Abstract
The lack of expression of the Suppressor of Cytokine Signalling-3 (SOCS3) or inactivation of the negative regulatory capacity of SOCS3 has been well documented in rheumatoid arthritis, viral hepatitis and cancer. The specific qualitative and quantitative consequences of SOCS3-deficiency on IL-6-mediated pro- and anti-inflammatory responses remain controversial in vitro and unknown in vivo. Mice with a conditional deletion of SOCS3 in hematopoietic cells develop lethal inflammatory disease during adult life and develop gross histopathological changes during experimental arthritis, typified by elevated IL-6 levels. To clarify the nature of the IL-6 responses in vivo, we generated mice deficient in SOCS3 (SOCS3−/Δvav) or both SOCS3 and IL-6 (IL-6−/−/SOCS3−/Δ vav) and examined responses in models of acute and chronic inflammation. Acute responses to IL-1β were lethal to SOCS3−/Δ vav mice but not IL-6−/−/SOCS3−/Δ vav mice, indicating that IL-6 was required for the lethal inflammation induced by IL-1β. Administration of IL-1β to SOCS3−/Δ vav mice induced systemic apoptosis of lymphocytes in the thymus, spleen and lymph nodes that was dependent on the presence of IL-6. IL-6-deficiency prolonged survival of SOCS3−/Δ vav mice and ameliorated spontaneous inflammatory disease developing during adult life. Infection of SOCS3−/Δ vav mice with LCMV induced a lethal inflammatory response that was dependent on IL-6, despite SOCS3−/Δ vav mice controlling viral replication. We conclude that SOCS3 is required for survival during inflammatory responses and is a critical regulator of IL-6 in vivo.
Keywords: SOCS3, IL-6, IL-1β, Suppressor of Cytokine Signaling-3
INTRODUCTION
Interleukin-6 (IL-6) is a multifunctional cytokine, regulating diverse physiological and pathological phenomena including granulocyte development1, T cell differentiation2, hepatocyte generation, acute phase protein production 3, 4 and autoimmune disease5. The expression of IL-6 can be induced in multiple cell types following infection or in response to cytokines including IL-1β and TNFα 6.
IL-6 has a range of pro- and anti-inflammatory activities. IL-6 promotes multiple experimental inflammatory and autoimmune diseases, including autoimmune myocarditis7, experimental autoimmune encephalomyelitis8, experimental autoimmune arthritis9, 10, experimental autoimmune myasthenia gravis 11 and pristane-induced lupus 12. In contrast, IL-6 prevents chronic autoimmune myocarditis following viral infection13, suppresses neutrophilia and production of pro-inflammatory cytokines such as TNFα, GM-CSF and IFN-γ, and enhances production of anti-inflammatory mediators such as IL-10, IRAP, TNF soluble receptor and protease inhibitors4, 14–16. Control of IL-6 production and its signalling is therefore critical during inflammation, given such a broad spectrum of activities. The central role of IL-6 in acute and chronic inflammatory diseases is demonstrated by the successful introduction of tocilizumab, a neutralising humanised antibody to the IL-6 receptor, in patients with Castleman’s disease, rheumatoid arthritis and systemic-onset juvenile idiopathic arthritis 17–19.
The Suppressor of Cytokine Signalling 3 (SOCS3) is an essential negative regulator of IL-6-gp130 signal transduction20–22. SOCS3 activity in this context is dependent upon binding to phosphorylated Tyr759 (Tyr 757 in the mouse gp130 receptor) 23. An inactivating mutation of Y759 (Y759F) on gp130 (gp130F759) increased IL-6 signalling 24. Embryonic fibroblasts and T cells derived from gp130F759/F759 mutant mice displayed prolonged IL-6/sIL-6R-induced phosphorylation of gp130, Jak1 and STAT3, consistent with a role for SOCS3 in the negative regulation of IL-6-gp130 signalling 24. Mutant gp130F759/F759 mice develop autoimmune arthritis, splenomegaly, lymphadenopathy, and display defects in B and T lymphocyte function 24. This phenotype is similar, but not identical, to mice with a SOCS3-deficient hematopoietic system that succumb to a lethal inflammatory disease characterised by pericarditis and extensive inflammatory lesions in the peritoneal and pleural cavities 25.
While these data collectively demonstrate a critical role for SOCS3 in the negative regulation of IL-6, the precise physiological consequence of SOCS3 deficiency on IL-6-dependent cellular responses remains unknown. Particularly, it is unknown whether SOCS3 determines whether a cellular response to IL-6 is pro- or anti-inflammatory. Yasukawa et al. have proposed that IL-6 delivers an anti-inflammatory signal in the absence of SOCS3, decreasing TNF production from macrophages stimulated with LPS 22. Consistent with this, mice with a SOCS3 deficiency in macrophages were protected from the lethal effects of galactosamine and LPS administration, a model that is dependent on TNF-induced hepatocyte death and liver failure. It is not known if mice with a SOCS3-deficiency in hematopoietic cells are also resistant to the effects of high doses of LPS 22. In contrast, Lang et al. and Croker et al. demonstrated that IL-6 delivers an IFNγ-like signal in the absence of SOCS3, suggesting that IL-6 delivers a pro-inflammatory signal in the absence of SOCS3, and that SOCS3 thereby modulates the quality of the cellular response to IL-6 20, 21. In support of this view, mice lacking SOCS3 in blood cells develop severe antigen-induced arthritis and display massive increases in serum IL-6 compared to controls, suggesting that IL-6 is not acting in an anti-inflammatory manner in this model 26. To specifically address these opposing models of SOCS3 regulation of IL-6 signalling, we examined the pathophysiological consequences of a deficiency in both SOCS3 and IL-6 using both acute and chronic models of inflammatory disease. We find that in the absence of SOCS3, IL-6 does not act in an anti-inflammatory manner but rather can promote lethal acute and chronic inflammatory diseases.
MATERIALS AND METHODS
Mice
C57BL/6J, IL-6−/−, vav-Cre/SOCS3−/loxP (SOCS3−/Δ vav) 25 and IL-6/SOCS3−/Δ vav mice were bred at The Walter and Eliza Hall Institute of Medical Research with unlimited access to food and water. Experiments were conducted in accordance with institute animal ethics guidelines and approval.
IL-1 and IL-6 challenge
For IL-1 challenge experiments, 1 μg IL-1β (eBioscience) in 0.2 mL saline was injected intraperitoneally every 12 h for 36 h. For IL-6 challenge experiments, 1 μg IL-6 was injected intraperitoneally every 12 h for 7 days. The bioactivity of IL-6 was confirmed using clonogenic bone marrow progenitor cell assays 25. Tissues were fixed in 10% buffered formalin, embedded in paraffin and 1 μm (bone) or 2 μm (spleen, thymus and lymph node) sections were stained with hematoxylin and eosin. Serum cytokines were analysed using Luminex beads according to the manufacturer’s instructions (Biorad).
Bone marrow chimeras
For reconstitution experiments, congenic C57BL/6.SJL (Ptprca Pep3b (Ly5.1)) mice were reconstituted with 5 × 106 C57BL/6 (Ptprcb Pep3a (Ly5.2)) bone marrow cells from either vavCre+SOCS3−/fl or vavCre−SOCS3+/+ genotype after two 5.5 Gy doses of irradiation given 3 h apart. The reconstitution of recipients with donor cells was consistently greater than 80% in the bone marrow, peripheral blood and lymph node.
Flow cytometry
Hematopoietic cells from the bone marrow, spleen and peripheral blood were analysed using antibodies specific for CD45R (B220), IgM, CD11b, CD45.1, CD45.2, Gr1, Ter119 and Thy1 (provided by Dr A. Strasser, Walter and Eliza Hall Institute of Medical Research, Parkville, Australia). Blood was collected into tubes containing EDTA (Becton Dickinson) and analysed using an Advia 120 analyser (Bayer).
LCMV infection
Mice were infected with 2×106 plaque forming units (pfu) LCMV clone 13.27 Staining of LCMV-specific T cells with tetramers was performed as described previously28. For virus quantification, organs were weighed and homogenized using the Qiagen TissueLyser and LCMV titers were determined by focus forming assays using MC57 fibroblast cells as previously described 29.
RESULTS
IL-6 drives chronic inflammatory disease in mice lacking SOCS3
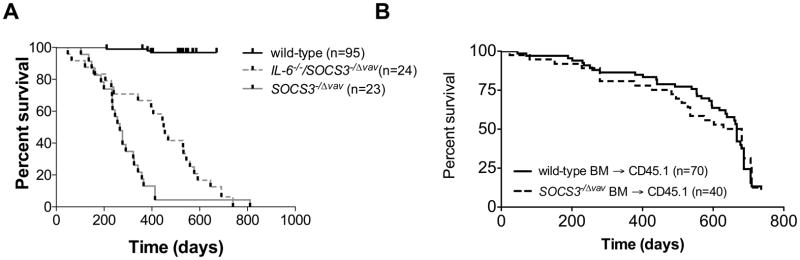
SOCS3−/Δ vav mice lack SOCS3 in hematopoietic and endothelial cells, and develop a lethal chronic inflammatory disease characterised by pericarditis, splenomegaly, hepatitis and severe fibrinous inflammation in the peritoneal and pleural cavities 25. Our studies and those of other investigators have demonstrated that SOCS3 negatively regulates IL-6, LIF and G-CSF signal transduction but the relative contributions of these cytokines to chronic inflammatory disease is unknown. To determine the role of IL-6 in the development of lethal chronic inflammatory disease in mice deficient in SOCS3, we established cohorts of SOCS3−/Δ vav mice and IL-6−/−/SOCS3−/Δ vav mice, and followed their survival and tissue pathology. IL-6−/−/SOCS3−/Δ vav mice survived for a significantly longer period than did SOCS3−/Δ vav mice (Figure 1A), indicating a role for IL-6 in the development of lethal inflammatory disease in mice lacking SOCS3. However, ultimately the majority of mice succumbed to inflammatory diseases. Histological examination of tissues from IL-6−/−/SOCS3−/Δ vav mice revealed a similar degree of pathology at the time of death in the liver, heart, lung, spleen and fibrinous inflammation surrounding organs in both the peritoneal and pleural cavities, as observed in SOCS3−/Δ vav mice (Table 1). To delineate the contribution of hematopoietic and non-hematopoietic cells to this chronic inflammatory disease, we established bone marrow chimeras by reconstituting lethally-irradiated wild-type recipients with wild-type or SOCS3−/Δ vav bone marrow cells. As Figure 1B demonstrates, no significant differences in survival were evident in mice receiving SOCS3−/Δ vav bone marrow cells compared to mice receiving WT bone marrow cells, indicating that non-hematopoietic cells also play a key role in the development of chronic inflammatory disease in SOCS3−/Δ vav mice.
Figure 1.
(A) Survival of SOCS3−/Δ vav mice is prolonged in the absence of IL-6. p<0.05, SOCS3−/Δ vav v IL-6−/−/SOCS3−/Δ vav, by log-rank test. (B) Survival of mice reconstituted with SOCS3−/Δ vav bone marrow is not different to mice reconstituted with wild-type bone marrow. Wild-type mice were reconstituted with SOCS3−/Δ vav or wild-type bone marrow. p>0.05, SOCS3−/Δ vav v wild-type, by log-rank test.
Table 1.
IL-6 deficiency in SOCS3−/Δ vav mice delays the onset but not the severity of inflammatory disease. The proportion of mice affected by inflammatory changes at sacrifice are shown for various organs. Detailed descriptions of the lesions in SOCS3−//Δvav mice are provided in 25. Inflammatory lesions found in the liver, lung, heart, spleen and peritoneal cavity of adult IL-6−/−/SOCS3−/Δ vav mice are indistinguishable from SOCS3−/Δ vav mice at the time of death.
| SOCS3−/Δvav | IL-6−/−/SOCS3−/Δ vav | |
|---|---|---|
| Pneumonitis | 3/3 | 10/10 |
| Hypergranulocytic marrow | 3/3 | 10/10 |
| Excess granulocytes in spleen | 3/3 | 10/10 |
| Leukocytic foci in liver | 3/3 | 7/10 |
| Pericarditis | 2/3 | 3/10 |
IL-6 does not induce acute inflammatory disease in mice lacking SOCS3
Several lines of evidence prompted us to examine if IL-6 administration could initiate acute inflammatory disease as a single agent or whether additional inflammatory cofactors are required for inflammatory disease. Firstly, as Figure 1 demonstrates, IL-6 plays a key role in development of lethal inflammatory disease. Secondly, serum IL-6 levels are approximately 20-fold higher in arthritic SOCS3-deficient animals than in arthritic control animals 26. To address the role of IL-6 in acute inflammatory disease, we injected SOCS3−/Δ vav mice (Table 2) with 1 μg IL-6, twice daily for up to 8 days. IL-6 induced mild splenomegaly in wild-type mice (Table 2), consistent with previous reports on its biological activity in vivo 30. However, no wild-type or SOCS3−/Δ vav mice developed illness during the course of IL-6 and no histopathological features were noted in response to IL-6 injections. These data demonstrate that, at least at the concentration used here, IL-6 does not induce acute inflammatory disease by itself. Rather, IL-6 seems likely to require the input of other upstream and parallel cytokine signalling pathways regulated directly or indirectly by SOCS3 for initiation and progression of pathology.
Table 2.
IL-6 challenge does not induce pathology in SOCS3−/Δvav mice
| SOCS3+/fl | SOCS3−/Δ vav | |||
|---|---|---|---|---|
| Peripheral Blood (×106/mL) | Platelets | Saline | 1272±399 | 1313±177 |
| IL-6 | 1863±289 | 1564±428 | ||
|
| ||||
| Leukocytes | Saline | 3±2 | 6±5 | |
| IL-6 | 5±2 | 5±1 | ||
|
| ||||
| Granulocytes | Saline | 0.3±0.1 | 0.4±0.5 | |
| IL-6 | 0.4±0.2 | 0.4±0.2 | ||
|
| ||||
| B cells | Saline | 2±2 | 4±3 | |
| IL-6 | 3±1 | 3±1 | ||
|
| ||||
| T cells | Saline | 0.7±0.3 | 2±1 | |
| IL-6 | 2±0.4 | 1±1 | ||
|
| ||||
| Spleen (×106 cells) | Weight (mg) | Saline | 73±6.4 | 133±77 |
| IL-6 | 121±18* | 169±26 | ||
|
| ||||
| Leukocytes | Saline | 75±3 | 142±94 | |
| IL-6 | 150±28 | 164±18 | ||
|
| ||||
| Granulocytes | Saline | 0.7±0.3 | 6±7 | |
| IL-6 | 0.8±0.2 | 4.3±0.9 | ||
|
| ||||
| Erythroid cells | Saline | 0.4±0.1 | 4.1±4.2 | |
| IL-6 | 14±4 | 16±6 | ||
|
| ||||
| B cells | Saline | 46±5 | 88±61 | |
| IL-6 | 87±18 | 87±10 | ||
|
| ||||
| T cells | Saline | 23±2 | 34±15 | |
| IL-6 | 33±5 | 38±4 | ||
|
| ||||
| BM (×106 cells) | Total cells | Saline | 19±0.1 | 14±6 |
| IL-6 | 22±3 | 16±3 | ||
|
| ||||
| Granulocytes | Saline | 6±0.7 | 7±2 | |
| IL-6 | 9±1 | 11±2 | ||
|
| ||||
| Erythroid cells | Saline | 6±1 | 3±2 | |
| IL-6 | 6±1 | 2±1 | ||
|
| ||||
| B cells | Saline | 4±0.3 | 2±1 | |
| IL-6 | 5±2 | 1±0.3 | ||
Mice were injected with 1 μg IL-6 twice daily for 7 days and analysed day 8. Gr1, Ter119, B220 and Thy1 were used as markers for granulocytes, erythroid cells, B cells and T cells, respectively. Leukocyte number from bone marrow is from 2 femurs. Figures represent mean and standard deviation from 2 (saline-treated) or 4 (IL-6-treated) mice per group.
p<0.05, saline-treated versus IL-6-treated.
IL-6 is required for the lethal effects of IL-1β in SOCS3-deficient mice
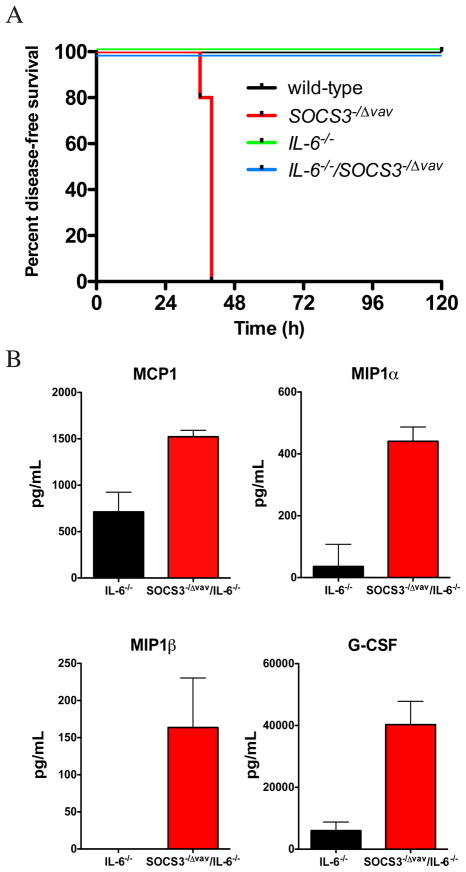
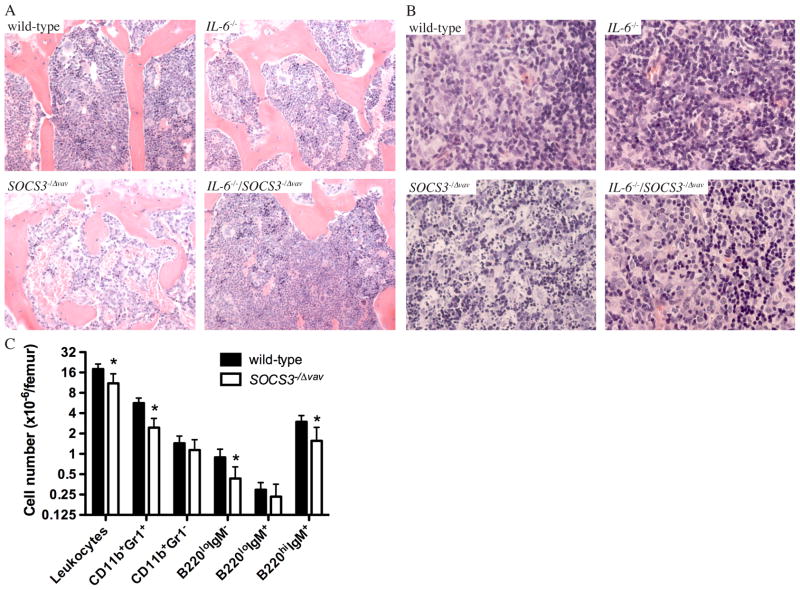
Patients with rheumatoid arthritis display high levels of expression of pSTAT3 in synovial tissue 31. Adenoviral expression of SOCS3 in the intra-articular joint alleviated arthritis in experimental models 31, and in mouse models of rheumatoid arthritis initiated by IL-1β, lack of SOCS3 results in exacerbated synovitis, pannus formation, cartilage and bone destruction and inflammatory exudate in joints 26. We therefore postulated that SOCS3 was a key negative regulator of IL-6 responses downstream of acute inflammation induced by IL-1β. To test this possibility, we administered a 1 μg dose of IL-1β on two consecutive days and monitored the incidence of inflammatory disease in SOCS3−/Δ vav mice or in IL-6−/−/SOCS3−/Δ vav mice. SOCS3−/Δ vav mice became moribund following injections with IL-1β in contrast to IL-6−/−/SOCS3−/Δ vav mice, IL-6−/− mice or wild-type controls, which survived the systemic administration of IL-1β (Figure 2A). This lethal systemic inflammatory response in SOCS3−/Δ vav mice injected with IL-1β was reflected by a dramatic increase in levels of MIP1α, MIP1β, MCP1 and G-CSF in IL-6−/−/SOCS3−/Δ vav mice compared to IL-6−/− controls (Figure 2B). A histological survey of tissues from IL-1β-injected SOCS3−/Δ vav mice revealed a dramatically reduced bone marrow cellularity (Figure 3A), and a high frequency of apoptotic cells in thymus (Figure 3B), spleen and lymph nodes (Supplementary Figure 1), that was not apparent in IL-6−/−/SOCS3−/Δ vav mice, IL-6−/− mice or wild-type controls (Figure 3 and Supplementary Figure 1). Analysis of haemopoietic cells in the bone marrow of IL-1β-injected SOCS3−/Δ vav mice indicated that the reduction in bone marrow cellularity could be attributed to a 50% reduction in CD11b+Gr1+ cells, B220loIgM− precursor B cells and B220hiIgM+ mature recirculating B cells (Figure 3C).
Figure 2.
SOCS3 regulates responses to IL-1β in vivo. (A) SOCS3−/Δ vav mice are hypersensitive to IL-1β, resulting from loss of regulation of IL-6. n=4–5 per group. (B) SOCS3 regulates cytokine production independently of IL-6 after challenge with IL-1β. Cytokine production was analysed in the serum of mice injected with IL-1β.
Figure 3.
IL-1β induces loss of cells in the bone marrow (A) and apoptosis of leukocytes in the thymus (B) of SOCS3−/Δ vav mice but not IL-6−/−/SOCS3−/Δ vav mice. Tissues were stained with haematoxylin and eosin. (C) Flow cytometric analysis of haemopoietic cells in the bone marrow from IL-1β-injected wild-type and SOCS3−/Δ vav mice, 36 h after injection.
SOCS3 is required for survival after LCMV challenge
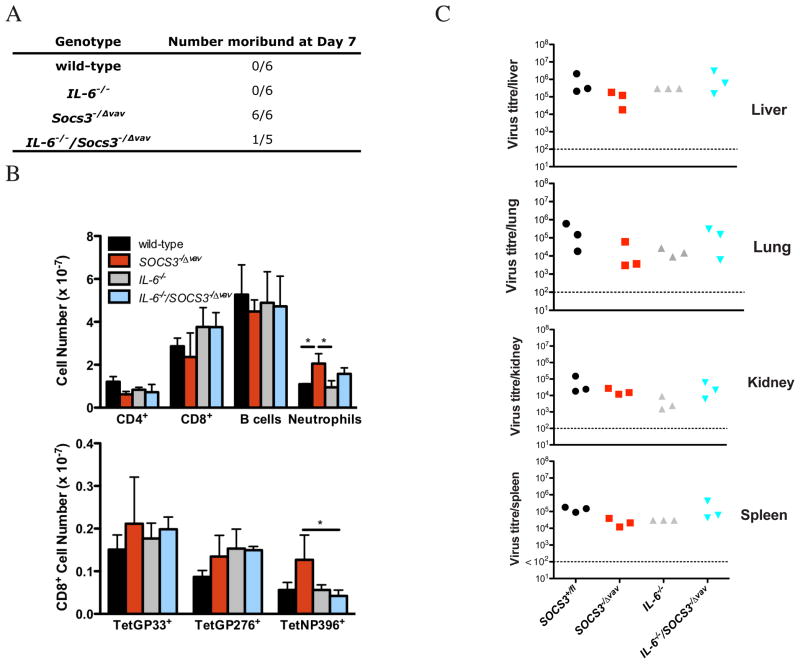
To investigate the role of SOCS3 in the regulation of IL-6 signaling during chronic active viral infection, we challenged mice with lymphocytic choriomeningitis virus (LCMV) clone 13. This infection causes persistent high level viremia and it mimics several human chronic active viral infections 32. IL-1β and IL-6 are highly expressed during the course of LCMV infection 28. Consistent with the sensitivity of SOCS3−/Δ vav mice to IL-1β, SOCS3−/Δ vav mice were moribund at day 7 of LCMV clone 13 infection in contrast to IL-6−/−/SOCS3−/Δ vav mice, of which only one of five became moribund, and wild-type mice which remained healthy (Figure 4A). To assess T cell responses to LCMV, we used tetramers specific for the LCMV epitopes (GP33-41, GP276-286 and NP396-404). A significant increase in total numbers of NP396-specific CD8+ T cells was found in SOCS3−/Δ vav mice compared to IL-6/SOCS3−/Δ vav mice (Figure 4B). No differences in viral titres were evident in the liver, lung, kidney and spleen of SOCS3−/Δ vav or IL-6/SOCS3−/Δ vav mice compared to controls (Figure 4C).
Figure 4.
SOCS3 is essential for survival to LCMV but not essential for the generation of LCMV-specific CD8+ T cell responses or the containment of viral replication. (A) Mice that were moribund at day 7 of LCMV infection. (B) Hematopoietic cell populations were measured by flow cytometry on day 7 of LCMV infection. LCMV-specific CD8+ T cells were identified using tetramers specific for LCMV epitopes GP33-41, GP276-286 and NP396-404. (C) Viral titres in lung, kidney, liver and spleen were assessed on day 7. *p<0.05, ANOVA and SNK.
DISCUSSION
Here we demonstrate a key role for SOCS3 in the regulation of IL-6-dependent inflammatory responses in vivo. The absence of SOCS3 expression promotes a pathophysiological response to IL-6 during viral infection, acute inflammation induced by IL-1β and adult life. The data indicate that the hypersensitivity of SOCS3−/Δvav mice to viral infection is not attributable to an inability to contain viral replication. Rather, the data supports a role for SOCS3 in the pathogenesis of viral infection by regulating responses to IL-6. We propose that an evaluation of the role of SOCS3 in regulating responses to cytokines, in addition to overall cytokine levels, will provide novel insight into the pathogenesis of acute and chronic inflammatory diseases.
SOCS3−/Δ vav mice develop a lethal inflammatory disease during adult life 25. We demonstrate that the survival of adult SOCS3−/Δ vav mice is prolonged in the absence of IL-6, supporting a pathological role of IL-6 in the absence of SOCS3. Monitoring of bone marrow chimeras reconstituted with wild-type or SOCS3-deficient hematopoietic cells indicated key roles for non-hematopoietic cells in the development of inflammatory disease in SOCS3−/Δ vav mice. These data are consistent with previous observations that non-hematopoietic tissues contribute to the pathological effects of G-CSF administration in SOCS3−/Δ vav mice 25. Because IL-6 alone appears unable to induce a lethal inflammatory response when injected in SOCS3−/Δ vav mice, we suggest that IL-6 synergises with IL-1β or that the biological effects of IL-6 require the actions of other cytokines induced by IL-1β, such as G-CSF. The data presented in Figure 2B indicate that SOCS3 is a critical regulator of IL-6-independent production of cytokines following IL-1β challenge, and we suggest that the synergistic actions of IL-6, G-CSF, MCP1, MIP1α and MIP1β drive the lethal systemic inflammatory response in SOCS3−/Δ vav mice. We have previously demonstrated that G-CSF, rather than being well tolerated, becomes toxic in the absence of SOCS3, causing neutrophilic infiltration and destruction of multiple tissues 25. Our previous studies also indicate that SOCS3 regulates both the quality and the quantity of signalling downstream of the IL-6 and G-CSF receptors20, 33. The enhanced activation of STAT3 and STAT1, and the profound changes in gene transcription profiles induced by IL-6 and G-CSF in SOCS3-deficient cells20–22, 25, 33, may underlie this lethal systemic inflammatory response by preventing appropriate resolution of inflammation triggered by IL-1β or LCMV.
The widespread induction of apoptosis of SOCS3-deficient lymphocytes in response to IL-1β may be a consequence of excessive STAT3 activation, converting pro-survival signals to pro-apoptotic signals, as demonstrated for SOCS3-deficient murine embryonic fibroblasts stimulated with leukemia inhibitory factor (LIF) 34. These in vivo studies suggest that SOCS3 may play key roles in regulating a hallmark condition of sepsis, the systemic apoptosis of lymphocytes 35. The data support key roles for SOCS3 in the regulation of inflammatory responses instigated by viral infection and IL-1β-driven inflammatory responses.
Supplementary Material
Acknowledgments
This research was supported by an NHMRC Program Grant (461219), NHMRC Project Grant (637367), NHMRC Independent Research Institutes Support Scheme Grant (361646), Victorian State Government Operational Infrastructure Support Grant, NIH Grant CA022556, NHMRC CDA (BAC, 575531), NHMRC CJ Martin Fellowship (BAC, 356262), ARC QEII Fellowship (BAC, DP1094854), NHMRC Biomedical Postgraduate Research Scholarship (HK, 461294), NHMRC CDA (MP), Carden Fellowship Fund of the Cancer Council, Victoria (DM), NHMRC Research Fellowship (WSA, 575501), NHMRC Research Fellowship (NAN, 637300), NHMRC Practitioner Fellowship (AWR, 637309) and a Victorian Cancer Agency Fellowship (AWR).
Footnotes
DISCLOSURES
The authors declare no financial conflicts of interest.
References
- 1.Liu F, Poursine-Laurent J, Wu HY, Link DC. Interleukin-6 and the granulocyte colony-stimulating factor receptor are major independent regulators of granulopoiesis in vivo but are not required for lineage commitment or terminal differentiation. Blood. 1997;90:2583–90. [PubMed] [Google Scholar]
- 2.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 3.Cressman DE, Greenbaum LE, DeAngelis RA, Ciliberto G, Furth EE, Poli V, et al. Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science. 1996;274:1379–83. doi: 10.1126/science.274.5291.1379. [DOI] [PubMed] [Google Scholar]
- 4.Kopf M, Baumann H, Freer G, Freudenberg M, Lamers M, Kishimoto T, et al. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature. 1994;368:339–42. doi: 10.1038/368339a0. [DOI] [PubMed] [Google Scholar]
- 5.Pflegerl P, Vesely P, Hantusch B, Schlederer M, Zenz R, Janig E, et al. Epidermal loss of JunB leads to a SLE phenotype due to hyper IL-6 signaling. Proc Natl Acad Sci U S A. 2009;106:20423–8. doi: 10.1073/pnas.0910371106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kishimoto T. Interleukin-6: from basic science to medicine--40 years in immunology. Annu Rev Immunol. 2005;23:1–21. doi: 10.1146/annurev.immunol.23.021704.115806. [DOI] [PubMed] [Google Scholar]
- 7.Eriksson U, Kurrer MO, Schmitz N, Marsch SC, Fontana A, Eugster HP, et al. Interleukin-6-deficient mice resist development of autoimmune myocarditis associated with impaired upregulation of complement C3. Circulation. 2003;107:320–5. doi: 10.1161/01.cir.0000043802.38699.66. [DOI] [PubMed] [Google Scholar]
- 8.Samoilova EB, Horton JL, Hilliard B, Liu TS, Chen Y. IL-6-deficient mice are resistant to experimental autoimmune encephalomyelitis: roles of IL-6 in the activation and differentiation of autoreactive T cells. J Immunol. 1998;161:6480–6. [PubMed] [Google Scholar]
- 9.Ohshima S, Saeki Y, Mima T, Sasai M, Nishioka K, Nomura S, et al. Interleukin 6 plays a key role in the development of antigen-induced arthritis. Proc Natl Acad Sci U S A. 1998;95:8222–6. doi: 10.1073/pnas.95.14.8222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alonzi T, Fattori E, Lazzaro D, Costa P, Probert L, Kollias G, et al. Interleukin 6 is required for the development of collagen-induced arthritis. J Exp Med. 1998;187:461–8. doi: 10.1084/jem.187.4.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deng C, Goluszko E, Tuzun E, Yang H, Christadoss P. Resistance to experimental autoimmune myasthenia gravis in IL-6-deficient mice is associated with reduced germinal center formation and C3 production. J Immunol. 2002;169:1077–83. doi: 10.4049/jimmunol.169.2.1077. [DOI] [PubMed] [Google Scholar]
- 12.Richards HB, Satoh M, Shaw M, Libert C, Poli V, Reeves WH. Interleukin 6 dependence of anti-DNA antibody production: evidence for two pathways of autoantibody formation in pristane-induced lupus. J Exp Med. 1998;188:985–90. doi: 10.1084/jem.188.5.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poffenberger MC, Straka N, El Warry N, Fang D, Shanina I, Horwitz MS. Lack of IL-6 during coxsackievirus infection heightens the early immune response resulting in increased severity of chronic autoimmune myocarditis. PLoS ONE. 2009;4:e6207. doi: 10.1371/journal.pone.0006207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xing Z, Gauldie J, Cox G, Baumann H, Jordana M, Lei XF, et al. IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J Clin Invest. 1998;101:311–20. doi: 10.1172/JCI1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fattori E, Cappelletti M, Costa P, Sellitto C, Cantoni L, Carelli M, et al. Defective inflammatory response in interleukin 6-deficient mice. J Exp Med. 1994;180:1243–50. doi: 10.1084/jem.180.4.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tilg H, Trehu E, Atkins MB, Dinarello CA, Mier JW. Interleukin-6 (IL-6) as an anti-inflammatory cytokine: induction of circulating IL-1 receptor antagonist and soluble tumor necrosis factor receptor p55. Blood. 1994;83:113–8. [PubMed] [Google Scholar]
- 17.Matsuyama M, Suzuki T, Tsuboi H, Ito S, Mamura M, Goto D, et al. Anti-interleukin-6 receptor antibody (tocilizumab) treatment of multicentric Castleman’s disease. Internal medicine (Tokyo, Japan) 2007;46:771–4. doi: 10.2169/internalmedicine.46.6262. [DOI] [PubMed] [Google Scholar]
- 18.Yokota S, Imagawa T, Mori M, Miyamae T, Aihara Y, Takei S, et al. Efficacy and safety of tocilizumab in patients with systemic-onset juvenile idiopathic arthritis: a randomised, double-blind, placebo-controlled, withdrawal phase III trial. Lancet. 2008;371:998–1006. doi: 10.1016/S0140-6736(08)60454-7. [DOI] [PubMed] [Google Scholar]
- 19.Smolen JS, Beaulieu A, Rubbert-Roth A, Ramos-Remus C, Rovensky J, Alecock E, et al. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet. 2008;371:987–97. doi: 10.1016/S0140-6736(08)60453-5. [DOI] [PubMed] [Google Scholar]
- 20.Croker BA, Krebs DL, Zhang JG, Wormald S, Willson TA, Stanley EG, et al. SOCS3 negatively regulates IL-6 signaling in vivo. Nat Immunol. 2003;4:540–5. doi: 10.1038/ni931. [DOI] [PubMed] [Google Scholar]
- 21.Lang R, Pauleau AL, Parganas E, Takahashi Y, Mages J, Ihle JN, et al. SOCS3 regulates the plasticity of gp130 signaling. Nat Immunol. 2003;4:546–50. doi: 10.1038/ni932. [DOI] [PubMed] [Google Scholar]
- 22.Yasukawa H, Ohishi M, Mori H, Murakami M, Chinen T, Aki D, et al. IL-6 induces an anti-inflammatory response in the absence of SOCS3 in macrophages. Nat Immunol. 2003;4:551–6. doi: 10.1038/ni938. [DOI] [PubMed] [Google Scholar]
- 23.Nicholson SE, De Souza D, Fabri LJ, Corbin J, Willson TA, Zhang JG, et al. Suppressor of cytokine signaling-3 preferentially binds to the SHP-2-binding site on the shared cytokine receptor subunit gp130. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:6493–8. doi: 10.1073/pnas.100135197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohtani T, Ishihara K, Atsumi T, Nishida K, Kaneko Y, Miyata T, et al. Dissection of signaling cascades through gp130 in vivo: reciprocal roles for STAT3- and SHP2-mediated signals in immune responses. Immunity. 2000;12:95–105. doi: 10.1016/s1074-7613(00)80162-4. [DOI] [PubMed] [Google Scholar]
- 25.Croker BA, Metcalf D, Robb L, Wei W, Mifsud S, DiRago L, et al. SOCS3 is a critical physiological negative regulator of G-CSF signaling and emergency granulopoiesis. Immunity. 2004;20:153–65. doi: 10.1016/s1074-7613(04)00022-6. [DOI] [PubMed] [Google Scholar]
- 26.Wong PK, Egan PJ, Croker BA, O’Donnell K, Sims NA, Drake S, et al. SOCS-3 negatively regulates innate and adaptive immune mechanisms in acute IL-1-dependent inflammatory arthritis. J Clin Invest. 2006;116:1571–81. doi: 10.1172/JCI25660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahmed R, Salmi A, Butler LD, Chiller JM, Oldstone MB. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J Exp Med. 1984;160:521–40. doi: 10.1084/jem.160.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pellegrini M, Calzascia T, Elford AR, Shahinian A, Lin AE, Dissanayake D, et al. Adjuvant IL-7 antagonizes multiple cellular and molecular inhibitory networks to enhance immunotherapies. Nat Med. 2009;15:528–36. doi: 10.1038/nm.1953. [DOI] [PubMed] [Google Scholar]
- 29.Battegay M, Cooper S, Althage A, Banziger J, Hengartner H, Zinkernagel RM. Quantification of lymphocytic choriomeningitis virus with an immunological focus assay in 24- or 96-well plates. J Virol Methods. 1991;33:191–8. doi: 10.1016/0166-0934(91)90018-u. [DOI] [PubMed] [Google Scholar]
- 30.Gainsford T, Roberts AW, Kimura S, Metcalf D, Dranoff G, Mulligan RC, et al. Cytokine production and function in c-mpl-deficient mice: no physiologic role for interleukin-3 in residual megakaryocyte and platelet production. Blood. 1998;91:2745–52. [PubMed] [Google Scholar]
- 31.Shouda T, Yoshida T, Hanada T, Wakioka T, Oishi M, Miyoshi K, et al. Induction of the cytokine signal regulator SOCS3/CIS3 as a therapeutic strategy for treating inflammatory arthritis. J Clin Invest. 2001;108:1781–8. doi: 10.1172/JCI13568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilson EB, Brooks DG. Translating insights from persistent LCMV infection into anti-HIV immunity. Immunol Res. 2010 doi: 10.1007/s12026-010-8162-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Croker BA, Mielke LA, Wormald S, Metcalf D, Kiu H, Alexander WS, et al. Socs3 maintains the specificity of biological responses to cytokine signals during granulocyte and macrophage differentiation. Exp Hematol. 2008;36:786–98. doi: 10.1016/j.exphem.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu Y, Fukuyama S, Yoshida R, Kobayashi T, Saeki K, Shiraishi H, et al. Loss of SOCS3 gene expression converts STAT3 function from anti-apoptotic to pro-apoptotic. J Biol Chem. 2006;281:36683–90. doi: 10.1074/jbc.M607374200. [DOI] [PubMed] [Google Scholar]
- 35.Wang SD, Huang KJ, Lin YS, Lei HY. Sepsis-induced apoptosis of the thymocytes in mice. J Immunol. 1994;152:5014–21. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.