.61 ± 6.04 μU/ml, P = 0.96), but from 2 to 30 min, insulin concentrations were higher in African-American than white women (all P < 0.05) (Fig. 2B). Between 10 and 60 min, FFA concentrations tended to be lower in African-American than white women but did not reach significance (Fig. 2C).
FFA minimal model
The minimal model results are presented in Table 2. X2 is the activation threshold for insulin's action on FFA appearance (i.e. inhibition of lipolysis), and it did not differ by race (2.73 ± 0.78 vs. 2.58 ± 0.72 μU/ml, P = 0.39). Similarly the rate of appearance of FFA (l0, l2, or l2+l0) was not significantly different in African-American and white women. However the FFA clearance rate constant, cf, was greater in African-American than white women (0.13 ± 0.06 vs. 0.08 ± 0.05 min−1, P = 0.02). The difference remained significant (P = 0.01) after adjusting for fat mass. However and most importantly, the race difference in cf was not present when adjusted for AIRg (P = 0.51).
Table 2.
Insulin and FFA model characteristics
| Variable (mean ± sd) | African-Americans (n = 17) | Whites (n = 17) | P value |
|---|---|---|---|
| Fasting insulin (μU/ml) | 6.5 ± 7.4 | 6.6 ± 6.0 | 0.96 |
| AIRg (mU/liter−1 · min) | 642 ± 379 | 263 ± 206 | <0.01 |
| X2 (μU/ml) | 2.73 ± 0.78 | 2.58 ± 0.72 | 0.39 |
| Fasting FFA (mEq/liter) | 575 ± 149 | 626 ± 159 | 0.34 |
| 0.21 adjusted for fat mass | |||
| l0 (μm/min) | 1.9 ± 1.7 | 1.1 ± 1.5 | 0.17 |
| 0.17 adjusted for fat mass | |||
| 0.53 adjusted for AIRg | |||
| l2 (μm/min) | 41 ± 19 | 30 ± 17 | 0.10 |
| 0.10 adjusted for fat mass | |||
| 0.49 adjusted for AIRg | |||
| l0+l2 (μm/min) | 43 ± 19 | 32 ± 18 | 0.08 |
| 0.09 adjusted for fat mass | |||
| 0.46 adjusted for AIRg | |||
| cf (min−1) | 0.13 ± 0.06 | 0.08 ± 0.05 | 0.02 |
| <0.01 adjusted for fat mass | |||
| 0.51 adjusted for AIRg |
Student t tests were performed for comparison of unadjusted variables. For conversion of insulin to international units, multiply by 6.95. Comparisons by race variables adjusted for fat mass and AIRg were analyzed by analysis of covariance. X2 is the activation threshold for insulin's action on FFA appearance, lo is the baseline nonsuppressible lipolysis rate, l2 is the difference between maximal and nonsuppressible lipolysis rates, lo+l2 is the maximum rate of appearance, and cf is the clearance rate constant.
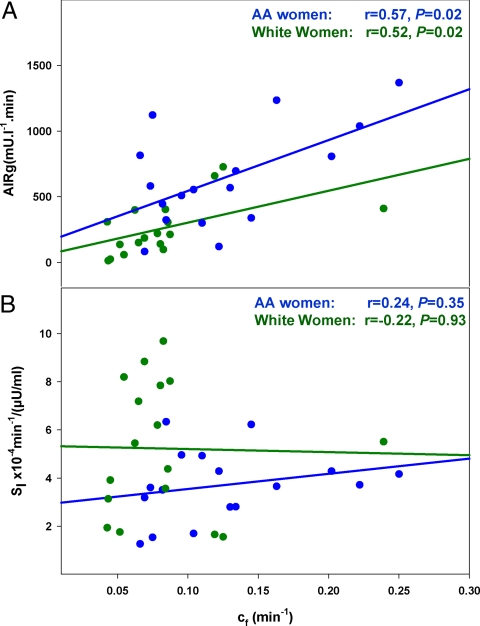
The overall dependence of cf on AIRg is reenforced by the finding that the correlation of cf with AIRg was significant (r = 0.64, P < 0.01). The correlation between cf and AIRg remained significant when African-Americans and whites were studied separately (Fig. 5A). In contrast, cf was not dependent on SI because the correlation between cf and SI was not significant (r = −0.07, P = 0.71). This remained true when African-Americans and whites were examined separately (Fig. 5B).
Fig. 5.
Correlation between AIRg and cf (A) and SI vs. cf (B). African-American women are represented by blue lines and symbols and white women by green lines and symbols.
Discussion
Performing an IM-FSIGT and simultaneously applying the minimal model for glucose and FFA, we found that insulin-induced FFA suppression is greater in African-American than white women. Higher insulin concentrations in African-American women rather than a difference in sensitivity to insulin action (i.e. SI) accounted for the greater FFA suppression in African-American than white women. Evidence implicating high insulin concentrations in African-American women is 3-fold. First, the race difference in cf was no longer presented when adjusted for AIRg. Second, there was no race difference in X2, the activation threshold for insulin's action on FFA appearance. Third, the correlation between AIRg and cf was significant while the correlation between cf and SI was not. Similarly, Koutsari et al. (15), using IM-FSIGT in a study of sex differences in FFA metabolism reported no correlation between SI and nonoxidative FFA clearance. Yet they did not go further and provide data on the relationship of FFA clearance to AIRg. We did take that next step, and our data suggest that in the evaluation of FFA metabolism in African-American women, instead of taking the more traditional approach and focusing almost exclusively on insulin resistance, the contribution of high acute insulin concentrations must also be considered.
The observation that AIRg was higher in African-Americans than whites has also been made by the Insulin Resistance and Atherosclerosis Investigators (16). It is important to follow up on this finding. Insulin is both a glucoregulatory and antilipolytic hormone. We propose that that the high insulin concentrations occurring in African-American women link these two actions of insulin. All the women enrolled in this study are nondiabetic. African-American women are more resistant to insulin as a glucoregulatory hormone; this is clearly demonstrated by both their higher peak glucose concentrations and lower SI. The high acute insulin concentrations observed in African-American women, which are necessary to overcome insulin resistance, lead to greater FFA clearance (i.e. higher cf). Yet FFA clearance in African-American women is enhanced further because the AIRg is actually elevated to levels beyond the degree necessary to compensate for the degree to insulin resistance. As a consequence, DI is higher in African-American than white women and the race difference in resistance to insulin as a both glucoregulatory hormone and sensitivity to insulin as antilipolytic hormone is magnified. This is best understood by comparing DI in African-American and white women graphically (Fig. 4).
Indeed, the hyperbolic curve, which is the graphic representation of the relationship between AIRg and SI, was visually higher in African-American women, i.e. above and to the right of the curve for white women (Fig. 4). This difference in the hyperbolic curves demonstrates that at every level of insulin resistance, the β-cell response was greater, or more robust, in African-American than white women. Rasouli et al. (17) also found that relative to whites, AIRg was out of proportion to SI in African-Americans. In addition, they and others have reported that compared with whites, the most likely etiology of high AIRg in African-Americans was reduced hepatic insulin extraction (18, 19). In our investigation, we examined the overall effect of AIRg on FFA concentration and did not distinguish between the factors that control AIRg levels, specifically β-cell secretion, hepatic extraction, and peripheral clearance.
High DI, representing AIRg out of proportion to SI, may not be beneficial to African-American women. Although others have also reported that AIRg and DI are higher in African-American than white women (17), these earlier investigations did not go further and explore the potential consequences of AIRg out of proportion to SI in African-American women. In the current study, we took this finding and examined the consequences vis-à-vis FFA metabolism. In our study we show that the high AIRg is likely to be contributing to race differences in FFA suppression (i.e. higher cf values in African-American than white women). High cf in African-American women may be a consequence of greater insulin-induced suppression of hormone sensitive lipase (20, 21). Therefore, the propensity to obesity observed in African-American women could be enhanced. Furthermore, peripheral fat is more sensitive to inhibition of lipolysis than central fat (22, 23). As a result, high insulin concentrations may contribute to the development of the high thigh circumferences and low VAT to SAT ratios we observed in the African American women. In terms of glucose metabolism, excessively high AIRg in African-American women may be a risk factor for the development of β-cell failure and ultimately T2DM. Clearly prospective studies are necessary to explore the unanswered question of whether high DI in African-Americans is beneficial or represents a risk factor for obesity and T2DM.
In addition, high insulin concentrations may contribute to the low TG levels frequently observed in obese, insulin-resistant African-American women (6). Insulin is required for in vivo activation of the enzyme, lipoprotein lipase (LPL) (24). LPL is located on proteoglycan strands attached to capillary basement membranes. By promoting the hydrolysis of fatty acids from the glycerol backbone of TG, LPL removes TG containing particles from the circulation. The Health, Risk Factors, Exercise Training, and Genetics study has established that postheparin LPL activity is higher in African Americans than whites (25). However, there is a race difference in the effect of insulin resistance on LPL activity. In whites, insulin resistance is associated with an impairment in the ability of insulin to activate LPL (26). In African-Americans insulin resistance does not interfere with LPL activity (27). Therefore, LPL activity could be higher in African-Americans for two reasons; first, higher AIRg could lead to greater activation of LPL (28), and second, insulin resistance in African-Americans is not associated with a decline in LPL activity (27).
A strength of this study is that even at this sample size (n = 17 in each group), there are statistically significant differences in those variables known to be different by race, specifically SI, AIRg, VAT, and the lipids, TG, and high-density lipoprotein cholesterol (6, 16). Another important feature of this study is the similarity between the African-American and white women in regard to family history of T2DM, lifestyle, and socioeconomic factors that could affect either insulin resistance or FFA metabolism. Differences in family history, lifestyle, and socioeconomic factors often confound investigations of this type. A weakness of the study is that in the absence of tracers, we were not able to distinguish between lipolysis, reesterification, and FFA oxidation. Rather we examined acute clearance of FFA from plasma as the net effect of these three processes on FFA.
A limitation of this study is that the effect of acute hyperglycemia on the decline of FFA cannot be separated from the effect of acute hyperinsulinemia. At the onset of the IM-FSIGT, iv glucose is administered. This is rapidly followed by the second intervention, which is the secretion of endogenous insulin by pancreatic β-cells. FFA suppression begins at approximately 10 min, with the two key stimuli being both acute hyperglycemia and endogenous hyperinsulinemia. The effect of the endogenous hyperinsulinemia on FFA suppression is the dominant influence, but acute hyperglycemia does play a minor role (29). A third intervention occurs at 20 min and is the bolus injection of iv insulin. The administration of exogenous insulin given at 20 min does not appear to influence FFA clearance. In an earlier investigation, frequently sampled iv glucose tolerance tests were performed with and without a dose of exogenous insulin and the suppression of FFA was the same (14). Hence, endogenous insulin maximally suppressed FFA levels and the introduction of exogenous insulin at 20 min had no additive effect (14).
Another factor to consider is the effect of FFA on β-cell function. Due to the rapid sequence of interventions during an IM-FSIGT, it is not possible with this technique to examine the effect of FFA on insulin secretion. However, methods used to examine the effect of FFA on insulin secretion are in general problematic. Most investigations that focus on determining an effect of FFA on β-cell function are performed by artificially raising circulating FFA concentration. This is usually done with the administration of exogenous FFA, usually but not always in the form of Liposyn with heparin (30–32). Studies that provide a constant infusion of FFA over several days, even when the FFA concentration is held at a level considered to be physiological, may not be optimal. in vivo FFA levels are not constant but vary widely, not only in response to iv glucose as well as meals but also because of diurnal variation (14, 33, 34). Furthermore, FFA release from adipose tissue is pulsatile (35, 37). Therefore, the relevance of studies based on constant infusion of FFA is unclear. Due to the limitations of available study techniques, the initiating factor in the circular relationship between FFA concentration and β-cell function is unresolved.
The essential next step in this investigation would be to perform low-dose insulin clamps. IM-FSIGT cannot be used to compare FFA suppression at similar insulin concentrations. We have shown that at higher insulin concentrations, FFA suppression is greater in African-American than white women. However, it is only if insulin concentrations were held constant would it be possible to determine whether race differences in tissue sensitivity also exist.
In conclusion, premenopausal African-American women have an acute insulin response to glucose that is greater than in white women and out of proportion to their degree of insulin resistance. Because the race difference in cf disappears when adjusted for AIRg, high AIRg in African-Americans is an important factor in race differences in FFA clearance. Due to the effect of high acute insulin concentration on FFA clearance, the role of high acute insulin concentrations in the development of obesity and diabetes in African-American women is worthy of consideration.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases (to C.C.C., V.P., M.R., B.V.M., and A.E.S.) and the National Institutes of Health Clinical Center (to G.C.). The Moss Heart Foundation, D.W. Reynolds Foundation, and Veterans Administration provided support (to G.L.V.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AIRg
- Acute insulin response to glucose
- BMI
- body mass index
- cf
- clearance rate
- CV
- coefficient of variation
- DI
- disposition index
- FFA
- free fatty acid
- IM-FSIGT
- insulin-modified frequently sampled iv glucose tolerance test
- LPL
- lipoprotein lipase
- SAT
- sc adipose tissue
- SI
- insulin sensitivity index
- T2DM
- type 2 diabetes mellitus
- TG
- triglyceride
- VAT
- visceral adipose tissue
- WC
- waist circumference
- WHR
- waist to hip ratio.
References
- 1. Cowie CC, Rust KF, Byrd-Holt DD, Eberhardt MS, Flegal KM, Engelgau MM, Sayhad SH, Williams DE, Geiss LS, Gregg EW. 2006. Prevalence of diabetes and impaired fasting glucose in adults in the U.S. population: National Health And Nutrition Examination Survey, 1999–2002. Diabetes Care 29:1263–1268 [DOI] [PubMed] [Google Scholar]
- 2. Flegal KM, Carroll MD, Ogden CL, Curtin LR. 2010. Prevalence and trends in obesity among U.S. adults, 1999–2008. JAMA 303:235–241 [DOI] [PubMed] [Google Scholar]
- 3. Bergman RN, Ader M. 2000. Free fatty acids and pathogenesis of type 2 diabetes mellitus. Trends Endocrinol Metab 11:351–356 [DOI] [PubMed] [Google Scholar]
- 4. Boden G. 2008. Obesity and free fatty acids. Endocrinol Metab Clin North Am 37:635–646, viii-ix [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guerrero R, Vega GL, Grundy SM, Browning JD. 2009. Ethnic differences in hepatic steatosis: an insulin resistance paradox? Hepatology 49:791–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sumner AE, Cowie CC. 2008. Ethnic differences in the ability of triglyceride levels to identify insulin resistance. Atherosclerosis 196:696–703 [DOI] [PubMed] [Google Scholar]
- 7. Sumner AE, Micklesfield LK, Ricks M, Tambay AV, Avila NA, Thomas F, Lambert EV, Levitt NS, Evans J, Rotimi CN, Tulloch-Reid MK, Goedecke JH. 2011. Waist circumference, BMI, and visceral adipose tissue in white women and women of African descent. Obesity (Silver Spring) 19:671–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Periwal V, Chow CC, Bergman RN, Ricks M, Vega GL, Sumner AE. 2008. Evaluation of quantitative models of the effect of insulin on lipolysis and glucose disposal. Am J Physiol Regul Integr Comp Physiol 295:R1089–R1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bergman RN, Ider YZ, Bowden CR, Cobelli C. 1979. Quantitative estimation of insulin sensitivity. Am J Physiol 236:E667–E677 [DOI] [PubMed] [Google Scholar]
- 10. Heiling VJ, Jensen MD. 1992. Free fatty acid metabolism in the follicular and luteal phases of the menstrual cycle. J Clin Endocrinol Metab 74:806–810 [DOI] [PubMed] [Google Scholar]
- 11. Boston RC, Stefanovski D, Moate PJ, Sumner AE, Watanabe RM, Bergman RN. 2003. MINMOD Millennium: a computer program to calculate glucose effectiveness and insulin sensitivity from the frequently sampled intravenous glucose tolerance test. Diabetes Technol Ther 5:1003–1015 [DOI] [PubMed] [Google Scholar]
- 12. Bergman RN, Ader M, Huecking K, Van Citters G. 2002. Accurate assessment of β-cell function: the hyperbolic correction. Diabetes 51(Suppl 1):S212–S220 [DOI] [PubMed] [Google Scholar]
- 13. Sumner AE, Farmer NM, Tulloch-Reid MK, Sebring NG, Yanovski JA, Reynolds JC, Boston RC, Premkumar A. 2002. Sex differences in visceral adipose tissue volume among African Americans. Am J Clin Nutr 76:975–979 [DOI] [PubMed] [Google Scholar]
- 14. Sumner AE, Bergman RN, Vega GL, Genovese DJ, Cochran CS, Pacak K, Watanabe RM, Boston RC. 2004. The multiphasic profile of free fatty acids during the intravenous glucose tolerance test is unresponsive to exogenous insulin. Metabolism 53:1202–1207 [DOI] [PubMed] [Google Scholar]
- 15. Koutsari C, Basu R, Rizza RA, Nair KS, Khosla S, Jensen MD. 2011. Nonoxidative free fatty acid disposal is greater in young women than men. J Clin Endocrinol Metab 96:541–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Haffner SM, D'Agostino R, Saad MF, Rewers M, Mykkänen L, Selby J, Howard G, Savage PJ, Hamman RF, Wagenknecht LE. 1996. Increased insulin resistance and insulin secretion in nondiabetic African-Americans and Hispanics compared with non-Hispanic whites. The Insulin Resistance Atherosclerosis Study. Diabetes 45:742–748 [DOI] [PubMed] [Google Scholar]
- 17. Rasouli N, Spencer HJ, Rashidi AA, Elbein SC. 2007. Impact of family history of diabetes and ethnicity on β-cell function in obese, glucose-tolerant individuals. J Clin Endocrinol Metab 92:4656–4663 [DOI] [PubMed] [Google Scholar]
- 18. Harris MI, Cowie CC, Gu K, Francis ME, Flegal K, Eberhardt MS. 2002. Higher fasting insulin but lower fasting C-peptide levels in African Americans in the U.S. population. Diabetes Metab Res Rev 18:149–155 [DOI] [PubMed] [Google Scholar]
- 19. Osei K, Schuster DP. 1994. Ethnic differences in secretion, sensitivity, and hepatic extraction of insulin in black and white Americans. Diabet Med 11:755–762 [DOI] [PubMed] [Google Scholar]
- 20. Arner P, Langin D. 2007. The role of neutral lipases in human adipose tissue lipolysis. Curr Opin Lipidol 18:246–250 [DOI] [PubMed] [Google Scholar]
- 21. Frayn KN, Arner P, Yki-Järvinen H. 2006. Fatty acid metabolism in adipose tissue, muscle and liver in health and disease. Essays Biochem 42:89–103 [DOI] [PubMed] [Google Scholar]
- 22. Kelley DE, Thaete FL, Troost F, Huwe T, Goodpaster BH. 2000. Subdivisions of subcutaneous abdominal adipose tissue and insulin resistance. Am J Physiol Endocrinol Metab 278:E941–E948 [DOI] [PubMed] [Google Scholar]
- 23. Martin ML, Jensen MD. 1991. Effects of body fat distribution on regional lipolysis in obesity. J Clin Invest 88:609–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Eckel RH. 1989. Lipoprotein lipase. A multifunctional enzyme relevant to common metabolic diseases. N Engl J Med 320:1060–1068 [DOI] [PubMed] [Google Scholar]
- 25. Després JP, Couillard C, Gagnon J, Bergeron J, Leon AS, Rao DC, Skinner JS, Wilmore JH, Bouchard C. 2000. Race, visceral adipose tissue, plasma lipids, and lipoprotein lipase activity in men and women: the Health, Risk Factors, Exercise Training, and Genetics (HERITAGE) family study. Arterioscler Thromb Vasc Biol 20:1932–1938 [DOI] [PubMed] [Google Scholar]
- 26. Maheux P, Azhar S, Kern PA, Chen YD, Reuven GM. 1997. Relationship between insulin-mediated glucose disposal and regulation of plasma and adipose tissue lipoprotein lipase. Diabetologia 40:850–858 [DOI] [PubMed] [Google Scholar]
- 27. Sumner AE, Vega GL, Genovese DJ, Finley KB, Bergman RN, Boston RC. 2005. Normal triglyceride levels despite insulin resistance in African Americans: role of lipoprotein lipase. Metabolism 54:902–909 [DOI] [PubMed] [Google Scholar]
- 28. Brown MS, Goldstein JL. 2008. Selective versus total insulin resistance: a pathogenic paradox. Cell Metab 7:95–96 [DOI] [PubMed] [Google Scholar]
- 29. Park KS, Rhee BD, Lee KU, Lee HK, Koh CS, Min HK. 1990. Hyperglycemia per se can reduce plasma free fatty acid and glycerol levels in the acutely insulin-deficient dog. Metabolism 39:595–597 [DOI] [PubMed] [Google Scholar]
- 30. Boden G. 2005. Free fatty acids and insulin secretion in humans. Curr Diab Rep 5:167–170 [DOI] [PubMed] [Google Scholar]
- 31. Carpentier A, Mittelman SD, Bergman RN, Giacca A, Lewis GF. 2000. Prolonged elevation of plasma free fatty acids impairs pancreatic β-cell function in obese nondiabetic humans but not in individuals with type 2 diabetes. Diabetes 49:399–408 [DOI] [PubMed] [Google Scholar]
- 32. Kashyap S, Belfort R, Gastaldelli A, Pratipanawatr T, Berria R, Pratipanawatr W, Bajaj M, Mandarino L, DeFronzo R, Cusi K. 2003. A sustained increase in plasma free fatty acids impairs insulin secretion in nondiabetic subjects genetically predisposed to develop type 2 diabetes. Diabetes 52:2461–2474 [DOI] [PubMed] [Google Scholar]
- 33. Gibson T, Stimmler L, Jarrett RJ, Rutland P, Shiu M. 1975. Diurnal variation in the effects of insulin on blood glucose, plasma non-esterified fatty acids and growth hormone. Diabetologia 11:83–88 [DOI] [PubMed] [Google Scholar]
- 34. Jensen MD. 1995. Gender differences in regional fatty acid metabolism before and after meal ingestion. J Clin Invest 96:2297–2303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Getty L, Panteleon AE, Mittelman SD, Dea MK, Bergman RN. 2000. Rapid oscillations in omental lipolysis are independent of changing insulin levels in vivo. J Clin Invest 106:421–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hsu IR, Zuniga E, Bergman RN. 2010. Pulsatile changes in free fatty acids augment hepatic glucose production and preserves peripheral glucose homeostasis. Am J Physiol Endocrinol Metab 299:E131–E136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hücking K, Hamilton-Wessler M, Ellmerer M, Bergman RN. 2003. Burst-like control of lipolysis by the sympathetic nervous system in vivo. J Clin Invest 111:257–264 [DOI] [PMC free article] [PubMed] [Google Scholar]