Abstract
Background
Skin cells produce soluble factors which influence keratinocyte proliferation, angiogenesis, nerve innervation and immunocyte response.
Objective
To test the hypothesis that epidermal-dermal interactions influence neural outgrowth, vascular survival, immunocyte recruitment and keratinocyte proliferation.
Methods
We genetically manipulated the epidermis to express excess vascular endothelial growth factor (VEGF) and/or angiopoietin-1 (Ang1) and then examined the epidermal and dermal phenotypes. We compared these findings with those occurring following overexpression of the Ang1 receptor Tie2 in endothelial cells or keratinocytes.
Results
Keratinocyte-overexpression of Ang1 resulted in increased epidermal thickness compared to control littermates. Keratinocyte-specific overexpression of Ang1 or VEGF increased dermal angiogenesis compared to control animals and combined Ang1-VEGF lead to further increases. Cutaneous leukocyte examination revealed increases in CD4+ T cell infiltration in mice with keratinocyte-specific overexpression of Ang1, VEGF and Ang1-VEGF combined; in contrast only keratinocyte-specific Ang1 overexpression increased cutaneous F4/80+ macrophage numbers. Interestingly, combined keratinocyte-derived Ang1-VEGF overexpression reduced significantly the number of F4/80+ and Cd11c+ cells compared to mice overexpressing epidermal Ang1 alone. Endothelial cell-specific Tie2 overexpression increased dermal angiogenesis but failed to influence the epidermal and immune cell phenotypes. Keratinocyte-specific Tie2 expressing mice had the highest levels of CD4+, CD8+ and CD11c+ cell numbers and acanthosis compared to all animals. Finally, increases in the number of cutaneous nerves were found in all transgenic mice compared to littermate controls.
Conclusion
These findings demonstrate that change to one system (vascular or epidermal) results in change to other cutaneous systems and suggest that individual molecules can exert effects on multiple systems.
Introduction
Nervous system components and blood vessels develop similar anatomical patterning in the adult body, follow similar routes and modes of migration during development; and in skin, epithelial skin stem cells, neuronal stem cells (neuroblasts) and vascular stem cells (angioblasts/hemangioblasts) all localize to regions undergoing repair [1]. In spite of the blatant similarities between the vascular and nervous systems, and the extensive examination of each system independently, little is known about the co-dependence and/or interactions between the two systems [2–4]. Even less well understood is how these interactions are influenced within a specific environmental niche, such as that found in the skin.
Secreted growth factors mutually affect and are released by both blood vessel components (endothelial cells (ECs), pericytes, smooth muscle cells) and nervous system components (neurons and Schwann cells) (i.e. the neurovascular unit). In skin, KCs also secrete soluble factors that influence vessels and nerves, and conversely growth factors secreted by vessels and nerves can in turn influence epidermal KCs (i.e. the cutaneous neurovascular unit, CNU) [2–4]. The regulated expression of these factors and their cellular interactions ultimately dictates cutaneous homeostasis.
The vascular growth factor, Angiopoietin-1 (Ang1) is a member of the angiopoietin family and has been shown to play a role in vascular development and vascular related disease processes [5]. Ang1 is the best characterized member of the angiopoietin family and binds to Tie2, a receptor tyrosine kinase that is expressed on ECs lining blood vessels [6, 7]. In the vascular system, Ang1 is involved in EC survival and migration and peri-endothelial cell recruitment and tubule formation [5]; moreover Ang1 can elicit context dependent pro-inflammatory [8, 9] or anti-inflammatory events [10–12], is expressed by KCs [13] (unpublished observations) and is capable of inducing neurite outgrowth of cultured dorsal root ganglion (DRG) neurons [3, 14]. Overexpression of Ang1 in KCs results in increases in dermal angiogenesis [15, 16]; however the ability to affect other members of the CNU remains unclear. Transgenic overexpression of the Ang1 receptor, Tie2 in skin also changes multiple members of the CNU. We recently demonstrated that transgenic expression of Tie2 in ECs or KCs significantly increases dermal angiogenesis and that KC proliferation and immunocyte infiltration occurs in the animals where Tie2 was expressed in the KCs (KC-Tie2) but not in mice where Tie2 was expressed in ECs (EC-Tie2)[17]. Whether KC-Tie2 or EC-Tie2 expression leads to alterations in cutaneous innervation remains unknown.
The vascular growth factor, vascular endothelial growth factor (VEGF) also has distinct and overlapping roles in the skin including its expression by KCs [18], dermal nerves [19], vascular pericytes and smooth muscle cells [20, 21] and almost all immunocytes [22–24]. Originally identified for its role in vascular permeability and vascular development [25], overexpression of VEGF in KCs results in increases in dermal angiogenesis [26, 27], and sustains a pro-inflammatory environment following exposure to oxazolone [28], after wounding or in aged mice that leads to the development of a thickened epidermis [29]. Some reports suggest it has the ability to induce KC proliferation directly [30]. Recent work has detailed roles for VEGF in the nervous system, including neurotrophic, neuroprotective, and neurogenic functions such as axonal growth and cell survival [31–37]. However its effects on cutaneous innervation remain unknown. Overexpression of VEGF in combination with Ang1 leads to the development of larger and more blood vessels that are less leaky than in VEGF overexpressing animals [15, 38] however the effect of this combination on other members of the CNU has not been reported.
We hypothesized that epidermal-dermal interactions govern neural outgrowth, vascular survival, immunocyte recruitment and KC proliferation and that modification of one system within the CNU would result in alterations to the others. To test this hypothesis we genetically overexpressed Ang1, VEGF, and combined Ang1-VEGF in KCs and determined the effects on the cutaneous vasculature, innervation, immunocyte infiltrate and epidermal phenotypes. These findings were compared and contrasted with our previously reported cutaneous phenotypes resulting from overexpression of Tie2 in KCs or ECs [17].
Materials and Methods
Transgenic mice
A conditional mouse model approach [39, 40] was developed and utilized for the current experiments. This approach uses a doxycycline-based binary transgenic expression system [39], in which controlled expression (on/off) of a transgene occurs by exposure to doxycycline. Two individual lines of mice are used, with cell specificity dictated by a “driver” mouse line, using an appropriate promoter upstream of a tetracycline transactivator (tTA). Transgene specificity is dictated by the “responder” mouse, using the transgene of interest downstream of the tetracycline operator sequence (TetOS). On their own, each gene is silent. Following the appropriate matings, mice containing both genes express the transgene in a cell specific manner.
Driver lines used included the previously engineered KC-specific, K5-tTA mouse [41] and the EC-specific, Tie1-tTA mouse [39] lines. KC-specific overexpression of Tie2, Ang1 and VEGF was accomplished by breeding the K5tTA driver line with the TetosTie2, TetosAng1 and TetosVEGF responder lines which have been previously described [42–44]. The TetosAng1 and TetosVEGF lines were mated to each other to generate a new TetosAng1-VEGF double responder line which was then mated with the K5tTA driver line resulting in concurrent overexpression of both Ang1 and VEGF in KCs. EC-specific overexpression of Tie2 was accomplished by breeding the Tie1tTA driver line with the TetosTie2 responder line.
Mice were genotyped using polymerase chain reaction (PCR) using DNA extracted from ear biopsies. DNA was prepared and PCR performed using primers as previously described [42, 44]. Littermates that inherited one or no transgenes served as experimental controls. All animal protocols were approved by the Case Western Reserve University institutional animal care and use committee (IACUC) and conformed to the American Association for Accreditation of Laboratory Animal Care guidelines.
Tissue collection, histology, immunofluorescence and image analyses
Sexually mature adult mice were euthanized; their hair shaved and skin from the back was processed for either thin frozen, thick frozen or paraffin sectioning. For paraffin sectioning, skin was placed in 10% buffered formalin (Surgipath Medical Industries, Richmond, IL), overnight at 4º Celsius (C) prior to dehydration and embedding (Sakura Finetech, Torrance, CA). For frozen sectioning, skin was either fixed in the non-cross-linking fixative Histochoice (Amresco, Solon, OH) for 4 hours at 4ºC or in Zamboni’s Fixative (Newcomer Supply, Middleton, WI) overnight at room temperature, and then transferred to 5% sucrose for 1 hour at 4ºC and placed in 20% sucrose overnight at 4ºC, and then embedded in Tris buffered saline (TBS) Tissue Freezing Medium (TFM; Triangle Biomedical Sciences, Durham, NC), and then flash frozen in liquid nitrogen.
H&E staining was completed on 5μm thick paraffin sections using standard protocols [45]. Images were captured using a Leica DM L82 microscope with an attached Q Imaging MicroPublisher 3.3 Mega Pixel camera and Q-capture Pro software. Epidermal thickness was quantified using Image Pro Plus software (MediaCybernetics, Bethesda, MD). For each animal, 5 measurements were taken from at least five different fields of view from one section (25 measurements per animal). Epidermal thickness was measured from the stratum basale to stratum granulosum, and excluded the stratum corneum and hair follicles.
Immunohistochemistry against CD4, CD8, CD11c, F4/80 and NCAM (CD56) was performed on TFM-embedded frozen skin sectioned at 8 μm, using specific anti-CD4, anti-CD8, anti-CD11c, anti-CD56 (BD Biosciences, San Jose, CA), and anti-F4/80 (eBioscience, San Diego, CA) antibodies. Antibodies were detected using either rabbit anti-rat IgG biotinylated (CD4, F4/80, CD56; Vector Labs, Burlingame, CA) or goat anti-hamster IgG biotinylated (CD11c; Jackson Immunoresearch Labs, West Grove, PA), amplified with Avidin/Biotinylated Enzyme Complex (Vector Labs) and were visualized using the enzyme substrate diaminobenzidine (Vector Labs). The slides were counterstained with hematoxylin. Images were captured as above. For quantification of CD11c+ and F4/80+ cells, image analyses was completed in a blinded fashion using an automated Metamorph software program (Molecular Devices, Sunnyvale, CA) from at least five fields of view per animal. Quantification of CD4+ and CD8+ cells were hand counted in blinded fashion from at least five fields of view per animal.
Blood vessel analyses was completed on histochoice fixed tissue sectioned at 8μM using a specific primary antibody targeting the pan mouse endothelial cell antigen (MECA; Developmental Studies Hybridoma Bank, Iowa City, IA), followed by detection using a rabbit anti-rat IgG biotinylated antibody, amplified with Avidin/Biotinylated Enzyme Complex, and visualized using the enzyme substrate diaminobenzidine. The slides were counterstained with hematoxylin. Photographs were taken and image analyses completed in a blinded fashion using an automated Metamorph software program (Molecular Devices, Sunnyvale, USA). Background staining was minimal therefore colour thresholds were not altered between samples. Four fields of view from 4 individual sections were analyzed per animal. A separate set of thickly cut (50 μM) histochoice fixed skin sections was examined using the same antibody (MECA) with immunofluorescence. Visualization was completed using Texas Red Streptavidin (Vector) or goat anti-rabbit Alexa Flour 488 (Molecular Probes, Eugene, OR).
Immunofluorescence staining using two independent nerve markers was completed using the pan neuronal marker protein gene product (PGP)9.5 on free-floating Zamboni fixed tissue sectioned at 50μM using a specific antibody (Ultraclone, UK), or using the non-C fiber cutaneous nerve marker, neurofilament (NF) 200 (Sigma, St. Louis, MO) on fresh frozen tissue sectioned at 8μM followed by labeling with AlexaFluor 488 goat anti-rabbit IgG (Invitrogen, Carlsbad, CA). The slides were cover-slipped with Vectashield mounting medium (Vector Labs) and antigen-antibody complexes were detected under a Carl Zeiss Axiophot fluorescent microscope and images captured with Carl Zeiss Axiocam HRC. PGP9.5+ nerve fiber staining was quantitated in a blinded fashion using the Metamorph program and reported as number of PGP9.5+ nerves per field of view.
Statistical analysis
All data are represented as mean ± standard error of the mean (SEM). At least four animals for each group were used for statistical analyses. Between group comparisons were analyzed using an unpaired, two tailed Student’s t-test and statistical significance was defined as p<0.05.
Results
Overexpression of Ang1, VEGF, Ang1-VEGF and Tie2 in skin leads to increased dermal angiogenesis compared to littermate controls
The phenotypic characteristics of the dermal vasculature was analyzed in skin sections collected from KC-Ang1, KC-VEGF, KC-Ang1-VEGF and littermate controls and was also compared with newly collected, stained and analyzed skin tissues from our previously published KC-Tie2 and EC-Tie2 mice [17]. Mouse endothelial cell antigen (MECA) stained sections demonstrated that skin from all lines of mice had more numerous vessels (Figure 1A-D, Supplemental Figure 1). All of the KC-driven transgenic mice showed altered blood vessel patterns compared to littermate controls and EC-Tie2 mice, that included larger and more numerous blood vessels running parallel to, and just below, the dermal epidermal junction (DEJ; Figure 1C-D, Supplemental Figure 1A). The vessels in KC-VEGF and KC-Ang1-VEGF mice also appeared more tortuous and more tightly abutted to the DEJ compared to all other mouse lines (Figure 1C-D).
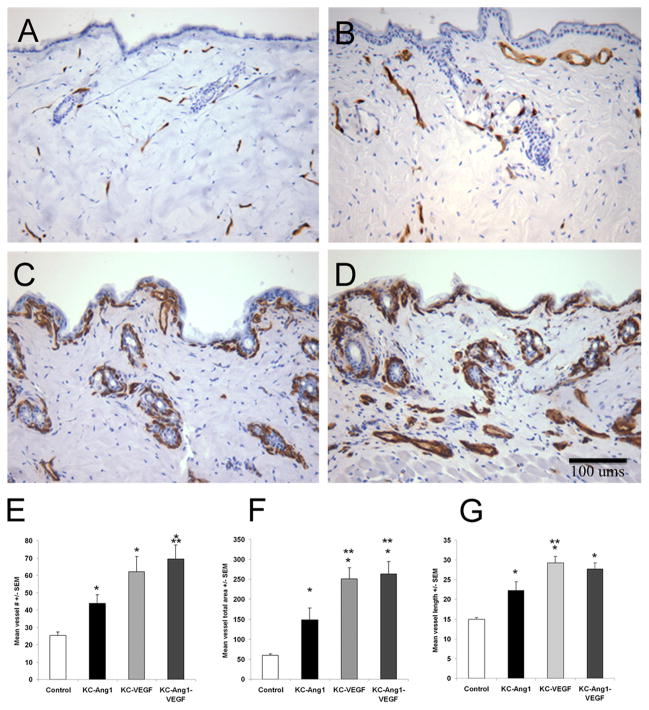
Figure 1. KC-Ang1, KC-VEGF, and KC-Ang1-VEGF animals have increased dermal angiogenesis compared to CD1 control mice.
(A–D) Back skin sections from control (A), KC-Ang1 (B), KC-VEGF (C) and KC-Ang1-VEGF (D) mice were immunostained with antibodies targeting the pan EC marker, MECA. Distinct and unique changes in blood vessel number, size and location were found between the different mouse strains (A–D). Quantification of blood vessel number (E), total blood vessel area (F) and blood vessel diameter (G) revealed KC-Ang1, KC-VEGF and KC-Ang1-VEGF mice have increases in the number, area and diameter of blood vessels compared to control mice. KC-VEGF mice have increases in vessel area and vessel length and KC-Ang1-VEGF mice have increases in vessel number and vessel area compared to KC-Ang1 animals. * p<0.05 compared to control mice; ** p<0.05 compared to KC-Ang1 mice.
Quantitative analyses of the vasculature revealed a 1.8-, 2.5-, and 2.8-fold increase in blood vessel number in KC-Ang1, KC-VEGF, and KC-Ang1-VEGF mice, respectively when compared to control animals (CD1 background littermates; p<0.002; Figure 1E). In addition, the combined expression of Ang and VEGF also lead to an amplified angiogenic response compared to Ang1 expressing mice alone (p=0.038), providing independent confirmation of work done by others [15]. Similar observations were found for total stained-vessel area (Figure 1F) and average vessel length (Figure 1G) for KC-Ang1, KC-VEGF and KC-Ang1-VEGF mice. Confirming our prior work, KC-Tie2 and EC-Tie2 mice had a 1.5- and 1.6-fold increase in the number of MECA-stained vessels in the dermis compared to littermate controls (p=0.002; Supplemental Figure 1C)[17]; however only EC-Tie2 and not KC-Tie2 animals had significantly longer MECA-stained vessels compared to controls (Supplemental Figure 1E), and these were significantly shorter than all other animals (p<0.004) except KC-Tie2 mice. These findings indicate that alteration of either the KC environment or the vasculature itself results in increased dermal angiogenesis.
Ang1 or Tie2 overexpression in KCs leads to modest and robust epidermal hyperplasia respectively
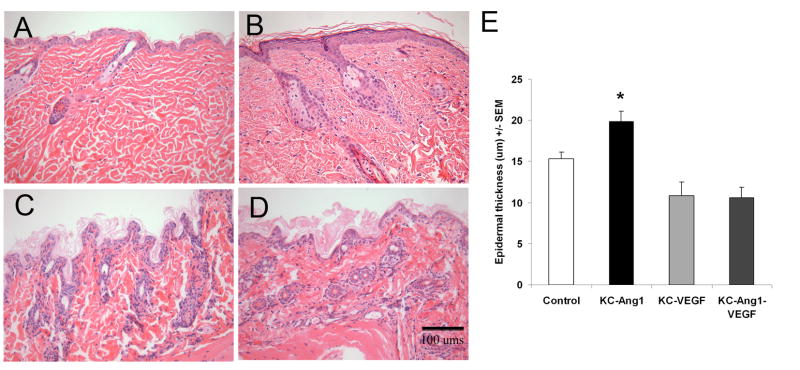
To study whether altering KCs alone would lead to epidermal hyperplasia, or whether changes in the vasculature resulted in increased KC proliferation, we measured the thickness of the epidermis from each line of transgenic mice and compared it to littermate controls. A modest, but statistically significant increase in the epidermis was observed in KC-Ang1 mice compared to littermate controls (19.9±1.3 vs. 15.3±0.8; p=0.018; Figure 2). KC-VEGF and KC-Ang1-VEGF skin appeared extremely thin, with only a single layer of KCs visible, and was not significantly different from controls (p=0.07 and p=0.08, respectively), but was thinner compared to KC-Ang1 animals (p<0.0001). KC-Tie2 animals had the thickest epidermis compared to all animals (Supplemental Figure 2; p<0.003) consistent with previous findings [17]. Taken together, these results suggest that altering KCs alone can, in some instances lead to changes in KC proliferation and in others not; whereas increasing the vasculature alone appears to be insufficient to induce epidermal hyperplasia.
Figure 2. Histological analyses of KC-Ang1, KC-VEGF, and KC-Ang1-VEGF and CD1 control mouse skin demonstrates modest levels of epidermal hyperplasia in KC-Ang1 mouse skin.
H&E stained skin taken from backs of control (A) KC-Ang1 (B), KC-VEGF (C) and KC-Ang1-VEGF (D) mice. Epidermal thickness (in μm) was quantified using Adobe Photoshop Analysis Ruler Tool software (E). KC-Ang1 mice have a slightly thicker epidermal thickness compared to control, KC-VEGF and KC-Ang1-VEGF skin. * p<0.05 compared to control mice.
Mice with increased dermal angiogenesis have more cutaneous nerves
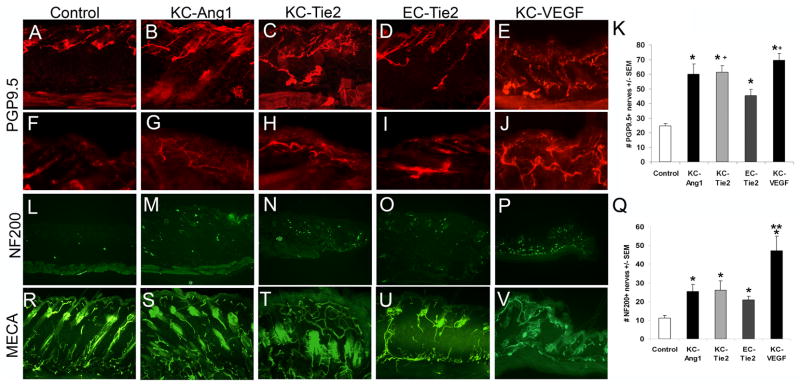
We hypothesized that changes in the dermal vasculature or epidermal environment would result in changes to the cutaneous nerve. KC-Ang1, KC-Tie2, EC-Tie2 and KC-VEGF mice showed a 2.4-, 2.5-, 1.8- and 2.8-fold increase in the number of total cutaneous nerves as indicated by staining with the pan neuronal marker, PGP9.5+ compared to littermate controls (Figure 3; 24.7±1.5 vs KC-Ang1 59.9±7.0 vs KC-Tie2 61.5±4.5 vs EC-Tie2 45.2±4.2 vs KC-VEGF 69.4±4.6, p<0.0006). Interestingly, unique changes in nerve patterning detected between the different transgenic lines mimicked the patterns observed for the dermal vasculature (Figure 3R-V). Mice with increased cutaneous angiogenesis also demonstrated increases in their innervation. The increase in the number of PGP9.5+ nerves in both KC-Ang1 and KC-VEGF animals was apparent in both the dermis (Figure 3A-E) and epidermis (Figure 3F-J) with many nerve fibers localized to the dermal-epidermal junction (DEJ) and significantly more fibers penetrating into the epidermis in KC-Ang1 and KC-VEGF mice. KC-Tie2 animals also had increases in the number of PGP9.5+ nerves in the dermis with these fibers appearing more tortuous, similar to what was seen for the dermal vasculature (Figure 3N). In addition, many fibers lined up at the DEJ and more fibers were observed penetrating into the epidermis, although not to the same degree as observed in KC-Ang1 animals (Figure 3F-G). EC-Tie2 mice showed thin linear nerves projecting towards the epidermis imitating the cutaneous vasculature (Figure 3D, O), and despite an overall increase in the number of PGP9.5+ nerves in the skin compared to control mice, no obvious change in the number of fibers was seen projecting into the epidermis in these mice when compared to control animals (Figure 3I). EC-Tie2 mice had significantly fewer PGP9.5+ nerves than KC-Tie2 (p=0.014) and KC-VEGF (p=0.013) animals (Figure 3K). These results were confirmed using a second independent neural specific marker, neurofilament 200 (NF200), which stains larger caliber nerve fibers and does not stain C-fibers. KC-Ang1, KC-Tie2, EC-Tie2 and KC-VEGF animals all had more NF200+ cutaneous nerve fibers than control mice (Figure 3L-P) and KC-VEGF animals had significantly more NF200+ fibers than KC-Ang1, KC-Tie2 and EC-Tie2 mice (Figure 3Q) consistent with increases in the larger lightly myelinated cutaneous nerve population. The alterations in both nerve and vessel phenotypes within the CNU of transgenic mice was further confirmed in tissues stained with antibodies targeting neural cell adhesion molecule (NCAM; CD56), a marker of both neural and vascular tissues in mice (Supplemental Figure 3).
Figure 3. Cutaneous nerves are increased in number in KC-Ang1, KC-Tie2, EC-Tie2, and KC-VEGF skin compared to CD1 control animals and pattern similarly to cutaneous vessels.
Cutaneous nerves stained with the pan peripheral nerve marker PGP9.5 in (A, F) control, (B, G) KC-Ang1, (C, H) KC-Tie2, (D, I) EC-Tie2, and (E, J) KC-VEGF mice. Low magnification (A-E) images of thick skin (50μm) demonstrate PGP9.5+ nerve patterning throughout the dermis and epidermis and high magnification (F–J) images at the dermal-epidermal junction illustrate epidermal-specific nerve staining. Quantification of the number of nerves in each mouse strain (K) reveals all transgenic mice have more PGP9.5+ cutaneous nerves than control mice and KC-Tie2 and KC-VEGF animals have significantly more nerves than EC-Tie2 animals. Thin (8um) skin sections stained with antibodies targeting neurofilament 200 (NF200), a non-C fiber sensory nerves marker and quantitation demonstrates increases in NF200+ nerve fibers in all transgenic mice compared to control animals (L–Q). KC-VEGF mice have significantly more NF200+ nerves than KC-Ang1, KC-Tie2 and EC-Tie2 mice. Back skin sections stained with MECA antibodies (R–V) demonstrate moderate similarities between vascular and neural patterning as compared with the PGP9.5+ stained sections. * p< 0.05 compared to control animals; + p<0.05 compared to EC-Tie2 mice; ** p<0.05 compared to KC-Ang1, KC-Tie2 and EC-Tie2 mice.
Genetic manipulation of KCs or ECs changes the cutaneous immune cell environment
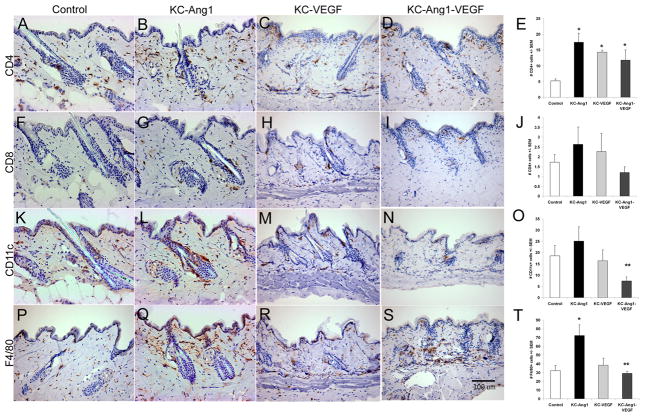
We previously reported that KC-Tie2 overexpression results in increased inflammatory infiltrates consisting of T cells, dendritic cells, neutrophils, and macrophages [17] when compared to littermate controls. Although Ang1 and VEGF combined with Ang1 have been genetically overexpressed in KCs previously [15, 16], alterations to the cutaneous immune cell milieu under non-pathological conditions has not been reported, we therefore quantitated the inflammatory cell infiltration in KC-Ang1, KC-VEGF, and KC-Ang1-VEGF animals and compared these findings with that observed for KC-Tie2, EC-Tie2 and control animals. KC-Ang1, KC-VEGF and KC-Ang1-VEGF animals had 3.4-, 2.75- and 2.3-fold more CD4+ T cells compared to control littermates (p<0.004; Figure 4A-E) and no differences in the number of CD8+ T cells (Figure 4F-J). KC-Tie2 animals (Supplemental Figure 4) had a 9.8-fold increase in CD4+ and a 6.5-fold increase in CD8+ T cells, however no change in either cell population was found for EC-Tie2 animals, consistent with our previous report [17]. KC-Tie2 animals had significantly more CD4+ and CD8+ T cells than any of the other transgenic mouse lines (p<0.05) and were the only animals showing any accumulation of epidermal T cells amongst the lines (Supplemental Figure 4). Analyses of CD11c+ antigen presenting cells revealed no differences between control littermates and KC-Ang1, KC-VEGF and KC-Ang-1-VEGF mouse lines; however KC-Ang1-VEGF mice had fewer positively stained cells (p=0.035) compared to KC-Ang1 mice (Figure 4K-O). Analyses of the F4/80+ cell population failed to identify differences between control littermates, KC-VEGF and KC-Ang1-VEGF animals; however KC-Ang1 mice had a 3-fold increase in the number of F4/80+ cells compared to control littermates (p=0.0048) and a 2.5-fold increase compared to KC-Ang1-VEGF animals (p=0.013) (Figure 4P-T). Consistent with prior observations [17], we confirmed a 3.2- and a 2.6-fold increase (p<0.002) in the number of CD11c+ and F4/80+ cells in KC-Tie2 animals (Supplemental Figure 4), and no differences between EC-Tie2 and control animals. KC-Tie2 animals had significantly more CD11c+ cells than any of the other transgenic mouse lines (p<0.05) except KC-Ang1 mice (p=0.07); and EC-Tie2 mice had significantly more CD11c+ cells than KC-Ang1-VEGF animals. KC-Tie2 and EC-Tie2 mouse skin both showed significant increases in F4/80+ cell numbers compared to KC-Ang1-VEGF mice (p=0.03).
Figure 4. Inflammatory cell infiltrates in KC-Ang1, KC-VEGF, KC-Ang1-VEGF and control CD1 mouse skin.
Back skin from KC-Ang1 (B, G, L, O), KC-VEGF (C, H, M, R), KC-Ang1-VEGF (D, I, N, S) and control animals (A, F, K, P) was stained for CD4+ T cells (A–D), CD8+ T cells (F–I), CD11c+ (K–N) and F4/80+ macrophages (P–S). The mean number of cells present per field of view was quantified (E, J, O, T). KC-Ang1, KC-VEGF and KC-Ang1-VEGF mice have increases in the number of CD4+ T cells (E) compared to control mice. No differences were observed between any mice for CD8+ T cells (J) and KC-Ang1-VEGF animals have significantly fewer CD11c+ cells than KC-Ang1 mice alone (O). KC-Ang1 mice have significantly increased F4/80+ macrophages compared with control and KC-Ang1-VEGF mice (T). * p<0.05 compared to control mice; ** p< 0.05 compared to KC-Ang1 mice.
Discussion
We demonstrated that genetic alterations to either KCs or ECs results in changes to the CNU. Alteration of KCs themselves, via the overexpression of Ang1, VEGF, or Ang1-VEGF or by the ectopic expression of Tie2 resulted in changes to each of the CNU systems regardless of which protein was expressed. In contrast, modification of ECs via the overexpression of Tie2 only lead to changes in the vasculature and the cutaneous innervation, with little change observed to the immune cell milieu or the epidermal anatomy.
KC-specific overexpression of Ang1, VEGF and Ang1-VEGF all resulted in increases in angiogenesis, with the combined Ang1-VEGF further amplifying this response compared to Ang1 alone, confirming prior reports [15, 16, 27, 38], Moreover, all three lines of mice also had increases in cutaneous innervation, thus providing further in vivo evidence for Ang1 [46–49] and VEGF [19, 31, 50, 51] as neurotrophic factors, specifically in skin. This innervation may occur via Tie2, VEGFR2 or neuropilin receptors on the neurites themselves [14, 50, 51] or possibly, via Ang1-β1 integrin signaling, which we recently have shown to induce neurite outgrowth in an in vitro neurite outgrowth assay utilizing differentiated rat pheochromocytoma PC12 neurons [48].
We observed subtle but significant increases in epidermal thickness of KC-Ang1 mice compared to control littermates perhaps reflective of downstream mitogenic signaling events resulting from autocrine Ang1-β1 integrin interactions [52], or alternatively via mitogenic signals derived from the cutaneous nerves, such that the presence of nerves is positively correlated to epidermal thickness [53], and that derived peptides including CGRP can directly elicit KC proliferation [54]. Our work suggests it is more complicated than that, as KC-Tie2 animals have more nerves and have a thicker epidermis; however KC-VEGF and EC-Tie2 animals also have more cutaneous nerves, but fail to demonstrate epidermal hyperplasia.
The lack of acanthosis we observed in KC-VEGF mouse skin contradicts previous reports [29] yet is consistent with others [26, 28, 38]. The different outcomes obtained amongst groups most likely reflect expression levels of VEGF. Animals engineered to express very high levels of VEGF in the skin during development die embryonically [26], and adult mice receiving moderate to high levels of exogenous VEGF-containing adenovirus became moribund and died within days in a dose-dependent manner [38], resulting most likely from pervasive tissue edema, tissue swelling and separation of cellular elements by interstitial fluid. In contrast, mice containing lower levels of VEGF [28, 29] survive, and with time or an inflammatory stimulus eventually develop acanthosis. Despite the increase in dermal angiogenesis and innervation in addition to the influx of CD4+ T cells, KC-VEGF animals failed to develop epidermal hyperplasia; this may be a reflection of insufficient time for this to occur, but may also reflect the lack of CD8+ cytotoxic T cells, as others have previously demonstrated the importance for and presence of CD3+ or CD8+ T cells in the epidermis when it is hyperplastic [28, 55, 56].
Although Ang1 has previously been shown to be capable of eliciting an inflammatory response, this effect appears to be context dependent, such that it can serve as either a pro-inflammatory molecule [8, 9] or as an anti-inflammatory agent [10–12]. In our case, Ang1 on its own appears to increase the number of immune cells infiltrating into the skin, either directly by Ang1 binding to Tie2 on monocytes [57], or indirectly via increasing the numbers of nerves and their derived neuropeptides, which in turn can recruit leukocytes to the skin [58–60]. However, these pro-inflammatory effects, specifically on myeloid cells, disappear when combined with VEGF. This would be consistent with what others have reported for VEGF, such that pathophysiologic levels of VEGF are capable of inhibiting dendritic cell maturation, function and infiltration [61–65]. It can not be ruled out that Ang1 and VEGF overexpression in KCs also leads to increases in other KC-derived factors that themselves can draw in immune cells, induce angiogenesis, and affect neurite outgrowth.
KC-Tie2 expression also resulted in increases in angiogenesis confirming our previous findings [17], an increase in cutaneous innervation, a large influx of immune cell infiltrate and the highest levels of acanthosis. Time course analyses on the KC-Tie2 mice indicate that immune cell infiltration preceded epidermal hyperplasia, followed by increases in nerve innervation and then increases in dermal angiogenesis (data not shown). These data suggest that a soluble factor derived from transgenic KCs may recruit immune cells into the skin which then contribute to the development of epidermal hyperplasia which in turn provides additional KC-derived soluble factors which then increase cutaneous innervation. VEGF is a solid candidate for the KC-derived soluble factor, as we previously demonstrated this factor to be significantly increased in the epidermis of KC-Tie2 mice [17] and VEGF can serve directly and indirectly as a chemoattractant for immune cells [66–68], is capable of eliciting KC proliferation [30] and is a potent angiogenic and neurogenic factor [69]. It is also plausible that cutaneous nerves increase in number at the same time as CD8+ cells increase, perhaps reflecting neurogenic-mediated inflammation and subsequent immunocyte recruitment via nerve derived factors, including neuropeptides. These findings are in contrast to what was observed in KC-VEGF animals, where increased dermal angiogenesis, cutaneous innervation and influx of CD4+ T cells occurred despite the lack of acanthosis, perhaps reflective of direct influences of VEGF on both the vascular and neural systems.
As expected, modification of ECs alone (EC-Tie2) lead to increases in dermal angiogenesis, however this change also lead to increases in the number of cutaneous nerves, consistent with the idea that vessel-derived factors can mediate nerve-vessel interactions. Of further interest was the observation that in EC-Tie2 mouse skin, vessels and nerves appeared to pattern each other, with the appearance of both vessels and nerves projecting in a linear pattern towards the epidermis. The concept of nerves and vessel patterning off each other is a relatively new concept. Mukoyama et al. [19] originally showed sensory nerves determine the pattern of arterial differentiation and blood vessel patterning, that arteries, and not veins, were specifically aligned with the peripheral cutaneous nerves in embryonic mice, and that the elimination of the nerve itself or the surrounding support (Schwann) cells resulted in disorganized nerves and improper arteriogenesis. Others however, have shown that in grafted skin, angiogenesis precedes innervation [70, 71] and that both are influenced by the presence or absence of KCs [72]. The differences reported between these studies may reflect events that occur embryonically [19] versus those occurring in adult skin [70–72]. Interestingly, during embryonic skin development, it is nerve-derived VEGF that leads to the parallel patterning of the arteries [73] and examination of nerve and vessel patterns in the KC-VEGF mice show similar areas of dermal vascularization and innervation.
As the etiology and treatment strategies of many dermatologic diseases involve and target the epidermal KCs, dermal blood vessels and nerves, the infiltrating and resident immune cells and their derived products, understanding the individual contributions to the homeostasis of the CNU becomes important. Skin xenografts used for treating severe burns have intrinsic challenges associated with vascularization, reinnervation of dermal structures, immune response, trophic support, and cell survival of the grafted skin [1, 74]. Psoriasis is characterized by recurrent erythematous lesions, KC proliferation, inflammatory cell accumulation, unscheduled angiogenic growth and alterations to the dermal nerves [17, 75–84]. Wound healing is impaired in individuals with decreased angiogenesis, compromised immune response and in denervated skin [85–92]. Moreover other roles for the CNU in skin disease or in skin manifestations also exist outside of the obvious. Port wine stains contain a significant reduction in nerve density which has been correlated to blood vessel size, consistent with the idea that vascular ectasia observed in port wine stain birthmarks occurs as a result of reduced autonomic neural innervation and stimulation [93]. Rosacea is also well known to have increased angiogenesis, increases in VEGF [94], and contains vasodilation that could be mediated by autonomic nerve innervation. One study reported increased nerve fiber density and neuropeptides, including substance P in patients with Rosacea [95] and patients with rosacea often complain of more sensitive skin [96, 97] and that their rosacea is exacerbated when they are under stress [98].
Our results suggest that that each system within the CNU has the ability to affect the others and that individual molecules exert effects on multiple systems. Future experiments will allow a better understanding of the basic mechanisms underlying the relationship between members of the CNU, will enable a clearer comprehension of how CNU components interact, and will define whether one compartment leads and others follow, or perhaps (and most likely) that all compartments of the CNU are in constant communication with each other, responding both interdependently and independently to similar and different growth factors.
Supplementary Material
Acknowledgments
This work was supported in part by grants to NLW from the Juvenile Diabetes Research Foundation (JDRF), Dermatology Foundation, and the NIH (P30AR39750 and P50AR05508). JAW is supported by training grant T32 GM-08056-23. The K5-tTA mouse was provided by Dr. Adam Glick (Pennsylvania State University, USA) the TetOSTie2, Tie1-tTA, and pTetOSAng1 mice by Dr. Daniel Dumont (Sunnybrook & Women’s Research Center, Toronto, Canada), and the TetOSVEGF mouse from Drs. Ann Akeson and Jeffrey Whitsett (Cincinnati Children’s Hospital Research Foundation, Cincinatti, Ohio, USA). We would like to thank Doina Diaconu for her technical assistance, Dr. Scott Howell for his guidance with the image analyses, and Dr. Thomas McCormick for helpful discussion, suggestions and feedback throughout the completion of this work.
Abbreviations used
- Ang1
angiopoietin
- KCs
keratinocytes
- VEGF
vascular endothelial growth factor
- VEGFR2
vascular endothelial growth factor receptor 2
- DNA
deoxyribonucleic acid
- PCR
polymerase chain reaction
- ECs
endothelial cells
- CNU
cutaneous neurovascular unit
- PGP9.5
protein gene product 9.5
- TBS
tris buffered saline
- TFM
tissue freezing medium
- C
Celsius
- DRG
dorsal root ganglion
- MECA
mouse endothelial cell antigen
- ABC
avidin biotin complex
- SEM
standard error of the mean
- DEJ
dermal epidermal junction
- PC12 cells
rat pheochromocytoma cells
- CGRP
calcitonin gene related peptide
- tTA
tetracycline transactivator
- TetOS
Tetracycline operator sequence
- IRES
internal ribosomal entry site
- IACUC
institutional animal care and use committee
- Tie2
Tunica interna endothelial cell kinase
- H&E
hematoxylin and eosin
References
- 1.Brouard M, Barrandon Y. Controlling skin morphogenesis: hope and despair. Curr Opin Biotechnol. 2003;14:520–5. doi: 10.1016/j.copbio.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 2.Park JA, Choi KS, Kim SY, Kim KW. Coordinated interaction of the vascular and nervous systems: from molecule- to cell-based approaches. Biochem Biophys Res Commun. 2003;311:247–53. doi: 10.1016/j.bbrc.2003.09.129. [DOI] [PubMed] [Google Scholar]
- 3.Ward N, LaManna JC. The neurovascular unit and its growth factors: coordinated response in the vascular and nervous systems. Neurological Research. 2004;26:879–83. doi: 10.1179/016164104X3798. [DOI] [PubMed] [Google Scholar]
- 4.Zacchigna S, de Almodovar CR, Carmeliet P. Similarities between angiogenesis and neural development: what small animal models can tell us. Curr Top Dev Biol. 2008;80:1–55. doi: 10.1016/S0070-2153(07)80001-9. [DOI] [PubMed] [Google Scholar]
- 5.Ward NL, Dumont DJ. The angiopoietins and Tie2/Tek: adding to the complexity of cardiovascular development. Seminars in cell & developmental biology. 2002;13(1):19–27. doi: 10.1006/scdb.2001.0288. [DOI] [PubMed] [Google Scholar]
- 6.Dumont DJ, Gradwohl G, Fong GH, Puri MC, Gertsenstein M, Auerbach A, et al. Dominant-negative and targeted null mutations in the endothelial receptor tyrosine kinase, tek, reveal a critical role in vasculogenesis of the embryo. Genes & development. 1994;8(16):1897–909. doi: 10.1101/gad.8.16.1897. [DOI] [PubMed] [Google Scholar]
- 7.Nourhaghighi N, Teichert-Kuliszewska K, Davis J, Stewart DJ, Nag S. Altered expression of angiopoietins during blood-brain barrier breakdown and angiogenesis. Lab Invest. 2003;83:1211–22. doi: 10.1097/01.lab.0000082383.40635.fe. [DOI] [PubMed] [Google Scholar]
- 8.Lemieux C, Maliba R, Favier J, Theoret J-F, Merhi Y, Sirois MG. Angiopoietins can directly activate endothelial cells and neutrophils to promote proinflammatory responses. Blood. 2005;105:1523–30. doi: 10.1182/blood-2004-09-3531. [DOI] [PubMed] [Google Scholar]
- 9.Brkovic A, Pelletier M, Girard D, Sirois MG. Angiopoietin chemotactic activities on neutrophils are regulated by PI-3K activation. J Leukoc Biol. 2007;81:1093–101. doi: 10.1189/jlb.0906580. [DOI] [PubMed] [Google Scholar]
- 10.Gamble JR, Drew J, Trezise L, Underwood A, Parsons M, Kasminkas L, et al. Angiopoietin-1 is an antipermeability and anti-inflammatory agent in vitro and targets cell junctions. Circulation research. 2000;87(7):603–7. doi: 10.1161/01.res.87.7.603. [DOI] [PubMed] [Google Scholar]
- 11.Kim I, Oh JL, Ryu YS, So JN, Sessa WC, Walsh K, et al. Angiopoietin-1 negatively regulates expression and activity of tissue factor in endothelial cells. The FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2002;16(1):126–8. doi: 10.1096/fj.01-0556fje. [DOI] [PubMed] [Google Scholar]
- 12.Kim I, Moon SO, Park SK, Chae SW, Koh GY. Angiopoietin-1 reduces VEGF-stimulated leukocyte adhesion to endothelial cells by reducing ICAM-1, VCAM-1, and E-selectin expression. Circulation research. 2001;89(6):477–9. doi: 10.1161/hh1801.097034. [DOI] [PubMed] [Google Scholar]
- 13.Larcher F, Franco M, Bolontrade M, Rodriguez-Puebla M, Casanova L, Navarro M, et al. Modulation of the angiogenesis response through Ha-ras control, placenta growth factor, and angiopoietin expression in mouse skin carcinogenesis. Mol Carcinog. 2003;37:83–90. doi: 10.1002/mc.10126. [DOI] [PubMed] [Google Scholar]
- 14.Kosacka J, Figiel M, Engele J, Hilbig H, Majewski M, Spanel-Borowski K. Angiopoietin-1 promotes neurite outgrowth from dorsal root ganglion cells positive for Tie-2 receptor. Cell Tissue Res. 2005;320:11–9. doi: 10.1007/s00441-004-1068-2. [DOI] [PubMed] [Google Scholar]
- 15.Thurston G, Suri C, Smith K, McClain J, Sato TN, Yancopoulos GD, et al. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science. 1999;286(5449):2511–4. doi: 10.1126/science.286.5449.2511. [DOI] [PubMed] [Google Scholar]
- 16.Suri C, McClain J, Thurston G, McDonald DM, Zhou H, Oldmixon EH, et al. Increased vascularization in mice overexpressing angiopoietin-1. Science. 1998;282(5388):468–71. doi: 10.1126/science.282.5388.468. [DOI] [PubMed] [Google Scholar]
- 17.Wolfram JA, Diaconu D, Hatala DA, Rastegar J, Knutsen DA, Lowther A, et al. Keratinocyte but not endothelial cell specific overexpression of Tie2 leads to the development of psoriasis. Am J Pathol. 2009;174:1443–58. doi: 10.2353/ajpath.2009.080858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frank S, Hubner G, Breier G, Longaker MT, Greenhalgh DG, Werner S. Regulation of vascular endothelial growth factor expression in cultured keratinocytes. Implications for normal and impaired wound healing. J Biol Chem. 1995;270:12607–13. doi: 10.1074/jbc.270.21.12607. [DOI] [PubMed] [Google Scholar]
- 19.Mukouyama YS, Shin D, Britsch S, Taniguchi M, Anderson DJ. Sensory nerves determine the pattern of arterial differentiation and blood vessel branching in the skin. Cell. 2002;109:693–705. doi: 10.1016/s0092-8674(02)00757-2. [DOI] [PubMed] [Google Scholar]
- 20.Wakui S, Yokoo K, Muto T, Suzuki Y, Takahashi H, Furusato M, et al. Localization of Ang-1, -2, Tie-2, and VEGF expression at endothelial-pericyte interdigitation in rat angiogenesis. Lab Invest. 2006;86:1172–84. doi: 10.1038/labinvest.3700476. [DOI] [PubMed] [Google Scholar]
- 21.Dumont DJ, Fong GH, Puri MC, Gradwohl G, Alitalo K, Breitman ML. Vascularization of the mouse embryo: a study of flk-1, tek, tie, and vascular endothelial growth factor expression during development. Dev Dyn. 1995;203:80–92. doi: 10.1002/aja.1002030109. [DOI] [PubMed] [Google Scholar]
- 22.Mor F, Quintana FJ, Cohen IR. Angiogenesis-inflammation cross-talk: vascular endothelial growth factor is secreted by activated T cells and induces Th1 polarization. J Immunol. 2004;172:4618–23. doi: 10.4049/jimmunol.172.7.4618. [DOI] [PubMed] [Google Scholar]
- 23.Stockmann C, Kerdiles Y, Nomaksteinsky M, Weidemann A, Takeda N, Doedens A, et al. Loss of myeloid cell-derived vascular endothelial growth factor accelerates fibrosis. Proc Natl Acad Sci U S A. 107:4329–34. doi: 10.1073/pnas.0912766107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kasama T, Miwa Y, Isozaki T, Odai T, Adachi M, Kunkel SL. Neutrophil-derived cytokines: potential therapeutic targets in inflammation. Curr Drug Targets Inflamm Allergy. 2005;4:273–9. doi: 10.2174/1568010054022114. [DOI] [PubMed] [Google Scholar]
- 25.Senger DR, Perruzzi CA, Feder J, Dvorak HF. A highly conserved vascular permeability factor secreted by a variety of human and rodent tumor cell lines. Cancer Res. 1986;46:5629–32. [PubMed] [Google Scholar]
- 26.Detmar M, Brown LF, Schon MP, Elicker BM, Velasco P, Richard L, et al. Increased microvascular density and enhanced leukocyte rolling and adhesion in the skin of VEGF transgenic mice. J Invest Dermatol. 1998;111:1–6. doi: 10.1046/j.1523-1747.1998.00262.x. [DOI] [PubMed] [Google Scholar]
- 27.Larcher F, Murillas R, Bolontrade M, Conti CJ, Jorcano JL. VEGF/VPF overexpression in skin of transgenic mice induces angiogenesis, vascular hyperpermeability and accelerated tumor development. Oncogene. 1998;17:303–11. doi: 10.1038/sj.onc.1201928. [DOI] [PubMed] [Google Scholar]
- 28.Kunstfeld R, Hirakawa S, Hong YK, Schacht V, Lange-Asschenfeldt B, Velasco P, et al. Induction of cutaneous delayed-type hypersensitivity reactions in VEGF-A transgenic mice results in chronic skin inflammation associated with persistent lymphatic hyperplasia. Blood. 2004;104:1048–57. doi: 10.1182/blood-2003-08-2964. [DOI] [PubMed] [Google Scholar]
- 29.Xia YP, Li B, Hylton D, Detmar M, Yancopoulos GD, Rudge JS. Transgenic delivery of VEGF to mouse skin leads to an inflammatory condition resembling human psoriasis. Blood. 2003;102:161–8. doi: 10.1182/blood-2002-12-3793. [DOI] [PubMed] [Google Scholar]
- 30.Wilgus TA, Matthies AM, Radek KA, Dovi JV, Burns AL, Shankar R, et al. Novel Function for Vascular Endothelial Growth Factor Receptor-1 on Epidermal Keratinocytes. Am J Pathol. 2005;167:1257–66. doi: 10.1016/S0002-9440(10)61213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sondell M, Lundborg G, Kanje M. Vascular endothelial growth factor has neurotrophic activity and stimulates axonal outgrowth, enhancing cell survival and Schwann cell proliferation in the peripheral nervous system. J Neurosci. 1999;19:5731–40. doi: 10.1523/JNEUROSCI.19-14-05731.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oosthuyse B, Moons L, Storkebaum E, Beck H, Nuyens D, Brusselmans K, et al. Deletion of the hypoxia-response element in the vascular endothelial growth factor promoter causes motor neuron degeneration. Nat Genet. 2001;28:131–8. doi: 10.1038/88842. [DOI] [PubMed] [Google Scholar]
- 33.Ogunshola OO, Antic A, Donoghue MJ, Fan SY, Kim H, Stewart WB, et al. Paracrine and autocrine functions of neuronal vascular endothelial growth factor (VEGF) in the central nervous system. J Biol Chem. 2002;277:11410–5. doi: 10.1074/jbc.M111085200. [DOI] [PubMed] [Google Scholar]
- 34.Silverman WF, Krum JM, Mani N, Rosenstein JM. Vascular, glial and neuronal effects of vascular endothelial growth factor in mesencephalic explant cultures. Neuroscience. 1999;90:1529–41. doi: 10.1016/s0306-4522(98)00540-5. [DOI] [PubMed] [Google Scholar]
- 35.Jin KL, Mao XO, Greenberg DA. Vascular endothelial growth factor: direct neuroprotective effect in in vitro ischemia. Proc Natl Acad Sci U S A. 2000;97:10242–7. doi: 10.1073/pnas.97.18.10242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci U S A. 2002;99:11946–50. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matsuzaki H, Tamatani M, Yamaguchi A, Namikawa K, Kiyama H, Vitek MP, et al. Vascular endothelial growth factor rescues hippocampal neurons from glutamate-induced toxicity: signal transduction cascades. Faseb J. 2001;15:1218–20. [PubMed] [Google Scholar]
- 38.Thurston G, Rudge JS, Ioffe E, Zhou H, Ross L, Croll SD, et al. Angiopoietin-1 protects the adult vasculature against plasma leakage. Nature medicine. 2000;6(4):460–3. doi: 10.1038/74725. [DOI] [PubMed] [Google Scholar]
- 39.Sarao R, Dumont DJ. Conditional transgene expression in endothelial cells. Transgenic research. 1998;7(6):421–7. doi: 10.1023/a:1008837410485. [DOI] [PubMed] [Google Scholar]
- 40.Kistner A, Gossen M, Zimmermann F, Jerecic J, Ullmer C, Lèubbert H, et al. Doxycycline-mediated quantitative and tissue-specific control of gene expression in transgenic mice. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(20):10933–8. doi: 10.1073/pnas.93.20.10933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Diamond I, Owolabi T, Marco M, Lam C, Glick A. Conditional gene expression in the epidermis of transgenic mice using the tetracycline-regulated transactivators tTA and rTA linked to the keratin 5 promoter. J Invest Dermatol. 2000;115:788–94. doi: 10.1046/j.1523-1747.2000.00144.x. [DOI] [PubMed] [Google Scholar]
- 42.Akeson AL, Greenberg JM, Cameron JE, Thompson FY, Brooks SK, Wiginton D, et al. Temporal and spatial regulation of VEGF-A controls vascular patterning in the embryonic lung. Dev Biol. 2003;264:443–55. doi: 10.1016/j.ydbio.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 43.Jones N, Voskas D, Master Z, Sarao R, Jones J, Dumont DJ. Rescue of the early vascular defects in Tek/Tie2 null mice reveals an essential survival function. EMBO Rep. 2001;2:438–45. doi: 10.1093/embo-reports/kve093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ward NL, Haninec AL, Van Slyke P, Sled JG, Sturk C, Henkelman RM, et al. Angiopoietin-1 Causes Reversible Degradation of the Portal Microcirculation in Mice: Implications for Treatment of Liver Disease. Am J Pathol. 2004;165:889–99. doi: 10.1016/S0002-9440(10)63351-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sheehan DCHBB. Theory and practice of Histotechnology. 2. Detroit: Battelle Press; 1980. [Google Scholar]
- 46.Kosacka J, Nowicki M, Kacza J, Borlak J, Engele J, Spanel-Borowski K. Adipocyte-derived angiopoietin-1 supports neurite outgrowth and synaptogenesis of sensory neurons. J Neurosci Res. 2006;83:1160–9. doi: 10.1002/jnr.20811. [DOI] [PubMed] [Google Scholar]
- 47.Ward NL, Putoczki T, Mearow K, Ivanco TL, Dumont DJ. Vascular-specific growth factor angiopoietin 1 is involved in the organization of neuronal processes. J Comp Neurol. 2004;482:244–56. doi: 10.1002/cne.20422. [DOI] [PubMed] [Google Scholar]
- 48.Chen X, Fu W, Tung CE, Ward NL. Angiopoietin-1 induces neurite outgrowth of PC12 cells in a Tie2-independent, beta1-integrin-dependent manner. Neurosci Res. 2009;64:348–54. doi: 10.1016/j.neures.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bai Y, Cui M, Meng Z, Shen L, He Q, Zhang X, et al. Ectopic expression of angiopoietin-1 promotes neuronal differentiation in neural progenitor cells through the Akt pathway. Biochem Biophys Res Commun. 2009;378:296–301. doi: 10.1016/j.bbrc.2008.11.052. [DOI] [PubMed] [Google Scholar]
- 50.Sondell M, Sundler F, Kanje M. Vascular endothelial growth factor is a neurotrophic factor which stimulates axonal outgrowth through the flk-1 receptor. Eur J Neurosci. 2000;12:4243–54. doi: 10.1046/j.0953-816x.2000.01326.x. [DOI] [PubMed] [Google Scholar]
- 51.Marko SB, Damon DH. VEGF promotes vascular sympathetic innervation. Am J Physiol Heart Circ Physiol. 2008;294:H2646–52. doi: 10.1152/ajpheart.00291.2008. [DOI] [PubMed] [Google Scholar]
- 52.Ismail NS, Pravda EA, Li D, Shih S-C, Dallabrida SM. Angiopoietin-1 Reduces H2O2-Induced Increases in Reactive Oxygen Species and Oxidative Damage to Skin Cells. J Invest Dermatol. 2010 doi: 10.1038/jid.2009.431. [DOI] [PubMed] [Google Scholar]
- 53.Huang IT, Lin WM, Shun CT, Hsieh ST. Influence of cutaneous nerves on keratinocyte proliferation and epidermal thickness in mice. Neuroscience. 1999;94:965–73. doi: 10.1016/s0306-4522(99)00210-9. [DOI] [PubMed] [Google Scholar]
- 54.Yu XJ, Li CY, Xu YH, Chen LM, Zhou CL. Calcitonin gene-related peptide increases proliferation of human HaCaT keratinocytes by activation of MAP kinases. Cell Biol Int. 2009 doi: 10.1016/j.cellbi.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 55.Nickoloff BJ, Wrone-Smith T. Injection of pre-psoriatic skin with CD4+ T cells induces psoriasis. Am J Pathol. 1999;155:145–58. doi: 10.1016/S0002-9440(10)65109-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Conrad C, Boyman O, Tonel G, Tun-Kyi A, Laggner U, de Fougerolles A, et al. Alpha1beta1 integrin is crucial for accumulation of epidermal T cells and the development of psoriasis. Nat Med. 2007;13:836–42. doi: 10.1038/nm1605. [DOI] [PubMed] [Google Scholar]
- 57.Venneri MA, Palma MD, Ponzoni M, Pucci F, Scielzo C, Zonari E, et al. Identification of proangiogenic TIE2-expressing monocytes (TEMs) in human peripheral blood and cancer. Blood. 2007;109:5276–85. doi: 10.1182/blood-2006-10-053504. [DOI] [PubMed] [Google Scholar]
- 58.Scott JR, Tamura RN, Muangman P, Isik FF, Xie C, Gibran NS. Topical substance P increases inflammatory cell density in genetically diabetic murine wounds. Wound Repair and Regeneration. 2008;16:529–33. doi: 10.1111/j.1524-475X.2008.00400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ding W, Stohl LL, Wagner JA, Granstein RD. Calcitonin gene-related peptide biases Langerhans cells toward Th2-type immunity. J Immunol. 2008;181:6020–6. doi: 10.4049/jimmunol.181.9.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Torii H, Yan Z, Hosoi J, Granstein RD. Expression of neurotrophic factors and neuropeptide receptors by Langerhans cells and the Langerhans cell-like cell line XS52: further support for a functional relationship between Langerhans cells and epidermal nerves. J Invest Dermatol. 1997;109:586–91. doi: 10.1111/1523-1747.ep12337516. [DOI] [PubMed] [Google Scholar]
- 61.Inoshima N, Nakanishi Y, Minami T, Izumi M, Takayama K, Yoshino I, et al. The influence of dendritic cell infiltration and vascular endothelial growth factor expression on the prognosis of non-small cell lung cancer. Clin Cancer Res. 2002;8:3480–6. [PubMed] [Google Scholar]
- 62.Huang Y, Chen X, Dikov MM, Novitskiy SV, Mosse CA, Yang L, et al. Distinct roles of VEGFR-1 and VEGFR-2 in the aberrant hematopoiesis associated with elevated levels of VEGF. Blood. 2007;110:624–31. doi: 10.1182/blood-2007-01-065714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mimura K, Kono K, Takahashi A, Kawaguchi Y, Fujii H. Vascular endothelial growth factor inhibits the function of human mature dendritic cells mediated by VEGF receptor-2. Cancer Immunol Immunother. 2007;56:761–70. doi: 10.1007/s00262-006-0234-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ohm JE, Shurin MR, Esche C, Lotze MT, Carbone DP, Gabrilovich DI. Effect of vascular endothelial growth factor and FLT3 ligand on dendritic cell generation in vivo. J Immunol. 1999;163:3260–8. [PubMed] [Google Scholar]
- 65.Oyama T, Ran S, Ishida T, Nadaf S, Kerr L, Carbone DP, et al. Vascular endothelial growth factor affects dendritic cell maturation through the inhibition of nuclear factor-kappa B activation in hemopoietic progenitor cells. J Immunol. 1998;160:1224–32. [PubMed] [Google Scholar]
- 66.Reinders ME, Sho M, Izawa A, Wang P, Mukhopadhyay D, Koss KE, et al. Proinflammatory functions of vascular endothelial growth factor in alloimmunity. J Clin Invest. 2003;112:1655–65. doi: 10.1172/JCI17712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee T-H, Avraham H, Lee S-H, Avraham S. Vascular Endothelial Growth Factor Modulates Neutrophil Transendothelial Migration via Up-regulation of Interleukin-8 in Human Brain Microvascular Endothelial Cells. J Biol Chem. 2002;277:10445–51. doi: 10.1074/jbc.M107348200. [DOI] [PubMed] [Google Scholar]
- 68.Hattori K, Dias S, Heissig B, Hackett NR, Lyden D, Tateno M, et al. Vascular endothelial growth factor and angiopoietin-1 stimulate postnatal hematopoiesis by recruitment of vasculogenic and hematopoietic stem cells. The Journal of experimental medicine. 2001;193(9):1005–14. doi: 10.1084/jem.193.9.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Carmeliet P, Storkebaum E. Vascular and neuronal effects of VEGF in the nervous system: implications for neurological disorders. Semin Cell Dev Biol. 2002;13:39–53. doi: 10.1006/scdb.2001.0290. [DOI] [PubMed] [Google Scholar]
- 70.Ferretti A, Boschi E, Stefani A, Spiga S, Romanelli M, Lemmi M, et al. Angiogenesis and nerve regeneration in a model of human skin equivalent transplant. Life Sci. 2003;73:1985–94. doi: 10.1016/s0024-3205(03)00541-1. [DOI] [PubMed] [Google Scholar]
- 71.Gu XH, Terenghi G, Kangesu T, Navsaria HA, Springall DR, Leigh IM, et al. Regeneration pattern of blood vessels and nerves in cultured keratinocyte grafts assessed by confocal laser scanning microscopy. Br J Dermatol. 1995;132:376–83. doi: 10.1111/j.1365-2133.1995.tb08670.x. [DOI] [PubMed] [Google Scholar]
- 72.Kangesu T, Manek S, Terenghi G, Gu XH, Navsaria HA, Polak JM, et al. Nerve and blood vessel growth in response to grafted dermis and cultured keratinocytes. Plast Reconstr Surg. 1998;101:1029–38. doi: 10.1097/00006534-199804040-00022. [DOI] [PubMed] [Google Scholar]
- 73.Mukouyama YS, Gerber HP, Ferrara N, Gu C, Anderson DJ. Peripheral nerve-derived VEGF promotes arterial differentiation via neuropilin 1-mediated positive feedback. Development. 2005;132:941–52. doi: 10.1242/dev.01675. [DOI] [PubMed] [Google Scholar]
- 74.Griffith LG, Naughton G. Tissue engineering--current challenges and expanding opportunities. Science. 2002;295:1009–14. doi: 10.1126/science.1069210. [DOI] [PubMed] [Google Scholar]
- 75.Jiang W-Y, Raychaudhuri SP, Farber EM. Double-labeled immunofluorescence study of cutaneous nerves in psoriasis. International Journal of Dermatology. 1998;37:572–4. doi: 10.1046/j.1365-4362.1998.00533.x. [DOI] [PubMed] [Google Scholar]
- 76.Naukkarinen A, Nickoloff BJ, Farber EM. Quantification of cutaneous sensory nerves and their substance P content in psoriasis. J Invest Dermatol. 1989;92:126–9. doi: 10.1111/1523-1747.ep13071340. [DOI] [PubMed] [Google Scholar]
- 77.Farber EM, Lanigan SW, Boer J. The role of cutaneous sensory nerves in the maintenance of psoriasis. Int J Dermatol. 1990;29:418–20. doi: 10.1111/j.1365-4362.1990.tb03825.x. [DOI] [PubMed] [Google Scholar]
- 78.Kuroda K, Sapadin A, Shoji T, Fleischmajer R, Lebwohl M. Altered expression of angiopoietins and Tie2 endothelium receptor in psoriasis. J Invest Dermatol. 2001;116:713–20. doi: 10.1046/j.1523-1747.2001.01316.x. [DOI] [PubMed] [Google Scholar]
- 79.Creamer D, Sullivan D, Bicknell R, Barker J. Angiogenesis in psoriasis. Angiogenesis. 2002;5:231–6. doi: 10.1023/a:1024515517623. [DOI] [PubMed] [Google Scholar]
- 80.Creamer JD, Barker JN. Vascular proliferation and angiogenic factors in psoriasis. Clin Exp Dermatol. 1995;20:6–9. doi: 10.1111/j.1365-2230.1995.tb01274.x. [DOI] [PubMed] [Google Scholar]
- 81.Baker BS, Fry L. The immunology of psoriasis. Br J Dermatol. 1992;126:1–9. doi: 10.1111/j.1365-2133.1992.tb08394.x. [DOI] [PubMed] [Google Scholar]
- 82.Mordovtsev VN, Albanova VI. Morphology of skin microvasculature in psoriasis. Am J Dermatopathol. 1989;11:33–42. doi: 10.1097/00000372-198902000-00006. [DOI] [PubMed] [Google Scholar]
- 83.Raychaudhuri SP, Raychaudhuri SK. Role of NGF and neurogenic inflammation in the pathogenesis of psoriasis. Prog Brain Res. 2004;146:433–7. doi: 10.1016/S0079-6123(03)46027-5. [DOI] [PubMed] [Google Scholar]
- 84.Hern S, Stanton AW, Mellor R, Levick JR, Mortimer PS. Control of cutaneous blood vessels in psoriatic plaques. J Invest Dermatol. 1999;113:127–32. doi: 10.1046/j.1523-1747.1999.00638.x. [DOI] [PubMed] [Google Scholar]
- 85.Menke NB, Ward KR, Witten TM, Bonchev DG, Diegelmann RF. Impaired wound healing. Clinics in Dermatology. 2007;25:19–25. doi: 10.1016/j.clindermatol.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 86.Fukai T, Takeda A, Uchinuma E. Wound healing in denervated rat skin. Wound Repair Regen. 2005;13:175–80. doi: 10.1111/j.1067-1927.2005.130208.x. [DOI] [PubMed] [Google Scholar]
- 87.Kampfer H, Pfeilschifter J, Frank S. Expressional regulation of angiopoietin-1 and -2 and the tie-1 and -2 receptor tyrosine kinases during cutaneous wound healing: a comparative study of normal and impaired repair. Lab Invest. 2001;81:361–73. doi: 10.1038/labinvest.3780244. [DOI] [PubMed] [Google Scholar]
- 88.Galeano M, Altavilla D, Cucinotta D, Russo GT, Calo M, Bitto A, et al. Recombinant Human Erythropoietin Stimulates Angiogenesis and Wound Healing in the Genetically Diabetic Mouse. Diabetes. 2004;53:2509–17. doi: 10.2337/diabetes.53.9.2509. [DOI] [PubMed] [Google Scholar]
- 89.Galiano RD, Tepper OM, Pelo CR, Bhatt KA, Callaghan M, Bastidas N, et al. Topical Vascular Endothelial Growth Factor Accelerates Diabetic Wound Healing through Increased Angiogenesis and by Mobilizing and Recruiting Bone Marrow-Derived Cells. Am J Pathol. 2004;164:1935–47. doi: 10.1016/S0002-9440(10)63754-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Martin A, Komada MR, Sane DC. Abnormal angiogenesis in diabetes mellitus. Med Res Rev. 2003;23:117–45. doi: 10.1002/med.10024. [DOI] [PubMed] [Google Scholar]
- 91.Loomans CJM, de Koning EJP, Staal FJT, Rookmaaker MB, Verseyden C, de Boer HC, et al. Endothelial Progenitor Cell Dysfunction: A Novel Concept in the Pathogenesis of Vascular Complications of Type 1 Diabetes. Diabetes. 2004;53:195–9. doi: 10.2337/diabetes.53.1.195. [DOI] [PubMed] [Google Scholar]
- 92.Echtermeyer F, Streit M, Wilcox-Adelman S, Saoncella S, Denhez F, Detmar M, et al. Delayed wound repair and impaired angiogenesis in mice lacking syndecan-4. J Clin Invest. 2001;107:R9–14. doi: 10.1172/JCI10559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chang CJ, Yu JS, Nelson JS. Confocal microscopy study of neurovascular distribution in facial port wine stains (capillary malformation) J Formos Med Assoc. 2008;107:559–66. doi: 10.1016/S0929-6646(08)60169-2. [DOI] [PubMed] [Google Scholar]
- 94.Lachgar S, Charveron M, Gall Y, Bonafe JL. Inhibitory effects of retinoids on vascular endothelial growth factor production by cultured human skin keratinocytes. Dermatology. 1999;199 (Suppl 1):25–7. doi: 10.1159/000051374. [DOI] [PubMed] [Google Scholar]
- 95.Lonne-Rahm S, Nordlind K, Edstrom DW, Ros AM, Berg M. Laser treatment of rosacea: a pathoetiological study. Arch Dermatol. 2004;140:1345–9. doi: 10.1001/archderm.140.11.1345. [DOI] [PubMed] [Google Scholar]
- 96.Draelos ZD. Noxious sensory perceptions in patients with mild to moderate rosacea treated with azelaic acid 15% gel. Cutis. 2004;74:257–60. [PubMed] [Google Scholar]
- 97.Draelos ZD. Assessment of skin barrier function in rosacea patients with a novel 1% metronidazole gel. J Drugs Dermatol. 2005;4:557–62. [PubMed] [Google Scholar]
- 98.Sowinska-Glugiewicz I, Ratajczak-Stefanska V, Maleszka R. Role of psychological factors in course of the rosacea. Rocz Akad Med Bialymst. 2005;50 (Suppl 1):49–53. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.