Abstract
Background
Hyperuricemia is prevalent in patients with chronic kidney disease (CKD). We explored the hypothesis that asymptmatic hyperuricemia may be associated with new-onset CKD.
Methods
The participants were all male factory workers in Kanagawa, Japan (n = 1,285). All were over 40 years of age and had undergone annual health examinations from 1990 to 2007. Individuals with a history of gouty attacks were excluded from the study. A retrospective cohort study was conducted by following the estimated glomerular filtration rate (eGFR) for each participant over a maximum period of 18 years. The endpoint was new-onset CKD defined as eGFR < 60 mL/min/1.73 m2. The associations between new-onset CKD and the presence of hyperuricemia, low serum high-density lipoprotein cholesterol, hypertension, diabetes, and obesity were analyzed.
Results
The mean (± standard deviation) follow-up period was 95.2 (± 66.7) months, and new-onset CKD was observed in 100 participants (7.8%) during this follow-up. Cox proportional hazards model revealed that the hazard ratio of new-onset CKD due to hyperuricemia, low serum high-density lipoprotein cholesterol, hypertension and obesity were 3.99 (95% confidence interval: 2.59-6.15), 1.69 (1.00-2.86), 2.00 (1.29-3.11) and 1.35 (0.87-2.10), respectively. Concerning hyperuricemia, low serum high-density lipoprotein cholesterol, hypertension and obesity, the log-rank tests showed P values of < 0.01, 0.01, < 0.01 and < 0.01, respectively.
Conclusion
The results of this study suggest that asymptomatic hyperuricemia is a predictive factor for new-onset CKD for Japanese male workers.
Keywords: chronic kidney disease, hyperuricemia, glomerular filtration rate, cohort, Japan
Background
End-stage renal disease (ESRD) poses a serious public health problem as a result of the costs of treatments such as dialysis and transplantation and harms the quality of life. Chronic kidney disease (CKD) precedes ESRD, and the progression of CKD to ESRD can be preventable by appropriate treatment. Prevention of new-onset CKD could effectively target ESRD. In 2002, the Kidney Disease Outcomes Quality Initiative of the National Kidney Foundation gave a definition and classification system for CKD, and CKD is defined as either glomerular filtration rate (GFR) < 60 mL/min/1.73 m2 or kidney damage lasting for at least 3 months [1]. The estimated prevalences of CKD measured as GFR < 60 mL/min/1.73 m2 are 8.1% in the United States [2] and 10.6% in Japan [3].
Previous studies have established hypertension [4-9] and diabetes [10-13] as predictive factors for CKD. Dyslipidemia [6,14,15], obesity [7,16] and hyperuricemia [17-20] have also been suggested to be predictive factors for CKD. However, it is not clear if asymptomatic hyperuricemia without gouty attacks is a risk factor for CKD. The aim of this study was to determine the associations between new-onset CKD and asymptomatic hyperuricemia, low serum high-density lipoprotein cholesterol (HDL-C), hypertension, diabetes and obesity in male factory workers over 40 years of age in Kanagawa, Japan.
Methods
Study Population
The participants were all male factory workers over 40 years of age who had undergone annual medical examinations from 1990 to 2007. This retrospective cohort study covered a maximum period of 18 years. To investigate the effects of asymptomatic hyperuricemia, 41 participants with a history of gouty attacks were excluded from the analysis. Participants with only one year of data, those lacking follow-up data, or those with incomplete data were also excluded.
Disease Criteria
The presence of hyperuricemia, low serum HDL-C, hypertension, diabetes, and obesity were based on data from the participants' first-year medical examinations. Hyperuricemia was defined as a uric acid level of > 7.0 mg/dL. Low serum HDL-C was defined as HDL-C < 40 mg/dL. Hypertension was defined as a systolic blood pressure of ≥ 140 mmHg or diastolic blood pressure of ≥ 90 mmHg. Diabetes was defined as a fasting blood sugar level of ≥ 126 mg/dL. Obesity was defined as a body mass index (BMI) of ≥ 25 kg/m2.
Outcomes
The endpoint was new-onset CKD. New-onset CKD was defined as the time point when estimated glomerular filtration rate (eGFR) fell below 60 mL/min/1.73 m2. The eGFR was calculated based on the participant's age and serum creatinine (Cr) level, using the equation:
determined by the Japanese Society of Nephrology [21]. In this study, serum Cr was measured using the Jaffe method from 1990 to 2002, and using the enzyme method from 2003 to 2007. Because the equation for estimating GFR used in this study is based on an enzyme method, eGFR for the period from 1990 to 2002 was calculated using the serum Cr level after subtraction of 0.2 mg/dL from the original serum Cr level [22]. Participants were excluded from the analysis if their earliest year's eGFR was < 60 mL/min/1.73 m2, if they had only 1 year's worth of data or if they lacked follow-up data.
Statistical Analysis
The associations between new-onset CKD and the presence of hyperuricemia, low serum HDL-C, hypertension, diabetes, and obesity were analyzed. The covariates included age, hyperuricemia, low serum HDL-C, hypertension, diabetes and obesity. The follow-up period (in months) was the survival variable and new-onset CKD was a state variable. To adjust for age and the presence of disease as confounders, multivariate analysis was performed using Cox regression analysis [23]. A hazard ratio and 95% confidential interval (CI) were derived for each covariate. The hazard ratio was determined to be significant when the P value was < 0.05. In addition, Kaplan-Meier curves and log-rank tests were used to estimate the cumulative incidence of the covariates showing a significant hazard ratio [24]. The Japanese version of SPSS 17.0 for Windows was used for the analyses [25].
Ethical Approval
This study was conducted with the approval of the ethics committee of Kitasato University School of Medicine.
Results
The study involved 1,285 participants with a mean (± standard deviation [SD]) follow-up period of 95.2 (± 66.7) months. Of these participants, 100 (7.8%) developed CKD during the follow-up period, and their mean (± SD) follow-up period was 89.3 (± 62.0) months. The mean (± SD) follow-up period for participants who did not develop CKD was 95.7 (± 67.1) months. Table 1 shows the baseline characteristics of the participants in terms of age, levels of uric acid, HDL-C, systolic blood pressure, diastolic blood pressure, fasting blood sugar, and BMI. Hyperuricemia was detected in 166 participants (12.9%), low serum HDL-C in 153 (11.9%), hypertension in 255 (19.8%), diabetes in 51 (4.0%), and obesity in 255 (19.8%).
Table 1.
Baseline characteristics of participants
| Participants (n = 1,285) |
% | |
|---|---|---|
| Age (yrs) | ||
| 40 | 433 | 33.7 |
| 41 - 45 | 273 | 21.2 |
| 46 - 50 | 276 | 21.5 |
| 51 - 55 | 220 | 17.1 |
| ≥ 56 | 83 | 6.5 |
| Uric acid (mg/dL) | ||
| > 7.0 | 166 | 12.9 |
| ≤ 7.0 | 1,119 | 87.1 |
| HDL-C (mg/dL) | ||
| < 40 | 153 | 11.9 |
| ≥ 40 | 1,132 | 88.1 |
| Blood pressure (mmHg) | ||
| SBP ≥ 140 or DBP ≥ 90 | 255 | 19.8 |
| SBP < 140 and DBP < 90 | 1,030 | 80.2 |
| Fasting blood sugar (mg/dL) | ||
| ≥ 126 | 51 | 4.0 |
| < 126 | 1,234 | 96.0 |
| Body mass index (kg/m2) | ||
| ≥ 25.0 | 255 | 19.8 |
| < 25.0 | 1,030 | 80.2 |
HDL-C, high-density lipoprotein cholesterol
SBP, systolic blood pressure; DBP, diastolic blood pressure.
Table 2 shows the prediction for new-onset CKD during followed-up period. Of the participants with hyperuricemia at baseline, 32 (19.3%) developed CKD during the follow-up period. The results of Cox regression analysis revealed that the hazard ratio for new-onset CKD in the participants with hyperuricemia was 3.99 (95% CI: 2.59-6.15), showing a significant association between hyperuricemia and new-onset CKD. Likewise, the hazard ratio for new-onset CKD was 1.69 (95% CI: 1.00-2.86) in the participants with low serum HDL-C and 2.00 (95% CI: 1.29-3.11) in those with hypertension, indicating significant associations between new-onset CKD and these variables. However, a hazard ratio of 0.56 (95% CI: 0.17-1.77) indicated no significant association between new-onset CKD and diabetes. The hazard ratio was 1.35 (95% CI: 0.87-2.10) in the participants with obesity, indicating a weak association.
Table 2.
Associations between predictors and new-onset CKD during a maximum period of 18 years follow-up
| Predictors | Duration ± SD (months) |
Cases | Incidence (%) |
Hazard ratio | 95% CI |
|---|---|---|---|---|---|
| Uric acid (mg/dL) | |||||
| > 7.0 | 76.3 ± 63.3 | 32 | 19.3 | 3.99 | 2.59, 6.15 |
| ≤ 7.0 | 98.0 ± 66.8 | 68 | 6.1 | 1.00 | |
| HDL-C (mg/dL) | |||||
| < 40 | 89.3 ± 61.5 | 18 | 11.8 | 1.69 | 1.00, 2.86 |
| ≥ 40 | 96.0 ± 67.4 | 82 | 7.2 | 1.00 | |
| Blood pressure (mmHg) | |||||
| SBP ≥ 140 or DBP ≥ 90 | 83.5 ± 62.8 | 33 | 12.9 | 2.00 | 1.29, 3.11 |
| SBP < 140 and DBP < 90 | 98.1 ± 67.4 | 67 | 6.5 | 1.00 | |
| Fasting blood sugar (mg/dL) | |||||
| ≥ 126 | 95.1 ± 66.0 | 3 | 5.9 | 0.56 | 0.17, 1.77 |
| < 126 | 95.2 ± 66.8 | 97 | 7.9 | 1.00 | |
| Body mass index (kg/m2) | |||||
| ≥ 25.0 | 93.2 ± 66.4 | 34 | 12.5 | 1.35 | 0.87, 2.10 |
| < 25.0 | 95.7 ± 66.8 | 66 | 6.5 | 1.00 |
CKD, chronic kidney disease; SD, standard deviation; CI, confidence interval;
HDL-C, high-density lipoprotein cholesterol; SBP, systolic blood pressure; DBP, diastolic blood pressure.
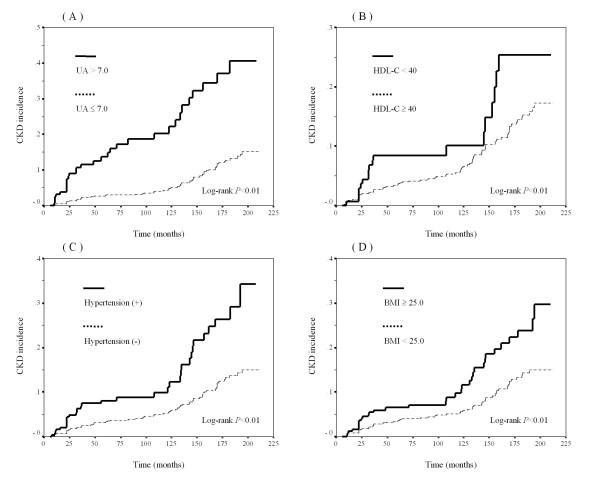
The cumulative incidence of CKD was analyzed by the Kaplan-Meier method using the three variables (hyperuricemia, low serum HDL-C, and hypertension) showing significant associations and the one variable (obesity) showing a weak association with new-onset CKD in the Cox proportional hazards model (Figure 1). Concerning hyperuricemia, low serum HDL-C, hypertension and obesity, the log-rank tests showed P values of < 0.01 (Figure 1-A), 0.01 (Figure 1-B), < 0.01 (Figure 1-C) and < 0.01 (Figure 1-D), respectively.
Figure 1.
Kaplan-Meier curves and Log-rank tests of CKD incidence. The Kaplan-Meier curves show the follow-up periods (in months) and cumulative incidence rate of CKD. Solid lines represent cumulative incidence rates of CKD in participants with hyperuricemia (A), low serum HDL-C (B), hypertension (C) and obesity (D) while dotted lines represent for the CKD rate in participants without these factors. Log-rank tests were performed to determine differences in cumulative incidence rates. P values are shown in the figures. (A) UA > 7.0 mg/dL versus UA ≤ 7.0 mg/dL. (B) HDL-C < 40 mg/dL versus HDL-C ≥ 40 mg/dL. (C) (SBP ≥ 140 mmHg or DBP ≥ 90 mmHg) versus (SBP < 140 mmHg and DBP < 90 mmHg). (D) BMI ≥ 25.0 kg/m2 versus BMI < 25.0 kg/m2. CKD, chronic kidney disease; UA, uric acid; HDL-C, high-density lipoprotein cholesterol; SBP, systolic blood pressure; DBP, diastolic blood pressure; BMI, body mass index.
Discussion
We investigated the associations between new-onset CKD and asymptomatic hyperuricemia without gouty attacks, low serum HDL-C, hypertension, diabetes and obesity in Japanese male factory workers over 40 years of age. The results showed a significantly higher incidence of new-onset CKD in participants with asymptomatic hyperuricemia. A significantly higher incidence of new-onset CKD was also found in participants with low serum HDL-C and hypertension. The incidence of new-onset CKD tended to be higher, but not significantly, in obese participants, whereas no significant increase in the incidence of new-onset CKD was found in those with diabetes.
Several studies have reported possible associations between gouty attacks and renal function [26,27] and have suggested an association between hyperuricemia and CKD [17-20]. However, previous studies were not noticed about asymptomatic hyperuricemia without gouty attacks. Hyperuricemia in this study was defined as a uric acid level above 7.0 mg/dL without gouty attacks, and the hazard ratio for new-onset CKD in participants with hyperuricemia was 3.99, indicating a significant association. In addition, significant negative correlations were found between the uric acid values at the start of follow-up and the GFR values at the start of follow-up, after follow-up for 5 years, and after follow-up for 10 years (correlation coefficient: at the start of follow-up, -0.21 p < 0.001; after 5 years, -0.20 p < 0.001; and after 10 years:-0.22 p < 0.001) (data not shown). A uric acid level of 7.0 mg/dL is the diagnostic standard for hyperuricemia in Japan [28]. Obermayr et al stated in their report on 21,475 participants followed for 7 years, that the risk of new-onset CKD was increased by a factor of 1.74 in those whose uric acid levels were 7.0-8.9 mg/dL, and by a factor of 3.12 in those whose uric acid levels were ≥ 9.0 mg/dL [17]. Iseki et al. conducted a study in Japan and reported that the hazard ratio for progression to ESRD was 5.77 in women with uric acid levels of ≥ 6.0 mg/dL, showing a significant association, whereas there was no significant association between progression to ESRD and uric acid levels in men with uric acid levels of ≥ 7.0 mg/dL [19]. A follow-up study of patients with immunoglobulin A nephropathy for at least 8 years reported that serum creatinine (Cr) levels were significantly elevated in patients with hyperuricemia [20]. Hyperuricemia appeared to be affected by other lifestyle-related diseases such as hypertension, diabetes and dyslipidemia, but a significant association between hyperuricemia and new-onset CKD remained even after adjusting for factors related to hypertension, low serum HDL-C, obesity and diabetes. In this study, the hazard ratio for asymptomatic hyperuricemia was greater than those for other factors, including hypertension. Asymptomatic hyperuricemia was therefore suggested not only to be a predictive factor for CKD, but also to be a stronger predictor than established factors such as hypertension and diabetes.
Dyslipidemia is thought to be a risk factor for new-onset CKD. Previous studies detected associations between CKD and decreased HDL-C [6,7,14,15,29], increased total cholesterol [29,30], increased low-density lipoprotein cholesterol [14,28,30], and increased triglycerides [6,15]. However, several other studies found no such significant associations, and further investigation may be needed to clarify the association between dyslipidemia and CKD. Low serum HDL-C in this study was classified as HDL-C levels < 40 mg/dL, which is the diagnostic standard for low HDL-C in the United States (NCEP ATP III)[31] and in Japan (Japan Atherosclerosis Society; Guidelines for Prevention of Atherosclerotic Cardiovascular Diseases)[32]. When serum HDL-C was low, the incidence of new-onset CKD increased significantly (hazard ratio: 1.69), supporting an association between dyslipidemia and CKD.
An association between hypertension and renal disease has been established in a number of previous studies [4-9]. Increases in blood pressure have also been reported to be associated with increases in the incidence of ESRD [8,33,34], and progression of CKD can be prevented by antihypertensive treatment [5,8,9]. In this study, hypertension was defined as a systolic blood pressure of ≥ 140 mmHg or diastolic blood pressure of ≥ 90 mmHg. This is the standard classification for stage I hypertension in the United States (JNC7)[35] and mild hypertension in Japan (JSH2009)[36] and is used as the diagnostic standard for hypertension. The hazard ratio for new-onset CKD in our study was 2.00, showing a significant association with hypertension. These results are consistent with those from several previous studies that also found an association between hypertension and CKD.
Diabetes is an established predictor of renal disease [10-13]. However, we found no significant association between diabetes and new-onset CKD. GFR decreases with the duration of diabetes, but it has been suggested that glomerular hyperfiltration occurs during the early stage of diabetes, leading to a temporary increase in GFR [37]. The mean (± SD) follow-up period was 95.1 (± 66.0) months in our participants with diabetes, but it is possible that the GFR was increased or not markedly decreased in some participants who were still in the hyperfiltration phase. Thus, it is necessary to determine the presence of new-onset CKD employing urinary albumin measurement in participants with diabetic nephropathy during the early stage. Diabetes reportedly did not significantly affect the progression of CKD in Japanese participants; significant reductions in GFR might not have been observed because of hyperfiltration, as observed in this study [6].
Obesity has been suggested to be a risk factor for ESRD [7,16]. Kramer et al. reported that the risk of new-onset CKD was increased by a factor of 1.21 in participants whose BMI was 25.0-29.9 kg/m2 and by a factor of 1.40 in those whose BMI was ≥ 30 kg/m2[38]. However, a study conducted in Japanese participants found that a BMI of ≥ 25 kg/m2 in male participants had no effect on the progression of CKD to stage III or more severe disease [6]. In our study, a BMI of 25 kg/m2 was used as the standard for defining obesity, according to the criteria for obesity in Japan (Guidelines for the Treatment of Obesity, 2006)[39]. The hazard ratio for new-onset CKD in participants with obesity was 1.35, showing no significant association; however, a weak association was observed, since the 95% CI was 0.87-2.10. Further studies with longer follow-up periods in more participants are needed to clarify the association between obesity and new-onset CKD.
In this study, new-onset CKD was defined as GFR < 60 mL/min/1.73 m2. It is therefore important to clarify the methods used for estimating the GFR; GFR-estimating equations for Japanese devised by the Japanese Society of Nephrology in March, 2008 [21]. Imai et al. previously reported a different equation for estimating GFR in Japanese participants [40], but it has been suggested that this might underestimate GFR values. The equation used in the current study was a revised version of the previous equation, and might produce more accurate estimates of GFR in the Japanese population. According to the diagnostic criteria for CKD, abnormal urinalysis results, such as proteinuria, and abnormal findings on echography, can also be used to diagnose CKD. However, in line with many previous studies, the current study defined CKD as GFR < 60 mL/min/1.73 m2.
Hyperuricemia, low serum HDL-C, hypertension, diabetes, and obesity were defined in this study based on the first year's data for each participant. Treatment for any of these diseases was not taken into account, except for the exclusion of any individuals with a history of gouty attacks. It is therefore possible that some of the participants who were classified as a uric acid level of ≤ 7.0 mg/dL were actually receiving treatment. In this case, compared with strictly extracting participants who have treatment of hyperuricemia, the power of statistically significance of the association between hyperuricemia and CKD could be low. However, hyperuricemia could be a predictive factor for new-onset CKD in this study, as suggested by the significant association between hyperuricemia and new-onset CKD. Conclusions
The results of this study suggest that asymptomatic hyperuricemia without gouty attacks is a predictive factor for new-onset CKD. Therefore, the appropriate treatment might reduce the number of patients of CKD.
Abbreviations
ESRD: end-stage renal disease; CKD: chronic kidney disease; GFR: glomerular filtration rate; HDL-C: high-density lipoprotein cholesterol; BMI: body mass index; eGFR: estimated glomerular filtration rate; Cr: creatinine; CI: confidence interval; SD: standard deviation.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
MK and KW designed the study, and completed the manuscript. HO, HT and YA undertook statistical analyses and assisted with drafting the manuscript from a clinical perspective. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Masatoshi Kawashima, Email: m-kawashima@umin.ac.jp.
Koji Wada, Email: kwada-sgy@umin.ac.jp.
Hiroshi Ohta, Email: ohta.hiroshi.md@gmail.com.
Hiroyuki Terawaki, Email: terawaki@jikei.ac.jp.
Yoshiharu Aizawa, Email: aizawa@kitasato-u.ac.jp.
Acknowledgements
We would like to express our gratitude to staff members of the clinic at Mitsubishi Heavy Industries, General Machinery and Special Vehicle Headquarters for help in collecting the participants' data during annual health examinations.
References
- K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1–266. [PubMed] [Google Scholar]
- Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298(17):2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- Imai E, Horio M, Watanabe T, Iseki K, Yamagata K, Hara S, Ura N, Kiyohara Y, Moriyama T, Ando Y, Fujimoto S, Konta T, Yokoyama H, Makino H, Hishida A, Matsuo S. Prevalence of chronic kidney disease in the Japanese general population. Clin Exp Nephrol. 2009;13(6):621–630. doi: 10.1007/s10157-009-0199-x. [DOI] [PubMed] [Google Scholar]
- Walker WG, Neaton JD, Cutler JA, Neuwirth R, Cohen JD. Renal function change in hypertensive members of the Multiple Risk Factor Intervention Trial. Racial and treatment effects. The MRFIT Research Group. JAMA. 1992;268(21):3085–3091. doi: 10.1001/jama.268.21.3085. [DOI] [PubMed] [Google Scholar]
- Bakris GL, Williams M, Dworkin L, Elliott WJ, Epstein M, Toto R, Tuttle K, Douglas J, Hsueh W, Sowers J. Preserving renal function in adults with hypertension and diabetes: a consensus approach. National Kidney Foundation Hypertension and Diabetes Executive Committees Working Group. Am J Kidney Dis. 2000;36(3):646–661. doi: 10.1053/ajkd.2000.16225. [DOI] [PubMed] [Google Scholar]
- Yamagata K, Ishida K, Sairenchi T, Takahashi H, Ohba S, Shiigai T, Narita M, Koyama A. Risk factors for chronic kidney disease in a community-based population: a 10-year follow-up study. Kidney Int. 2007;71(2):159–166. doi: 10.1038/sj.ki.5002017. [DOI] [PubMed] [Google Scholar]
- Fox CS, Larson MG, Leip EP, Culleton B, Wilson PW, Levy D. Predictors of new-onset kidney disease in a community-based population. JAMA. 2004;291(7):844–850. doi: 10.1001/jama.291.7.844. [DOI] [PubMed] [Google Scholar]
- Tozawa M, Iseki K, Iseki C, Kinjo K, Ikemiya Y, Takishita S. Blood pressure predicts risk of developing end-stage renal disease in men and women. Hypertension. 2003;41(6):1341–1345. doi: 10.1161/01.HYP.0000069699.92349.8C. [DOI] [PubMed] [Google Scholar]
- Vupputuri S, Batuman V, Muntner P, Bazzano LA, Lefante JJ, Whelton PK, He J. Effect of blood pressure on early decline in kidney function among hypertensive men. Hypertension. 2003;42(6):1144–1149. doi: 10.1161/01.HYP.0000101695.56635.31. [DOI] [PubMed] [Google Scholar]
- Adler AI, Stevens RJ, Manley SE, Bilous RW, Cull CA, Holman RR. Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64) Kidney Int. 2003;63(1):225–232. doi: 10.1046/j.1523-1755.2003.00712.x. [DOI] [PubMed] [Google Scholar]
- Araki S, Haneda M, Sugimoto T, Isono M, Isshiki K, Kashiwagi A, Koya D. Factors associated with frequent remission of microalbuminuria in patients with type 2 diabetes. Diabetes. 2005;54(10):2983–2987. doi: 10.2337/diabetes.54.10.2983. [DOI] [PubMed] [Google Scholar]
- Ruggenenti P, Fassi A, Ilieva AP, Bruno S, Iliev IP, Brusegan V, Rubis N, Gherardi G, Arnoldi F, Ganeva M, Ene-Iordache B, Gaspari F, Perna A, Bossi A, Trevisan R, Dodesini AR, Remuzzi G. Preventing microalbuminuria in type 2 diabetes. N Engl J Med. 2004;351(19):1941–1951. doi: 10.1056/NEJMoa042167. [DOI] [PubMed] [Google Scholar]
- Andersen S, Brochner-Mortensen J, Parving HH. Kidney function during and after withdrawal of long-term irbesartan treatment in patients with type 2 diabetes and microalbuminuria. Diabetes Care. 2003;26(12):3296–3302. doi: 10.2337/diacare.26.12.3296. [DOI] [PubMed] [Google Scholar]
- Schaeffner ES, Kurth T, Curhan GC, Glynn RJ, Rexrode KM, Baigent C, Buring JE, Gaziano JM. Cholesterol and the risk of renal dysfunction in apparently healthy men. J Am Soc Nephrol. 2003;14(8):2084–2091. doi: 10.1681/ASN.V1482084. [DOI] [PubMed] [Google Scholar]
- Muntner P, Coresh J, Smith JC, Eckfeldt J, Klag MJ. Plasma lipids and risk of developing renal dysfunction: the atherosclerosis risk in communities study. Kidney Int. 2000;58(1):293–301. doi: 10.1046/j.1523-1755.2000.00165.x. [DOI] [PubMed] [Google Scholar]
- Iseki K, Ikemiya Y, Kinjo K, Inoue T, Iseki C, Takishita S. Body mass index and the risk of development of end-stage renal disease in a screened cohort. Kidney Int. 2004;65(5):1870–1876. doi: 10.1111/j.1523-1755.2004.00582.x. [DOI] [PubMed] [Google Scholar]
- Obermayr RP, Temml C, Gutjahr G, Knechtelsdorfer M, Oberbauer R, Klauser-Braun R. Elevated uric acid increases the risk for kidney disease. J Am Soc Nephrol. 2008;19(12):2407–2413. doi: 10.1681/ASN.2008010080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madero M, Sarnak MJ, Wang X, Greene T, Beck GJ, Kusek JW, Collins AJ, Levey AS, Menon V. Uric acid and long-term outcomes in CKD. Am J Kidney Dis. 2009;53(5):796–803. doi: 10.1053/j.ajkd.2008.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iseki K, Ikemiya Y, Inoue T, Iseki C, Kinjo K, Takishita S. Significance of hyperuricemia as a risk factor for developing ESRD in a screened cohort. Am J Kidney Dis. 2004;44(4):642–650. [PubMed] [Google Scholar]
- Ohno I, Hosoya T, Gomi H, Ichida K, Okabe H, Hikita M. Serum uric acid and renal prognosis in patients with IgA nephropathy. Nephron. 2001;87(4):333–339. doi: 10.1159/000045939. [DOI] [PubMed] [Google Scholar]
- Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53(6):982–992. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- Hallan S, Asberg A, Lindberg M, Johnsen H. Validation of the Modification of Diet in Renal Disease formula for estimating GFR with special emphasis on calibration of the serum creatinine assay. Am J Kidney Dis. 2004;44(1):84–93. doi: 10.1053/j.ajkd.2004.03.027. [DOI] [PubMed] [Google Scholar]
- Cox D. Regression Models and Life-Tables. J R Stat Soc Ser B. 1972;34(2):187–220. [Google Scholar]
- Kaplan EL, Meier P. Nonparametric Estimation from Incomplete Observations. J Am Stat Assoc. 1958;53(282):457–481. doi: 10.2307/2281868. [DOI] [Google Scholar]
- SPSS. SPSS for Windows VJ. Chicago: SPSS Inc. 2008.
- Siu YP, Leung KT, Tong MK, Kwan TH. Use of allopurinol in slowing the progression of renal disease through its ability to lower serum uric acid level. Am J Kidney Dis. 2006;47(1):51–59. doi: 10.1053/j.ajkd.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Perez-Ruiz F, Calabozo M, Herrero-Beites AM, Garcia-Erauskin G, Pijoan JI. Improvement of renal function in patients with chronic gout after proper control of hyperuricemia and gouty bouts. Nephron. 2000;86(3):287–291. doi: 10.1159/000045783. [DOI] [PubMed] [Google Scholar]
- Guideline for the management of hyperuricemia and gout (in Japanese) Japanese Society of Gout and Nucleic Acid Metabolosm. 2002. [DOI] [PubMed]
- Ravid M, Brosh D, Ravid-Safran D, Levy Z, Rachmani R. Main risk factors for nephropathy in type 2 diabetes mellitus are plasma cholesterol levels, mean blood pressure, and hyperglycemia. Arch Intern Med. 1998;158(9):998–1004. doi: 10.1001/archinte.158.9.998. [DOI] [PubMed] [Google Scholar]
- Appel GB, Radhakrishnan J, Avram MM, DeFronzo RA, Escobar-Jimenez F, Campos MM, Burgess E, Hille DA, Dickson TZ, Shahinfar S, Brenner BM. Analysis of metabolic parameters as predictors of risk in the RENAAL study. Diabetes Care. 2003;26(5):1402–1407. doi: 10.2337/diacare.26.5.1402. [DOI] [PubMed] [Google Scholar]
- Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–3421. [PubMed] [Google Scholar]
- Teramoto T, Sasaki J, Ueshima H, Egusa G, Kinoshita M, Shimamoto K, Daida H, Biro S, Hirobe K, Funahashi T, Yokote K, Yokode M. Diagnostic criteria for dyslipidemia. Executive summary of Japan Atherosclerosis Society (JAS) guideline for diagnosis and prevention of atherosclerotic cardiovascular diseases for Japanese. J Atheroscler Thromb. 2007;14(4):155–158. doi: 10.5551/jat.E537. [DOI] [PubMed] [Google Scholar]
- Reynolds K, Gu D, Muntner P, Kusek JW, Chen J, Wu X, Duan X, Chen CS, Klag MJ, Whelton PK, He J. A population-based, prospective study of blood pressure and risk for end-stage renal disease in China. J Am Soc Nephrol. 2007;18(6):1928–1935. doi: 10.1681/ASN.2006111199. [DOI] [PubMed] [Google Scholar]
- Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL, Ford CE, Shulman NB, Stamler J. Blood pressure and end-stage renal disease in men. N Engl J Med. 1996;334(1):13–18. doi: 10.1056/NEJM199601043340103. [DOI] [PubMed] [Google Scholar]
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Roccella EJ Jr. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42(6):1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- Ogihara T, Kikuchi K, Matsuoka H, Fujita T, Higaki J, Horiuchi M, Imai Y, Imaizumi T, Ito S, Iwao H, Kario K, Kawano Y, Kim-Mitsuyama S, Kimura G, Matsubara H, Matsuura H, Naruse M, Saito I, Shimada K, Shimamoto K, Suzuki H, Takishita S, Tanahashi N, Tsuchihashi T, Uchiyama M, Ueda S, Ueshima H, Umemura S, Ishimitsu T, Rakugi H. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2009) Hypertens Res. 2009;32(1):3–107. [PubMed] [Google Scholar]
- Remuzzi G, Benigni A, Remuzzi A. Mechanisms of progression and regression of renal lesions of chronic nephropathies and diabetes. J Clin Invest. 2006;116(2):288–296. doi: 10.1172/JCI27699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer H, Luke A, Bidani A, Cao G, Cooper R, McGee D. Obesity and prevalent and incident CKD: the Hypertension Detection and Follow-Up Program. Am J Kidney Dis. 2005;46(4):587–594. doi: 10.1053/j.ajkd.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Guideline for the Treatment of Obesity (in Japanese) Japan Society for the Study of Obesity. 2006.
- Imai E, Horio M, Nitta K, Yamagata K, Iseki K, Tsukamoto Y, Ito S, Makino H, Hishida A, Matsuo S. Modification of the Modification of Diet in Renal Disease (MDRD) Study equation for Japan. Am J Kidney Dis. 2007;50(6):927–937. doi: 10.1053/j.ajkd.2007.09.004. [DOI] [PubMed] [Google Scholar]