Abstract
Background
Communicating prognosis to enable shared decision-making is strongly endorsed by heart failure (HF) guidelines. Patients are concerned with both their quantity and quality of life (QoL). To facilitate the recognition of patients at high risk for unfavorable future QoL or death, we created a simple prognostic tool to estimate this combined outcome.
Methods and Results
We identified factors associated with 6-month mortality or persistently unfavorable QoL, defined by Kansas City Cardiomyopathy Questionnaire (KCCQ) scores <45 at 1 and 24 weeks after hospital discharge, among 1458 patients from the Efficacy of Vasopressin Antagonism in HF Outcome Study with Tolvaptan (EVEREST). Within 24 weeks of discharge, 478 (32.8%) patients had died and 192 (13.2%) patients had serial KCCQ scores < 45. After adjusting for 23 pre-discharge covariates, independent predictors of the combined end point included low admission KCCQ score, high B-type natriuretic peptide, hyponatremia, tachycardia, hypotension, absence of β-blocker therapy, and history of diabetes mellitus and arrhythmia. A simplified pre-discharge HF score for subsequent death or unfavorable QoL had moderate discrimination (c-statistic 0.72). Pre-discharge clinical covariates were substantially different in predicting the QoL end point as compared with traditional death or rehospitalization end points.
Conclusions
At the time of hospital discharge, readily available clinical characteristics are associated with HF patients at high risk for suffering persistently unfavorable QoL or death over the next 6 months. Such information can target patients for whom aggressive treatment options (e.g., devices or transplantation) and/or end-of-life discussions should be strongly considered prior to discharge.
Keywords: heart failure, prognosis, risk factors, quality of life, health status
In the care of patients with heart failure (HF), estimating and communicating prognosis is endorsed by clinical guidelines1-3 and is considered to be an important component of high-quality healthcare.4 Without explicit education regarding future expectations regarding quantity and quality of life (QoL), patients and families are inadequately equipped to make important decisions about the optimal direction of their treatment. Yet despite the importance of estimating and discussing prognosis, this is seldom done in routine clinical practice5-7 and the majority of patients with HF underestimate their risk for adverse outcomes.8,9
Hospitalization is a critical event in the clinical course of HF. For some it represents a transient clinical deterioration, while for others it heralds a progressive phase of HF marked by recurrent hospitalizations, lower functional status, severe symptoms and death. This latter course, however, is common given the high rates of mortality (∼25%),10, 11 rehospitalization (∼50%),12, 13 and severe symptom burden14 in the 6 months following hospitalization for an episode of worsening HF. Thus, HF hospitalization represents an important opportunity to estimate patients' prognosis in an effort to identify those in whom health care providers should take the time to formally discuss treatment options and patients' end-of-life wishes.15
While there are numerous prognostic models to estimate survival and rehospitalization in the acute care setting,10, 11, 16-18 these models are fundamentally limited in that they fail to include measures of QoL, despite the availability of validated HF instruments and their frequent use in randomized trials.19, 20,21-25 By focusing singularly upon mortality, current prognostic models incorrectly presume that all survival represents a favorable outcome, even in light of evidence that many patients would make healthcare decisions that improve their QoL at the expense of its quantity.25-27
One needed component of improved communication and shared decision-making between health care providers and their patients is a means for recognizing patients at high risk for either death or persistently unfavorable QoL. To address this need, we analyzed the Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan (EVEREST).28, 29 EVEREST enrolled patients admitted with decompensated HF and collected serial health status measures at the time of hospitalization and after discharge. Our aim was to describe the frequency with which patients do not survive with favorable QoL following HF hospitalization, and then to create a simple clinical tool to enable health care providers to identify such high-risk patients approaching the time of hospital discharge. We also assessed the degree of discordance between predictors of this novel QoL end point with the more traditional outcomes of death and rehospitalization. Our goal was to create a simple tool that could potentially be used at the time of hospital discharge to facilitate communication between health care providers and their patients about prognosis—including expected QoL after discharge—to support timely discussions regarding treatment options and end-of-life care.
Methods
Study Population
The design of the EVEREST trial has been previously described.30 EVEREST examined both short-28 and long-term29 outcomes in patients hospitalized with a primary diagnosis of HF and randomized within 48 hours of admission to tolvaptan or placebo. From October 2003 to February 2006, patients were enrolled from 359 sites in North America, South America, and Europe. The date of the last follow-up was July 2006. Important eligibility criteria included history of chronic HF for at least 30 days prior to hospitalization, evidence of volume overload, and left ventricular ejection fraction (LVEF) ≤ 40%. Key exclusion criteria were expected survival < 6 months, cardiac mechanical support implantation, cerebrovascular accident in the last 30 days, dialysis, morbid obesity, substance abuse, systolic blood pressure < 90 mm Hg, serum creatinine >3.5 mg/dL, and hemoglobin <9 g/dL. We restricted the cohort to those patients who underwent formal assessments of health status. Because these assessments were introduced mid-way through the trial, health status measures were obtained consecutively on the last 2033 of the 4133 EVEREST participants. Among these eligible patients, 1458 (72%) survived to hospital discharge and had adequate follow up. All patients continued to receive standard therapy for HF while enrolled in the trial. The trial protocol was approved by the appropriate Institutional Review Board at each study site, and all enrollees provided written informed consent.
Study Variables
Baseline data were collected within 48 hours of hospital admission,30 including formal health status measures that were also obtained 1 week after discharge and 24 weeks later using the Kansas City Cardiomyopathy Questionnaire (KCCQ).19 The KCCQ is a validated, disease-specific, 23-item, self-administered questionnaire that quantifies health status in patients with HF. The KCCQ overall summary score, which includes information from the physical limitation, symptom, social limitation, and QoL scales, was used for all analyses. The KCCQ summary score ranges from 0 to 100, with higher scores reflecting better health status. Culturally and linguistically validated versions were used.31 Among other study covariates, glomerular filtration rate (GFR) was calculated using the Modification of Diet in Renal Disease equation.32 B-type natriuretic peptide (BNP) was measured at a centralized laboratory using the Triage Assay (Biosite, CA). History of arrhythmia was defined as any sustained atrial or ventricular arrhythmia.
Study Outcomes
The primary end point for this analysis was the composite of persistently unfavorable QoL (as defined by KCCQ <45 at weeks 1 and 24 following discharge) or all-cause mortality. The combined end point was chosen because both components were felt to warrant consideration of advanced therapies and/or end-of-life care; in a step-wise fashion, patients are first classified by level of risk for adverse future outcomes (i.e., failure to achieve favorable future QoL), and then, for those at sufficiently high risk, the type of advanced therapy (e.g., transplantation, mechanical circulatory support, hospice) is chosen based on certain patient characteristics and patient preferences. The KCCQ cut point of 45 was chosen a priori based upon an association of KCCQ <45 with advanced HF.19,33,34 We secondarily assessed unfavorable future QoL, all-cause mortality, and rehospitalization end points individually.
Statistical Analysis
Baseline characteristics were compared using Student's t-test or the Wilcoxon rank-sum test for continuous variables and Chi-square or Fisher's exact test for categorical variables.
The association of baseline clinical variables and the primary end point was assessed using multivariable regression models. Typical analyses utilize logistic regression to estimate adjusted odds ratios, which are then generally interpreted as relative risks. However, in this study the event being modeled was not rare, in which case odds ratios are poor estimated of relative risks. To address this issue, we estimated adjusted relative risks directly using hierarchical modified Poisson regression models,35 adjusted for site (given the reported association of site with outcome).36 For variables with multiple measurements during the index hospitalization, the most recent data point at the time of discharge was included. Model predictors were chosen a priori based on previous inpatient HF prognostic models10, 11, 16-18, 37, 38 and clinical factors related to QoL.19 These covariates included demographics (age, sex, race), clinical variables (current smoking; history of hypertension, diabetes mellitus, stroke, severe chronic obstructive pulmonary disease [COPD], arrhythmia, coronary artery disease as collected on the case report form at admission; LVEF; prescription of a β-blocker at discharge; heart rate, systolic blood pressure, and the degree of edema on the day of discharge), laboratory values (pre-discharge values for GFR, blood urea nitrogen, hemoglobin, sodium, b-type natriuretic peptide [BNP], and QRS width), and baseline KCCQ. NYHA functional class was not included because of the homogeneity in NYHA assessment at the time of hospital admission and known limitations in inter-rater reproducibility.39-42 Associations of continuous variables with outcomes were assessed for linearity using restricted cubic spine terms.43 Variables found to have non-linear relationships with the outcome were categorized using cut points chosen based on prior prognostic models and clinically meaningful values. Sensitivity analyses altering the cut point for KCCQ to < 40 or < 50 in defining the primary end point were performed to evaluate the robustness of our findings. An additional sensitivity analysis was performed for the full model using continuous variables whenever possible (transformed for non-linear relationships) to evaluate whether a significant loss of predictive ability had occurred by categorizing the continuous variables.
We then created a simplified risk score which could be more readily applied in clinical practice. First, the contributions of model predictors were assessed by F-statistics. Predictors were sequentially eliminated from the full model by removing the predictor with the smallest contribution until further variable elimination led to a greater than 5% loss in model prediction (i.e., the R2 for the predictions from the reduced model was >95% of the explained outcome from the full model).43 Second, the β regression estimates for each variable in the reduced model were divided by the smallest β weight to create standardized β weights, which were then rounded to the nearest integer. Finally, bootstrap model validation for the risk score was conducted by replicating our risk score model on 1000 datasets generated using random sampling with replacement. A risk score was developed for each of the 1000 datasets and these were used to predict outcomes in both the bootstrap and original datasets to describe potential model optimism. Risk score calibration was assessed by graphing the observed versus predicted rates of the combined end point of death or persistently unfavorable health status within deciles of predicted risk. C-statistics were calculated to characterize discriminatory accuracy. The Hosmer-Lemeshow statistic was calculated to determine goodness of fit, and the slope of the linear predictor was calculated to assess model calibration.43
In order to determine whether the predictors of future QoL differed from the more traditional clinical HF end points of death or rehospitalization (particularly since the rehospitalization is presumed to be driven by symptoms), we constructed separate risk models for the individual 24-week end points of (A) all-cause mortality, (B) persistently unfavorable QoL, and (C) rehospitalization. Mortality and rehospitalization were modeled using time to event analyses to produce hazard ratios, and QoL was modeled using modified Poisson regression models to produce relative risks. The relative contribution of each variable to each individual outcome was assessed using F-statistics.
The mean rate of missing data per patient was < 5%. Rates of missing data for individual variables was 10.5% for BNP levels, <3% for 5 variables, and <0.5% for all remaining variables. Missing data were imputed using IVEWARE.44 Imputation was predicated upon variables collected at surrounding time intervals and variables known to correlate with model covariates.
All analyses were performed using SAS software, release 9.1.3 (SAS Institute, Cary, NC), IVEWARE44, and R version 2.6.0.45 A p-value of <0.05 was used to define statistical significance.
Results
For the patients included in this analysis, the mean age was 66.5 ± 11.7 years, 75% were male, and 85% were Caucasian. The etiology of HF was primarily ischemic. Mean LVEF was 27.2 ± 8.0%. The average duration of HF diagnosis prior to study enrollment was 5.9 ± 4.9 years, and 82% reported a previous hospitalization for HF. Patients frequently had significant comorbidities, including 39% with diabetes, 19% with cerebrovascular disease, and 10% with severe COPD. Median length of stay for the index hospitalization was 7 days (IQR 3-12). Additional baseline characteristics, stratified by outcome, are described in Table 1. When comparing those eligible for the analysis to the overall EVEREST cohort, no significant differences in the Table 1 variables were observed.
Table 1. In-hospital characteristics of patients, stratified by the primary outcome.
| Characteristic (means ± SD or %, unless stated as median [IQR]) | Unfavorable QoL (Death or KCCQ <45 at both 1 and 24 weeks post discharge) | Favorable QoL (Survival to 24 weeks with KCCQ ≥45 at either 1 or 24 weeks post discharge) | P-value |
|---|---|---|---|
| N=670 | N=788 | ||
| KCCQ OS score at admission (0-100) | 24.0 ± 15.4 | 35.8 ± 19.5 | < 0.001 |
| Demographics | |||
| Age (years) | 68.1 ± 11.6 | 65.1 ± 11.6 | < 0.001 |
| Female | 25.7% | 24.9% | 0.726 |
| Race | 0.226 | ||
| Caucasian | 86.3% | 83.4% | |
| Black | 7.5% | 8.1% | |
| Other | 6.3% | 8.5% | |
| BMI at admission | 28.1 ± 5.7 | 28.5 ± 5.7 | 0.178 |
| Smoking, current | 9.7% | 12.9% | 0.054 |
| Hypertension, history of | 71.5% | 72.2% | 0.762 |
| Diabetes, history of | 43.1% | 36.9% | 0.016 |
| Chronic kidney disease, history of | 39.2% | 23.2% | < 0.001 |
| Stroke, history of | 14.8% | 10.1% | 0.006 |
| Peripheral vascular disease, history of | 24.6% | 22.2% | 0.285 |
| COPD, severe | 12.5% | 7.2% | < 0.001 |
| HF characteristics | |||
| Duration of HF (years) | 6.2 ± 5.2 | 5.6 ± 4.7 | 0.009 |
| Prior hospitalization for HF | 85.6% | 78.1% | < 0.001 |
| LVEF (percent) | 26.4 ± 8.1 | 27.9 ± 7.9 | < 0.001 |
| Coronary artery disease, history of | 74.7% | 70.4% | 0.067 |
| Arrhythmia, history of | 72.9% | 59.3% | < 0.001 |
| Atrial | 58.9% | 46.6% | < 0.001 |
| Ventricular | 32.2% | 26.6% | 0.019 |
| Therapies | |||
| ICD | 18.2% | 11.2% | < 0.001 |
| B-blocker at discharge | 67.8% | 79.9% | < 0.001 |
| ACEI/ARB at discharge | 78.7% | 88.5% | < 0.001 |
| Diuretic at discharge | 93.6% | 93.0% | 0.669 |
| Symptoms at discharge | |||
| NYHA functional class at discharge | < 0.001 | ||
| I | 1.9% | 5.6% | |
| II | 32.8% | 51.6% | |
| III | 54.1% | 41.5% | |
| IV | 11.2% | 1.4% | |
| Dyspnea at discharge, frequent or constant | 24.3% | 12.3% | < 0.001 |
| Fatigue at discharge, frequent or continuous | 39.7% | 18.0% | < 0.001 |
| Physical signs at discharge | |||
| Systolic BP at discharge (mmHg) | 116.1 ± 18.8 | 122.5 ± 19.0 | < 0.001 |
| Heart rate at discharge (bpm) | 75.6 ± 12.0 | 73.2 ± 11.7 | < 0.001 |
| Pedal edema, described as moderate or marked, at discharge | 9.5% | 3.9% | < 0.001 |
| Rales at discharge, any | 26.2% | 12.9% | < 0.001 |
| JVP ≥ 10 cm at discharge | 32.8% | 27.5% | 0.026 |
| Laboratory measures nearest to discharge | |||
| Sodium (mMol, median [IQR]) | 139.0 (136.0, 143.0) | 140.0 (138.0, 143.0) | < 0.001 |
| <135 | 18.3% | 8.6% | < 0.001 |
| 135-145 | 70.4% | 80.8% | |
| >145 | 11.4% | 10.7% | |
| BUN ≥43 (mMol)) | 31.9% | 15.1% | < 0.001 |
| GFR (ml/min, median [IQR]) | 49.8 (36.3, 65.9) | 58.2 (44.5, 72.6) | < 0.001 |
| ≥59 | 33.2% | 47.8% | < 0.001 |
| 30-59 | 53.1% | 45.8% | |
| 15-29 | 13.3% | 6.2% | |
| <15 | 0.3% | 0.1% | |
| Hemoglobin (g/dL) | 13.3 (11.8, 14.8) | 14.1 (12.6, 15.3) | < 0.001 |
| BNP (pg/mL, median [IQR]) | 794.0 (359.0, 1482.9) | 404.0 (169.0, 842.7) | < 0.001 |
| ≤500 | 35.6% | 58.7% | < 0.001 |
| 500-999 | 25.5% | 22.2% | |
| 1000+ | 38.9% | 19.1% | |
| QRS duration (msec, median [IQR] | 131 (106-161) | 124 (99-152) | < 0.001 |
| Index length of stay (days, median [IQR]) | 7.0 (4.0, 13.0) | 7.0 (3.0, 12.0) | 0.001 |
Continuous variables compared using Student's T-test, except for skewed variables expressed at medians which were compared using Wilcoxon rank-sum test, and categorical variables compared using Fisher exact test. QoL = quality of life; SD = standard deviation; IQR = interquartile range; KCCQ = Kansas City Cardiomyopathy Questionnaire; OS = Overall Summary; COPD = chronic obstructive pulmonary disease; HF = heart failure; LVEF = left ventricular ejection fraction; ICD = implantable cardioverter defibrillator; ACEI = angiotensin converting enzyme inhibitor; ARB = angiotensin II receptor blocker; NYHA = New York Heart Association; BP = blood pressure; JVP = jugular venous pressure estimation; BUN = blood urea nitrogen; GFR = estimated glomerular filtration rate; BNP = b-type natiuretic peptide
Outcomes
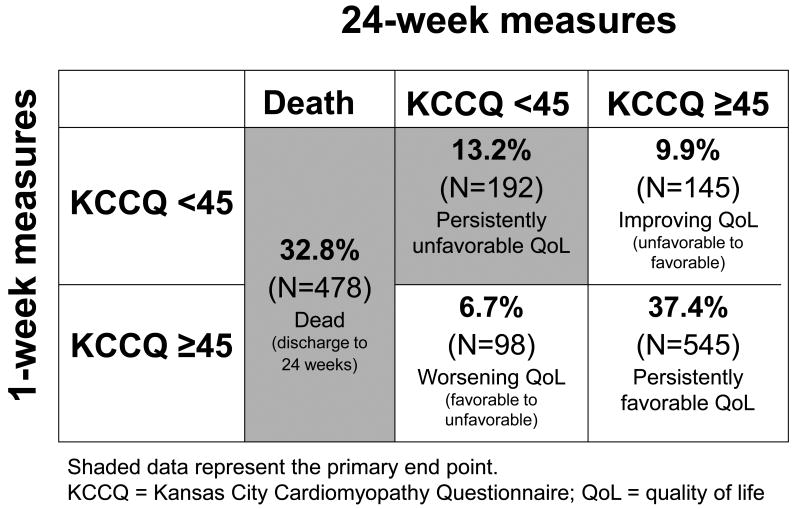
Mean KCCQ scores among survivors were 31.6 ± 19.0 at study enrollment, 52.9 ± 22.1 at 1 week post discharge, and 58.3 ± 23.9 at 24 weeks, indicating that significant gains in health status were typically made early with smaller improvements between 1 and 24 weeks. There were 478 deaths (32.8%) and an additional 192 (13.2%) patients who had persistently unfavorable QoL throughout follow-up (KCCQ <45 at week 1 and 24) (Figure 1). KCCQ measures of health status correlated well with NYHA assessments at 1 week after discharge: NYHA I, median KCCQ = 76 (IQR 58-87); NYHA II, median KCCQ = 63 (IQR 49-76); NYHA III, median KCCQ = 45 (IQR 33-60); NYHA IV, median KCCQ = 25 (IQR 17-36).
Figure 1.
Distribution of patients with KCCQ <45 versus >=45 at 1 week post discharge and 24 weeks post discharge.
Predictors of Persistently Unfavorable Future QoL or Death
Unadjusted associations between patient characteristics at discharge and the combined end point of persistently unfavorable QoL or death in the 24 weeks following hospital discharge are shown in Table 1. After adjusting for 23 covariates in the full model, independent predictors of the combined end point included low baseline KCCQ score (per 10 unit increase, or improvement in baseline QoL: risk ratio [RR] = 0.82, 95% confidence interval [CI] 0.78-0.87), high BNP (500-999 pg/ml: RR 1.27, CI 1.05-1.53; 1000+ pg/ml: RR = 1.41, CI 1.14-1.73; compared to <500 pg/ml), hyponatremia (sodium <135 mEq/L: RR = 1.30, CI 1.04-1.62; compared to sodium 135-145 mEq/L), increased heart rate at discharge (per 10 bpm increase: RR 1.08, CI 1.01-1.15), decreased systolic blood pressure at discharge (per 10 mmHg increase: RR = 0.92, CI 0.88-0.97), absence of β-blocker therapy at discharge (β-blocker prescribed: RR = 0.80, CI 0.64-0.99), history of diabetes (HR = 1.18, CI 1.01-1.39), and history of arrhythmia (RR = 1.32, CI 1.08-1.60) (see Appendix 1 for details). The full model had moderate discriminatory capacity (c-statistic = 0.73). Sensitivity analysis changing the cut point for the primary outcome from KCCQ < 45 to < 40 and then to < 50 did not substantially change the model predictors. Additional sensitivity analysis using continuous values for covariates, rather than dichotomous or ordinal transformations, resulted in the same covariates retaining significance with a c-statistic of 0.74.
Simplified Refractory Heart Failure Risk Score
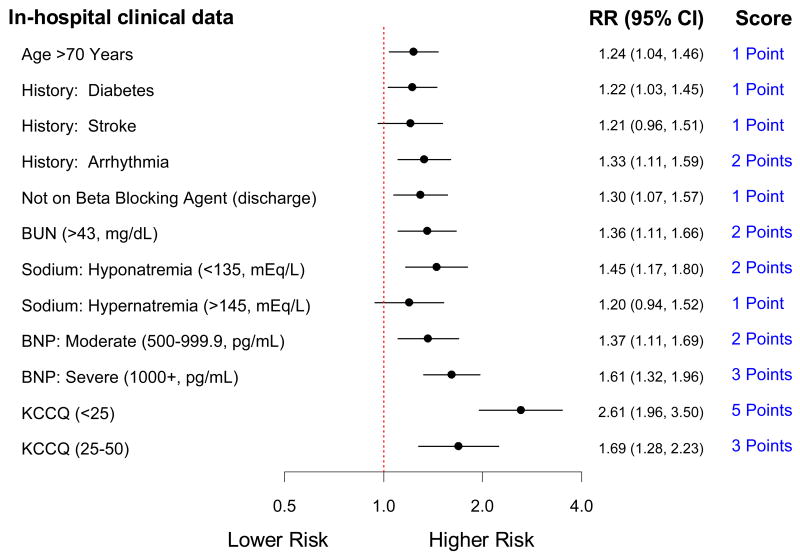
After variable elimination, the full model was reduced to 9 independent variables presented as 12 discrete risk predictors, each assigned a score between 1 and 5 points (Figure 2). The c-statistic for the reduced model was 0.72 and for the simplified risk score was 0.72 in this cohort. Similar to the full model, the individual predictors of baseline KCCQ and BNP carried the greatest discriminatory capacity for the combined end point, and thus were assigned the highest points. Bootstrap validation resulted in c-statistics of 0.74 for the bootstrap samples and 0.73 for the original cohort based on the risk scored developed from the bootstrap samples.
Figure 2.
The refractory heart failure score: a reduced model with a simplified clinical risk score predicting unfavorable future health status, defined as death or KCCQ persistently < 45 in the 24 weeks following heart failure hospitalization, determined using clinical information at the time of hospital discharge
Risk score distribution and corresponding predicted risk can be found in Figure 3. Risk score calibration stratified by deciles of predicted risk demonstrated a graded increase in the observed rates of death or persistently unfavorable health status. The Hosmer-Lemeshow statistic was 0.051, indicating no evidence for lack of fit. The model calibration slope of the linear predictor was 1.0 (CI 0.9-1.1) on 1000 bootstrap validation samples predicting onto the original dataset, indicating good calibration (Appendix 2). Importantly, the risk score stratified risk across a broad spectrum of risk, ranging from 7.6% in the lowest decile of predicted risk to 64.9% in the highest.
Figure 3.
Distribution of refractory heart failure risk scores and their probabilities
Comparing Predictors of the Future QoL to Traditional HF End Points
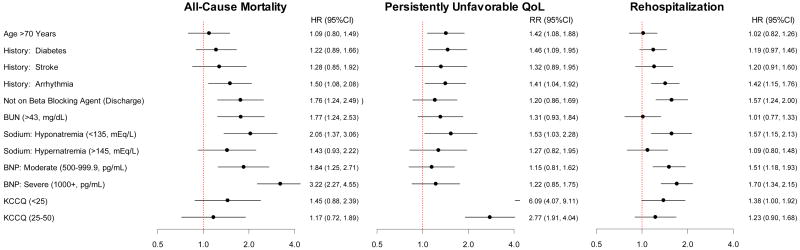
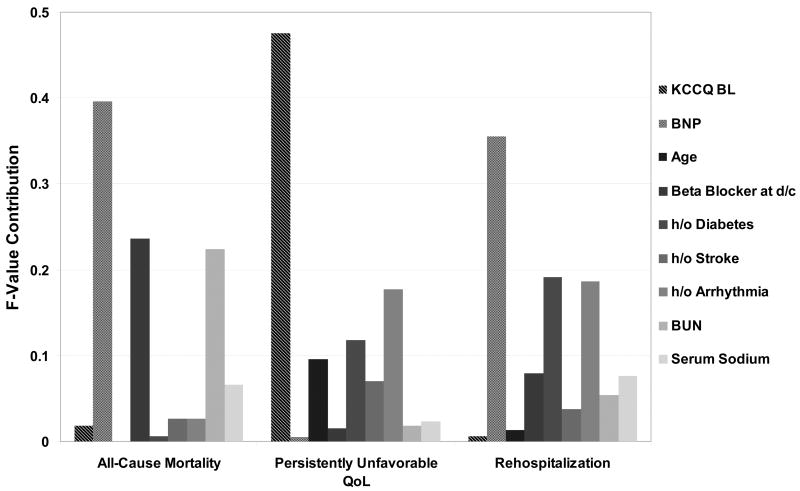
To clarify the unique information provided by a novel end point that includes persistently unfavorable QoL, separate multivariable-adjusted outcomes models were created for the individual end points of (A) all-cause mortality, (B) persistently unfavorable QoL, and (C) rehospitalization with 24 weeks of hospital discharge (Figure 4). Comparison of the hazards ratios and relative risks show that the predictors of these 3 end points are different. As assessed by the F-statistics (Figure 5), low baseline KCCQ is the dominant predictor of persistently unfavorable QoL and yet does not show a significant association with either death or rehospitalization. Conversely, elevated BNP is the dominant predictor of death and rehospitalization, but was not significantly associated with persistently unfavorable QoL. Other patient characteristics, including age, diabetes mellitus, inability to prescribe a β-blocker at discharge and sodium levels were associated to varying degrees with the different outcomes (e.g., age was strongly associated with poor QoL but not with death or rehospitalization).
Figure 4.
Separate adjusted multivariable risk models for the association of in-hospital clinical covariates with selected 24-week outcomes.
Figure 5.
Relative prognostic contribution of various in-hospital clinical covariates with selected 24-week outcomes as assessed by F-testing.
Discussion
Heart Failure patients and their health care providers are concerned not only with survival but also the quality of that survival.25 To our knowledge, this is the first study to provide formal estimates of future QoL in patients with HF and reduced LVEF. Modeling the combined end point of persistently low health status measures and mortality, rather than the more typical HF outcomes of rehospitalization and mortality, provides prognostic information that most directly relates to patients' concerns and experiences. Thus, these findings fundamentally extend the large body of existing literature regarding prognostication in HF by explicitly including patient-centered measures of QoL over time as part of the predicted clinical outcome. Given the growing importance of using objective evidence to engage patients in guiding their subsequent care so that decisions can be based on patients' individual goals and values,4, 46 we believe that these findings, once validated, may be used at the time of hospital discharge to improve the quality of HF care.
In the EVEREST cohort, we found that that nearly half of the patients discharged from the hospital did not survive to enjoy favorable QoL throughout the subsequent 24 weeks, despite eligibility criteria designed to exclude patients with end-stage HF or an expected survival < 6 months. Nearly a third of these patients survived with persistently unfavorable QoL (i.e., lived but with severe symptoms, poor function, and markedly impaired QoL). This subgroup is of particular concern because they may choose different therapies if made aware of their prognosis. Moreover, we were able to demonstrate that reasonable predictive discrimination could be accomplished using only 9 readily accessible clinical characteristics at the time of hospital discharge that could stratify patients' risk from a 7% to a 65% probability of either dying or having persistently unfavorable QoL. Importantly, the predictors of this clinically important, combined outcome differ from traditional risk models. It is noteworthy that while the KCCQ has been repeatedly demonstrated to be associated with survival, hospitalization, and costs in chronic heart failure,33, 47 it was not significantly associated with mortality or rehospitalization in the acute setting. Similarly, while age is one of the strongest predictors of mortality in the chronic setting, it was only associated with reduced QoL and not with mortality or rehospitalization in the setting of acute decompensated heart failure. Prior comparisons between chronic ambulatory and acute hospitalized phases of HF have shown similar differences in predictors of outcome.48
A recent article by Quill and colleagues underscores the importance of estimating prognosis as the foundation for identifying and following the treatment preferences of very ill patients.49 In the setting of severe HF despite optimal therapy, patients with a poor prognosis can be eligible for either more aggressive care (e.g., mechanical circulatory support and cardiac transplantation) or supportive measures to optimize their quality of life.15, 50 Only by transparently identifying which patients with HF are at risk for both unfavorable QoL and death can patients' preferences for the intensity of care be explicitly elicited and followed.4 Although the model presented here is not intended to dictate care, it can be potentially useful in identifying patients in whom an in-depth discussion of advanced treatment options or end-of-life care is most relevant.
Despite the importance of prognosis to patients with HF, studies show that patients and health care providers are relatively uninformed and overly optimistic about expectations for the future.5,8 Our findings suggest that the formal quantification of patients' QoL at the time of hospitalization, measurement of BNP, and the integration of these values with other clinically-available data can be useful in better identifying patients with an adverse prognosis at the time of discharge. Thus, this model is complementary to traditional mortality models by explicitly incorporating QoL with mortality.
The importance of this combined outcome is supported by prior studies that have examined how patients value QoL and survival. Individuals tend to fall into 3 groups: approximately half wish to maximize length of life and thus would not trade significant quantity of life in current health for better QoL; a quarter would trade the large majority of their remaining quantity of life for a higher QoL; and a quarter fall in the middle.26,51,52 This suggests that for approximately half of patients with symptomatic HF, patient-centered decisions about medical care will be significantly influenced by expectations and preferences for both quantity and quality of future survival. While patients' individual preferences may only be elicited by a detailed conversation between patients and their health care providers, the proposed risk-stratification model can identify those in whom such a conversation is most needed and provide a benchmark estimate of outcome to facilitate that conversation.3 Fundamentally, the data show that in order to provide expectations for future quality of life, risk models should include a baseline measure of health status.
Study Limitations
Several potential limitations should be considered when interpreting our results. The study findings are from a retrospective, post-hoc analysis of patients enrolled in a clinical trial. EVEREST excluded patients with end-stage HF, expected survival of < 6 months, significant hypotension, and severe renal dysfunction, thus eliminating some of the patients at the very highest risk for adverse outcomes. The cohort was also restricted to HF patients with reduced LVEF and who were younger, predominantly Caucasian and more likely to be male as compared with community HF populations.53 However, the trial included patients with a high degree of comorbidity, and thus the clinical profile of patients enrolled in EVEREST was more similar to patients captured in recent large HF registries than has generally been the case with earlier randomized trials.54-58 Additionally, the enrollment of patients from 359 sites on several continents, and from both academic and non-academic centers increases the generalizability of the findings. Serial KCCQ measures were obtained at 3 times, limiting the ability to assess changes in KCCQ prior to discharge and short-term fluctuations in QoL in follow up. We repeated our analysis with 1-week KCCQ scores as a model covariate in place of baseline enrollment KCCQ scores to address whether early improvements in KCCQ might reduce the predictive power of the model, and we found no major changes in model performance (data not shown). Due to the unique nature of the EVEREST data (i.e., high-quality clinical data including serial KCCQ measures in a large hospitalized HF cohort), model validation in a separate dataset is not possible at this time. External validation is a critical next step in the process of confirming the utility of this proposed risk score. In the meantime, clinical practice guidelines continue to advocate for communicating risk to patients hospitalized with heart failure. The general findings presented here help inform those discussions, suggesting a role for health status measurements in future quality of life considerations.
Conclusions
Nearly half of patients discharged after hospitalization for acute decompensated HF did not survive to enjoy favorable QoL during the subsequent 24 weeks. A risk score using commonly measured clinical data available at the time of hospital discharge, supplemented with a QoL measure, was able to identify patients at high risk for this outcome. Further work is needed to determine if this risk score can improve decisional quality and subsequent patient-centered outcomes for hospitalized patients with decompensated HF.
Supplementary Material
Acknowledgments
We thank the EVEREST investigators for their contributions to the acquisition of the data used in this analysis. In particular, we wish to acknowledge Drs Karl Swedberg and Aldo Pietro Maggioni for their assistance in completing this manuscript.
Funding Sources: EVEREST was funded by Otsuka.
Footnotes
Conflict of Interest Disclosures: The EVEREST trial was funded by Otsuka. Otsuka did not participate in the analysis or interpretation of the data for this post hoc analysis, and Otsuka had no role in the preparation, review, or approval of this manuscript. Dr Gheorghiade reports receiving research grants from research grants from the National Institutes of Health, Otsuka, Sigma Tau, Merck, and Scios Inc; being a consultant for Debbio Pharm, Errekappa Terapeutici, GlaxoSmithKline, Protein Design Laboratories, and Medtronic; and receiving honoraria from Abbott, AstraZeneca, GlaxoSmithKline, Medtronics, Otsuka, Protein Design Laboratories, Scios Inc, and Sigma Tau. Dr Hauptman reports being a member of the EVEREST CEC and consultant to Otsuka. Dr Zannad reports receiving research grants from Bayer; being a consultant for Servier and Johnson & Johnson; and receiving honoraria from AstraZeneca, Pfizer, Boehringer Ingelheim, Novartis, Abbott, Sanofi-Aventis, and Otsuka. Dr Konstam reports research grants and contracts from, being a consultant for, and receiving honoraria from Otsuka. Dr. Spertus owns the copyright for the KCCQ and reports having been a consultant for Otsuka in the past.
Clinical Trial Registration-clinicaltrials.gov; Identifier: NCT00071331
References
- 1.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW. 2009 Focused Update Incorporated Into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults. A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2009;119:e391–e479. doi: 10.1161/CIRCULATIONAHA.109.192065. [DOI] [PubMed] [Google Scholar]
- 2.Lindenfeld J, Albert NM, Boehmer JP, Collins SP, Ezekowitz JA, Givertz MM, Katz SD, Klapholz M, Moser DK, Rogers JG, Starling RC, Stevenson WG, Tang WH, Teerlink JR, Walsh MN. Heart Failure Society of America 2010 Comprehensive Heart Failure Practice Guideline. J Card Fail. 2010;16:e1–e194. doi: 10.1016/j.cardfail.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Goodlin SJ, Hauptman PJ, Arnold R, Grady K, Hershberger RE, Kutner J, Masoudi F, Spertus J, Dracup K, Cleary JF, Medak R, Crispell K, Pina I, Stuart B, Whitney C, Rector T, Teno J, Renlund DG. Consensus statement: Palliative and supportive care in advanced heart failure. J Card Fail. 2004;10:200–209. doi: 10.1016/j.cardfail.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Institute of Medicine Committee on Quality of Health Care in America: Crossing the Quality Chasm: A New Helath System for the 21st Century. Washington DC: National Acadamy Press; 2001. [Google Scholar]
- 5.Hauptman PJ, Swindle J, Hussain Z, Biener L, Burroughs TE. Physician attitudes toward end-stage heart failure: a national survey. Am J Med. 2008;121:127–135. doi: 10.1016/j.amjmed.2007.08.035. [DOI] [PubMed] [Google Scholar]
- 6.Murray SA, Boyd K, Kendall M, Worth A, Benton TF, Clausen H. Dying of lung cancer or cardiac failure: prospective qualitative interview study of patients and their carers in the community. BMJ. 2002;325:929. doi: 10.1136/bmj.325.7370.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.A controlled trial to improve care for seriously ill hospitalized patients.The study to understand prognoses and preferences for outcomes and risks of treatments (SUPPORT). The SUPPORT Principal Investigators. JAMA. 1995;274:1591–1598. [PubMed] [Google Scholar]
- 8.Allen LA, Yager JE, Funk MJ, Levy WC, Tulsky JA, Bowers MT, Dodson GC, O'Connor CM, Felker GM. Discordance between patient-predicted and model-predicted life expectancy among ambulatory patients with heart failure. JAMA. 2008;299:2533–2542. doi: 10.1001/jama.299.21.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hlatky MA, Greenland P, Arnett DK, Ballantyne CM, Criqui MH, Elkind MS, Go AS, Harrell FE, Jr, Hong Y, Howard BV, Howard VJ, Hsue PY, Kramer CM, McConnell JP, Normand SL, O'Donnell CJ, Smith SC, Jr, Wilson PW. Criteria for evaluation of novel markers of cardiovascular risk: a scientific statement from the American Heart Association. Circulation. 2009;119:2408–2416. doi: 10.1161/CIRCULATIONAHA.109.192278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'Connor CM, Abraham WT, Albert NM, Clare R, Gattis Stough W, Gheorghiade M, Greenberg BH, Yancy CW, Young JB, Fonarow GC. Predictors of mortality after discharge in patients hospitalized with heart failure: An analysis from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) Am Heart J. 2008;156:662–673. doi: 10.1016/j.ahj.2008.04.030. [DOI] [PubMed] [Google Scholar]
- 11.Lee DS, Austin PC, Rouleau JL, Liu PP, Naimark D, Tu JV. Predicting mortality among patients hospitalized for heart failure: derivation and validation of a clinical model. JAMA. 2003;290:2581–2587. doi: 10.1001/jama.290.19.2581. [DOI] [PubMed] [Google Scholar]
- 12.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Stafford R, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Heart Disease and Stroke Statistics--2010 Update: A Report From the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 13.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360:1418–1428. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 14.Bekelman DB, Rumsfeld JS, Havranek EP, Yamashita TE, Hutt E, Gottlieb SH, Dy SM, Kutner JS. Symptom burden, depression, and spiritual well-being: a comparison of heart failure and advanced cancer patients. J Gen Intern Med. 2009;24:592–598. doi: 10.1007/s11606-009-0931-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goodlin SJ. Palliative care in congestive heart failure. J Am Coll Cardiol. 2009;54:386–396. doi: 10.1016/j.jacc.2009.02.078. [DOI] [PubMed] [Google Scholar]
- 16.Felker GM, Leimberger JD, Califf RM, Cuffe MS, Massie BM, Adams KF, Jr, Gheorghiade M, O'Connor CM. Risk stratification after hospitalization for decompensated heart failure. J Card Fail. 2004;10:460–466. doi: 10.1016/j.cardfail.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 17.Fonarow GC, Adams KF, Jr, Abraham WT, Yancy CW, Boscardin WJ. Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA. 2005;293:572–580. doi: 10.1001/jama.293.5.572. [DOI] [PubMed] [Google Scholar]
- 18.O'Connor CM, Hasselblad V, Mehta RH, Tasissa G, Califf RM, Fiuzat M, Rogers JG, Leier CV, Stevenson LW. Triage after hospitalization with advanced heart failure: the ESCAPE (evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness) risk model and discharge score. J Am Coll Cardiol. 2010;55:872–878. doi: 10.1016/j.jacc.2009.08.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35:1245–1255. doi: 10.1016/s0735-1097(00)00531-3. [DOI] [PubMed] [Google Scholar]
- 20.Rector TS, Cohn JN. Assessment of patient outcome with the Minnesota Living with Heart Failure questionnaire: reliability and validity during a randomized, double-blind, placebo-controlled trial of pimobendan. Pimobendan Multicenter Research Group. Am Heart J. 1992;124:1017–1025. doi: 10.1016/0002-8703(92)90986-6. [DOI] [PubMed] [Google Scholar]
- 21.Taylor AL, Ziesche S, Yancy C, Carson P, D'Agostino R, Jr, Ferdinand K, Taylor M, Adams K, Sabolinski M, Worcel M, Cohn JN, the African-American Heart Failure Trial Investigators Combination of Isosorbide Dinitrate and Hydralazine in Blacks with Heart Failure. N Engl J Med. 2004;351:2049–2057. doi: 10.1056/NEJMoa042934. [DOI] [PubMed] [Google Scholar]
- 22.Spertus JA, Tooley J, Jones P, Poston C, Mahoney E, Deedwania P, Hurley S, Pitt B, Weintraub WS. Expanding the outcomes in clinical trials of heart failure: the quality of life and economic components of EPHESUS (EPlerenone's neuroHormonal Efficacy and SUrvival Study) Am Heart J. 2002;143:636–642. doi: 10.1067/mhj.2002.120775. [DOI] [PubMed] [Google Scholar]
- 23.Mark DB, Knight JD, Velazquez EJ, Howlett JG, Spertus JA, Djokovic LT, Harding TM, Rankin GR, Drew LA, Szygula-Jurkiewicz B, Adlbrecht C, Anstrom KJ. Quality of life and economic outcomes with surgical ventricular reconstruction in ischemic heart failure: results from the Surgical Treatment for Ischemic Heart Failure trial. Am Heart J. 2009;157:837–844. doi: 10.1016/j.ahj.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spertus JA. Evolving applications for patient-centered health status measures. Circulation. 2008;118:2103–2110. doi: 10.1161/CIRCULATIONAHA.107.747568. [DOI] [PubMed] [Google Scholar]
- 25.Lewis EF, Johnson PA, Johnson W, Collins C, Griffin L, Stevenson LW. Preferences for quality of life or survival expressed by patients with heart failure. J Heart Lung Transplant. 2001;20:1016–1024. doi: 10.1016/s1053-2498(01)00298-4. [DOI] [PubMed] [Google Scholar]
- 26.Stevenson LW, Hellkamp AS, Leier CV, Sopko G, Koelling T, Warnica JW, Abraham WT, Kasper EK, Rogers JG, Califf RM, Schramm EE, O'Connor CM. Changing preferences for survival after hospitalization with advanced heart failure. J Am Coll Cardiol. 2008;52:1702–1708. doi: 10.1016/j.jacc.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stewart GC, Brooks K, Pratibhu PP, Tsang SW, Semigran MJ, Smith CM, Saniuk C, Camuso JM, Fang JC, Mudge GH, Couper GS, Baughman KL, Stevenson LW. Thresholds of physical activity and life expectancy for patients considering destination ventricular assist devices. J Heart Lung Transplant. 2009;28:863–869. doi: 10.1016/j.healun.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 28.Gheorghiade M, Konstam MA, Burnett JC, Jr, Grinfeld L, Maggioni AP, Swedberg K, Udelson JE, Zannad F, Cook T, Ouyang J, Zimmer C, Orlandi C. Short-term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: the EVEREST Clinical Status Trials. JAMA. 2007;297:1332–1343. doi: 10.1001/jama.297.12.1332. [DOI] [PubMed] [Google Scholar]
- 29.Konstam MA, Gheorghiade M, Burnett JC, Jr, Grinfeld L, Maggioni AP, Swedberg K, Udelson JE, Zannad F, Cook T, Ouyang J, Zimmer C, Orlandi C. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA. 2007;297:1319–1331. doi: 10.1001/jama.297.12.1319. [DOI] [PubMed] [Google Scholar]
- 30.Gheorghiade M, Orlandi C, Burnett JC, Demets D, Grinfeld L, Maggioni A, Swedberg K, Udelson JE, Zannad F, Zimmer C, Konstam MA. Rationale and design of the multicenter, randomized, double-blind, placebo-controlled study to evaluate the Efficacy of Vasopressin antagonism in Heart Failure: Outcome Study with Tolvaptan (EVEREST) J Card Fail. 2005;11:260–269. doi: 10.1016/j.cardfail.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 31.Acquadro C, Conway K, Hareendran A, Aaronson N. Literature review of methods to translate health-related quality of life questionnaires for use in multinational clinical trials. Value Health. 2008;11:509–521. doi: 10.1111/j.1524-4733.2007.00292.x. [DOI] [PubMed] [Google Scholar]
- 32.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 33.Heidenreich PA, Spertus JA, Jones PG, Weintraub WS, Rumsfeld JS, Rathore SS, Peterson ED, Masoudi FA, Krumholz HM, Havranek EP, Conard MW, Williams RE. Health status identifies heart failure outpatients at risk for hospitalization or death. J Am Coll Cardiol. 2006;47:752–756. doi: 10.1016/j.jacc.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 34.Hauptman PJ, Masoudi FA, Weintraub WS, Pina I, Jones PG, Spertus JA. Variability in the clinical status of patients with advanced heart failure. J Card Fail. 2004;10:397–402. doi: 10.1016/j.cardfail.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 35.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 36.Blair JE, Zannad F, Konstam MA, Cook T, Traver B, Burnett JC, Jr, Grinfeld L, Krasa H, Maggioni AP, Orlandi C, Swedberg K, Udelson JE, Zimmer C, Gheorghiade M. Continental differences in clinical characteristics, management, and outcomes in patients hospitalized with worsening heart failure results from the EVEREST (Efficacy of Vasopressin Antagonism in Heart Failure: Outcome Study with Tolvaptan) program. J Am Coll Cardiol. 2008;52:1640–1648. doi: 10.1016/j.jacc.2008.07.056. [DOI] [PubMed] [Google Scholar]
- 37.Levy WC, Mozaffarian D, Linker DT, Sutradhar SC, Anker SD, Cropp AB, Anand I, Maggioni A, Burton P, Sullivan MD, Pitt B, Poole-Wilson PA, Mann DL, Packer M. The Seattle Heart Failure Model: prediction of survival in heart failure. Circulation. 2006;113:1424–1433. doi: 10.1161/CIRCULATIONAHA.105.584102. [DOI] [PubMed] [Google Scholar]
- 38.Kalogeropoulos AP, Georgiopoulou VV, Giamouzis G, Smith AL, Agha SA, Waheed S, Laskar S, Puskas J, Dunbar S, Vega D, Levy WC, Butler J. Utility of the Seattle Heart Failure Model in patients with advanced heart failure. J Am Coll Cardiol. 2009;53:334–342. doi: 10.1016/j.jacc.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 39.Bennett JA, Riegel B, Bittner V, Nichols J. Validity and reliability of the NYHA classes for measuring research outcomes in patients with cardiac disease. Heart Lung. 2002;31:262–270. doi: 10.1067/mhl.2002.124554. [DOI] [PubMed] [Google Scholar]
- 40.The Criteria Committee of the New York Heart Association. Nomenclature and criteria for diagnosis of diseases of the heart and blood vessels. Boston, MA: Little Brown; 1964. [Google Scholar]
- 41.Rafeal C, Briscoe C, Davies J, Ian Whinnett Z, Manistry C, Sutton R, Mayet J, Francis DP. Limitations of the New York Heart Association functional classification system and self-reported walking distances in chronic heart failure. Heart. 2007;93:476–482. doi: 10.1136/hrt.2006.089656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goldman L, Cook EF, Mitchell N, Flatley M, Sherman H, Cohn PF. Pitfalls in the serial assessment of cardiac functional status. How a reduction in “ordinary” activity may reduce the apparent degree of cardiac compromise and give a misleading impression of improvement. J Chronic Dis. 1982;35:763–771. doi: 10.1016/0021-9681(82)90087-x. [DOI] [PubMed] [Google Scholar]
- 43.Harrell FE. Regression Modeling Strategies with Applications to Linear Models, Logistic Regression and Survival Analysis. New York: Springer-Verlag; 2001. [Google Scholar]
- 44.Raghunathan TE, Solenberger PW, Van Hoewyk J. IVEware: Imputation and Variance Estimation Software - User Guide. Ann Arbor, MI: Survey Research Center, Institute for Social Research; 2002. [Google Scholar]
- 45.R: A language and environment for statistical computing [computer program] Vienna, Austria: Version. [Google Scholar]
- 46.Spertus JA, Eagle KA, Krumholz HM, Mitchell KR, Normand SL. American College of Cardiology and American Heart Association methodology for the selection and creation of performance measures for quantifying the quality of cardiovascular care. Circulation. 2005;111:1703–1712. doi: 10.1161/01.CIR.0000157096.95223.D7. [DOI] [PubMed] [Google Scholar]
- 47.Kosiborod M, Soto GE, Jones PG, Krumholz HM, Weintraub WS, Deedwania P, Spertus JA. Identifying heart failure patients at high risk for near-term cardiovascular events with serial health status assessments. Circulation. 2007;115:1975–1981. doi: 10.1161/CIRCULATIONAHA.106.670901. [DOI] [PubMed] [Google Scholar]
- 48.Fonarow GC. Epidemiology and risk stratification in acute heart failure. Am Heart J. 2008;155:200–207. doi: 10.1016/j.ahj.2006.10.043. [DOI] [PubMed] [Google Scholar]
- 49.Quill TE, Arnold R, Back AL. Discussing treatment preferences with patients who want “everything”. Ann Intern Med. 2009;151:345–349. doi: 10.7326/0003-4819-151-5-200909010-00010. [DOI] [PubMed] [Google Scholar]
- 50.Rogers JG, Aaronson KD, Boyle AJ, Russell SD, Milano CA, Pagani FD, Edwards BS, Park S, John R, Conte JV, Farrar DJ, Slaughter MS. Continuous flow left ventricular assist device improves functional capacity and quality of life of advanced heart failure patients. J Am Coll Cardiol. 2010;55:1826–1834. doi: 10.1016/j.jacc.2009.12.052. [DOI] [PubMed] [Google Scholar]
- 51.Havranek EP, McGovern KM, Weinberger J, Brocato A, Lowes BD, Abraham WT. Patient preferences for heart failure treatment: utilities are valid measures of health-related quality of life in heart failure. J Card Fail. 1999;5:85–91. doi: 10.1016/s1071-9164(99)90030-1. [DOI] [PubMed] [Google Scholar]
- 52.Stanek EJ, Oates MB, McGhan WF, Denofrio D, Loh E. Preferences for treatment outcomes in patients with heart failure: symptoms versus survival. J Card Fail. 2000;6:225–232. doi: 10.1054/jcaf.2000.9503. [DOI] [PubMed] [Google Scholar]
- 53.Heiat A, Gross CP, Krumholz HM. Representation of the elderly, women, and minorities in heart failure clinical trials. Arch Intern Med. 2002;162:1682–1688. doi: 10.1001/archinte.162.15.1682. [DOI] [PubMed] [Google Scholar]
- 54.Adams KF, Jr, Fonarow GC, Emerman CL, LeJemtel TH, Costanzo MR, Abraham WT, Berkowitz RL, Galvao M, Horton DP. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE) Am Heart J. 2005;149:209–216. doi: 10.1016/j.ahj.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 55.Cleland JG, Swedberg K, Follath F, Komajda M, Cohen-Solal A, Aguilar JC, Dietz R, Gavazzi A, Hobbs R, Korewicki J, Madeira HC, Moiseyev VS, Preda I, van Gilst WH, Widimsky J, Freemantle N, Eastaugh J, Mason J. The EuroHeart Failure survey programme-- a survey on the quality of care among patients with heart failure in Europe. Part 1: patient characteristics and diagnosis. Eur Heart J. 2003;24:442–463. doi: 10.1016/s0195-668x(02)00823-0. [DOI] [PubMed] [Google Scholar]
- 56.Nieminen MS, Brutsaert D, Dickstein K, Drexler H, Follath F, Harjola VP, Hochadel M, Komajda M, Lassus J, Lopez-Sendon JL, Ponikowski P, Tavazzi L. EuroHeart Failure Survey II (EHFS II): a survey on hospitalized acute heart failure patients: description of population. Eur Heart J. 2006;27:2725–2736. doi: 10.1093/eurheartj/ehl193. [DOI] [PubMed] [Google Scholar]
- 57.Tavazzi L, Maggioni AP, Lucci D, Cacciatore G, Ansalone G, Oliva F, Porcu M. Nationwide survey on acute heart failure in cardiology ward services in Italy. Eur Heart J. 2006;27:1207–1215. doi: 10.1093/eurheartj/ehi845. [DOI] [PubMed] [Google Scholar]
- 58.Fonarow GC, Abraham WT, Albert NM, Stough WG, Gheorghiade M, Greenberg BH, O'Connor CM, Pieper K, Sun JL, Yancy C, Young JB. Association between performance measures and clinical outcomes for patients hospitalized with heart failure. JAMA. 2007;297:61–70. doi: 10.1001/jama.297.1.61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.