Abstract
Estradiol and other steroid hormones modulate the nervous system and behavior on both acute and long-term time scales. Though estradiol was originally characterized as a regulator of gene expression through the action of nuclear estrogen receptors (ER) that directly bind DNA to regulate gene expression, research over the past thirty years has firmly established that estradiol can initiate signaling pathways via extra-nuclear ERs associated with the cellular membrane, producing changes in neurons through stimulation of various intracellular signaling pathways. Several studies have determined that the classical ERs, ERα and ERβ, mediate some of these fast-acting signaling pathways through activation of G proteins. Since ERα and ERβ are not G protein-coupled receptors, the mechanisms by which ERs can stimulate signal transduction pathways are a focus of recent research. Here we discuss recent studies illustrating one mechanism by which ERα and ERβ initiate these pathways: through direct association with metabotropic glutamate receptors (mGluRs). Estradiol binding to these membrane-localized estrogen receptors results in mGluR signaling independent of glutamate. ERs are organized with mGluRs into functional signaling microdomains via caveolin proteins. The pairing of ERs to specific mGluRs via caveolins is region specific, with ERs being linked to different mGluRs in hippocampal, striatal, and other neurons. It is becoming clear that ER signaling through mGluRs is one important mechanism by which estrogens can modulate neuron and glial physiology, ultimately impacting various aspects of nervous system function.
Keywords: Estradiol, metabotropic glutamate receptors, rapid, estrogen, caveolin, hippocampus, striatum
Introduction
Steroid sex hormone actions on brain and behavior have been studied for over 160 years, beginning with Arnold A. Berthold (1803–1861) and his studies with intact and castrated roosters (Berthold, 1944). The last 65 years in particular have seen an explosion in the number of studies focusing on the effects of gonadal hormones on brain function and behavior, heavily influenced by the work of Frank Beach (1911–1988) and his landmark book Hormones and Behavior, first published in 1948. It is now well established that steroid sex hormones influence the nervous system and behavior permanently, temporarily, slowly and rapidly, via changes in gene expression and other cellular processes. Steroid sex hormones have been shown to modulate brain anatomy and physiology, affect multiple behaviors, including sexual development and reproduction, and more recently, various processes outside of reproduction, including learning and memory, nociception, motor control, drug use and cognition.
The goal of this review is to focus on recent findings regarding the conversion of the intracellular estrogen receptors, ERα and ERβ into membrane associated signaling proteins, whereby they interact with metabotropic glutamate receptors (mGluRs) to rapidly trigger intracellular signaling pathways. In the course of reviewing this body of work, we will first provide background on the classical actions of ERs, and then follow with a brief discussion of the mechanisms of acute estrogen action with ERs working at the membrane surface. We will then discuss how ERs link to mGluRs to initiate signaling cascades, and how ERs and mGluRs are organized into functional signaling microdomains via caveolins. Finally, we will examine how ERs, mGluRs and caveolins are differentially organized by brain region.
Classical actions of estrogen receptors
The classical action of estrogens, including 17β-estradiol, is stimulating ERs to directly induce changes in gene expression and protein synthesis. Across many brain regions and animals, many ER-mediated effects are dependent on translation of mRNA into protein. Indeed, both types of classically described ERs, ERα and ERβ, can act as ligand-regulated transcription factors. ERα and ERβ are primarily localized in the nucleus, where after activation they can modulate gene expression by binding to specific estrogen response elements (ERE) on DNA. There they can also interact with many co-activators and other transcription factors to affect both ERE and non-ERE containing genes (Charlier et al., 2010), but all towards the ultimate action of regulating gene transcription.
Acute actions of estrogen receptors
This fairly straightforward and simple model of estrogen action is useful for explaining many estrogen actions, but has proven to be incomplete. Alongside the growing evidence that ERs act to modulate gene expression on relatively slow time scales, reports of estrogen action incompatible with this model were slowly being added to the literature. These reports generally fell within three categories: rapid actions of estrogen on both reproductive and non-reproductive behavior, actions that seemed to be initiated on or near the cellular membrane, and the focus of this review, rapid actions of estrogen on neuron physiology that was too fast to be induced by changes in gene transcription at the nucleus.
Some of the first experiments regarding fast actions of estrogens were actually done outside of the nervous system. One classic experiment was performed in uterine tissue, where estradiol exposure was found to rapidly increase cAMP within 15 seconds of exposure (Szego and Davis, 1967). In neurons, the first reports of rapid estrogen action came from studies of preoptic/septal neurons, whose electrophysiological properties were modulated within seconds of estrogen application (Kelly et al., 1976). Over the past 30 years, these initial findings have been augmented with much additional research (Woolley, 2007). Not only has it been repeatedly shown that estrogen can rapidly modulate neuronal electrophysiological properties (Mermelstein et al., 1996; Joels, 1997; Chaban et al., 2004; Woolley, 2007), but estrogen-exposure can also activate many intracellular signaling proteins, that often appeared dependent upon G protein signaling, despite the fact that classical ERs are clearly not G protein-coupled receptors. Through these signaling pathways, estrogen can not only rapidly modulate the electrophysiological properties of the neuron, but also activate transcription factors such as cAMP response element binding protein (pCREB) to affect gene expression. At this point it is well established that estrogens induce both rapid and long term, classically described, effects on neurons.
This consensus that estrogen exerts rapid effects on neuron biology has not extended to the underlying mechanisms, though one generally agreed-upon finding is that most of the rapid estrogen effects originate at the cellular membrane. This has been primarily established by the facts that many fast estrogen effects occur in brain regions that express little or no nuclear ER (Remage-Healey et al., 2009), that membrane-impermeant estrogen analogs stimulate rapid effects (Boulware et al., 2005), and that intracellular dialysis of neurons with 17β-estradiol does not block rapid effects (Mermelstein et al., 1996). Additionally, the generation of a membrane-estrogen receptor knock-out mouse determined that normal development requires both membrane and nuclear ER (Pedram et al., 2009).
Several candidates that have been proposed to function as the membrane estrogen receptors (mERs), including ER-X (Toran-Allerand et al., 2002), GPR30 (Terasawa et al., 2009), STX binding protein (Qiu et al., 2003; Qiu et al., 2006), ERα, and ERβ. These candidates are not necessarily mutually exclusive. Here we focus on the classical ERα and ERβ as a membrane associated ER, primarily they have been found to mediate the rapid estrogen actions on CREB phosphorylation studied in our laboratory (described below). The story actually begins long before we began our work in this field, when researchers in the 1980s found that steroid receptors could localize to the membrane surface in Xenopus oocytes (Sadler and Maller, 1982; Sadler et al., 1985). Though these experiments were often initially dismissed as an artifact (i.e., during the isolation procedure transposed receptors from the nucleus could have contaminated the membrane fractions), over time they have been validated using other techniques (Micevych and Mermelstein, 2008; Pedram et al., 2009). An additional criticism of these studies was that the structure of the ER seemed to preclude binding to the cellular membrane to activate intracellular signaling. However, experiments using over-expressed ERα and ERβ established that the ER could be targeted to the membrane and activate signaling pathways (Razandi et al., 1999). How exactly this occurs is an active area of research.
Additional evidence for the role of ERα and ERβ in mediating fast estrogen effects comes from the study of the transcription factor CREB, the phosphorylation of which at the serine 133 site is often a target of fast membrane-mediated estrogen effects via the MAPK/ERK signaling pathway (Gu and Moss, 1996; Zhou et al., 1996; Wade and Dorsa, 2003; Lee et al., 2004). This phosphorylation activates CREB to modulate gene transcription through interactions with DNA at CREB response elements (Lonze and Ginty, 2002; Carlezon et al., 2005). The ER antagonist ICI 182, 780 blocks the rapid effects of estradiol on CREB phosphorylation, while ERα and ERβ agonists generally mimic the effect of estradiol. Finally, experiments using ER knockout mice found that ERα and ERβ were necessary for estrogen-induced rapid CREB phosphorylation (Abraham et al., 2004). Though this evidence does not rule out other candidates for mediating other rapid mER actions, it does show that non-nuclear, membrane localized ERα and ERβ can induce rapid estrogen actions.
ERs interacts with mGluRs to activate intracellular signaling pathways
One of the questions generated by this research is how classical ERs trigger signal transduction pathways, given that they are not G protein-coupled receptors and are localized to the cellular membrane. Ours and other laboratories have found that ERα and ERβ can stimulate metabotropic glutamate receptors (mGluRs) to initiate intracellular signaling cascades. mGluRs are a family of G-protein coupled receptors that trigger G-protein activation after being bound by glutamate. There are at least three different mGluR families as determined by sequence homology and G-protein coupling (Niswender and Conn, 2010). These receptors link to separate second messenger systems (Figure 1A). The group I mGluRs are mGluR1 and mGluR5, and link to Gq. Group II mGluRs (mGluR2 and mGluR3), link to Gi/Go. Group III mGluRs (mGluR4, mGluR6, mGluR7, mGluR8) also link to Gi/Go.
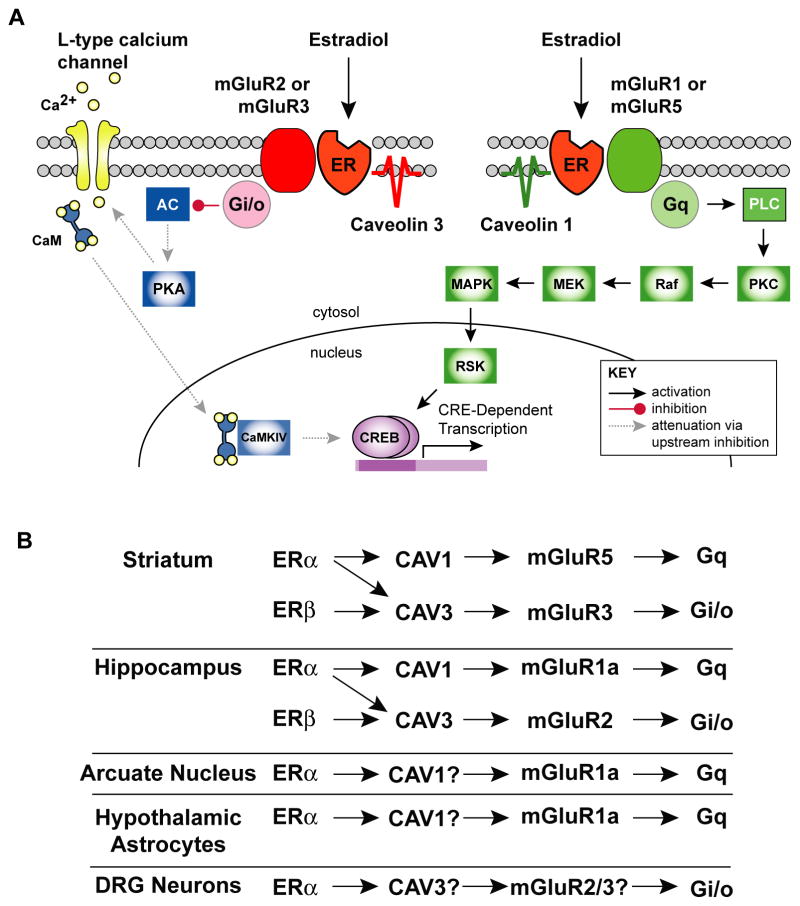
Figure 1.
ER activation of mGluRs is organized via caveolins into brain region specific functional microdomains. A. Schematic of proposed estradiol/ER/mGluR signaling microdomains as organized by caveolin proteins in hippocampal neurons. Caveolin 1 organizes ERα to mGluR1a to activate the MEK pathway which induces CREB phosphorylation. Caveolin 3 organizes ERα to mGluR2 which activates PKA/CaM signaling to attenuate CREB phosphorylation. Abbreviations: ER, estrogen receptor; AC, adenylyl cyclase; PKA, protein kinase A; CaM, Calmodulin; CaMKIV, calmodulin-dependent protein kinase IV; PLC, phospholipase C; MEK, mitogen-activated protein kinase kinase; MAPK, mitogen-activated protein kinase; RAF, rapidly accelerated fibrosarcoma kinase; RSK, p90 ribosomal protein S6 kinase; CREB, cyclic-AMP response element binding protein. B. Summary depicting how ERs link to different mGluRs depending on brain region. Question marks indicate as yet undetermined caveolins or mGluRs.
The first clues that hinted that ERα and ERβ stimulate mGluRs came from several studies which found that some rapid estrogen effects were sensitive to G protein manipulation (Mermelstein et al., 1996; Qiu et al., 2003). These results generated at least three different possibilities. The first being that ERα and ERβ directly bind to G proteins (Levin, 2005; Vasudevan and Pfaff, 2007), and indeed, evidence suggests that at least one G protein subunit can directly interact with ERα (Wyckoff et al., 2001). The second possibility is that there are separate classes of G protein-coupled receptors that are estrogen sensitive. Indeed, work from the Kelly laboratory has indicated that in some systems, this may well be the case (Roepke et al., 2009). The third possibility is that ERs activate other G protein-coupled receptors that then induce signaling pathways, as found both in and outside of the nervous system (Cardona-Gomez et al., 2000; Kahlert et al., 2000; Razandi et al., 2003; Song et al., 2007). We find this to be the case in our experimental model system of cultured hippocampal and striatal neurons from female rat pups.
In female cultured hippocampal neurons, exposure to 17β-estradiol increases CREB phosphorylation within 30 seconds, with a maximal response at 2 minutes (Boulware et al., 2005). The effect is only found in female pups, which is interesting given that both male and female hippocampus and cultured hippocampal neurons express ER. This increase in CREB phosphorylation requires picomolar concentrations of estradiol (a physiological concentration), and is blocked by the ER antagonist ICI 182,780. Because a non-permeable estrogen analog and ER agonist mimicked the effect, we concluded that this signaling pathway was mediated by membrane-associated ERα. We then found that group I mGluR1 antagonists/agonists either blocked or mimicked the effect of estradiol, and that inhibitors of the signal transduction molecules that link mGluR1 activation to CREB phosphorylation, such as PKC, IP3 and MEK (Choe and Wang, 2001; Warwick et al., 2005), eliminated the response to estradiol.
Estrogens stimulate a variety of signal transduction cascades, and linkage to one particular mGluR does not explain all of them (Figure 1). For example, estrogen acts through a G protein-coupled receptor to decrease L-type calcium channel currents (Mermelstein et al., 1996; Lee et al., 2002; Chaban et al., 2003). L-type calcium channel currents are known to rapidly trigger CREB phosphorylation via calcium calmodulin-dependent protein kinase IV (CaMKIV). As with ERα association with mGluR1, we found that estrogen inhibits L-type calcium channel mediated CREB phosphorylation in hippocampal neurons through ERα or ERβ activation of the group II mGluR, mGluR2 (Boulware et al., 2005).
ER association with particular mGluRs is brain region specific
The general finding that rapid ERα and ERβ effects of CREB phosphorylation are mediated through mGluRs, but not necessarily through the same mGluRs, has also been extended to neurons from other brain regions. For our next set of experiments we focused on striatal neurons, as the rapid estrogen actions reported in this brain region are also consistent with mGluR signaling (Becker and Hu, 2008). As with hippocampal neurons, we found that activation of ERα triggers CREB phosphorylation, and that activation of ERα and ERβ inhibits L-type calcium channel mediated CREB phosphorylation (Grove-Strawser et al., 2010). Unlike hippocampal neurons, however, ERα-mediated CREB phosphorylation in striatal neurons is via activation of the group I mGluR, mGluR5, and ERα/ERβ inhibition of L-type calcium channels is via activation of the group II mGluR, mGLuR3. This is particularly interesting as both hippocampus and striatum express all four mGluRs, mGluR1, mGluR2, mGluR3 and mGluR5. The across brain regions is currently being studied.
This nervous system region-specific linkage of ER to mGluR also holds true for other areas as well. In the arcuate nucleus, ERα is co-localized with mGluR1a. Furthermore, the rapid effects of estradiol on the arcuate nucleus and its impact on lordosis behavior have been attributed to ERα/mGluR1 signaling (Dewing et al., 2007). Estrogen exposure stimulates a rapid increase in intracellular calcium in hypothalamic astrocytes, thought to be necessary for the synthesis of neuroprogesterone and the luteinizing hormone surge (Sinchak et al., 2003; Micevych and Sinchak, 2008; Micevych et al., 2010). ERα activation of mGluR1a was found to be necessary for estrogen action (Kuo et al., 2009; Kuo et al., 2010), and estrogen’s effects were more pronounced in females than in males (Kuo et al., 2010). Estrogen inhibition of L-type calcium channels in small diameter dorsal root ganglia (DRG) neurons (Chaban et al., 2003; Chaban and Micevych, 2005), a subpopulation of which are nociceptors, is dependent on group II mGluR signaling (Chaban, 2007). mGluR signaling is also necessary for the estrogen-induced masculization of adult rat sex behavior and increases in dendritic spine density in the medial preoptic area (Wright and McCarthy, 2009). These examples together indicate that the ER/mGluR association is commonly found across the nervous system, and that the specific mGluR involved is dependent on brain region.
Caveolins organize ERs and mGluRs into functional microdomains
The preceding paragraphs describe how ERs link to different mGluRs both within the same neuron, and between different brain regions. Several different mechanisms are known to functionally organize signaling pathways such as this, with one prime candidate being caveolins (Stern and Mermelstein, 2010). Caveolin proteins are situated in the membrane and create functional microdomains of signaling proteins, They are well known to interact with both steroid sex hormone receptors and mGluRs (Patel et al., 2008), including ERα (Luoma et al., 2008), although most examples occur outside of the nervous system. Indeed, when studying the bidirectional affects of estradiol on CREB phosphorylation in hippocampal and striatal neurons, we found that we were able to independently block either pathway by manipulating caveolin expression and/or activity (Boulware et al., 2007; Grove-Strawser et al., 2010), with the caveolin-1 protein being necessary for coupling ERα to the group I mGluRs and caveolin-3 being necessary for ERα and ERβ association with the group II mGluRs. Similar to other signal transduction initiators (Stern and Mermelstein, 2010), we suspect that creating functional microdomains via caveolins will prove to be a general phenomenon. Supporting this, ERα associates with caveolin-1 protein in human hippocampus and cortex (Ramirez et al., 2009).
Physiological Impact of ERs signaling through mGluRs
Functional linkage of ERs with different mGluRs creates the potential for a vast diversity of estrogen-sensitive signaling pathways (Figure 1B). Though activation of Group I receptors stimulates CREB phosphorylation via the same pathway as estradiol (Choe and Wang, 2001; Warwick et al., 2005), mGluRs do much more to affect neuron physiology than just phosphorylate CREB (Niswender and Conn, 2010). With the added complexity of caveolin-created functional microdomains, the ER-mGluR relationship may over time be found to be the underlying mechanism of many of the diverse rapid estrogen actions reported in the nervous system. Furthermore, estrogen receptors may be linked to other G-protein coupled signaling receptors as well, similar to that reported for the A2A adenosine receptor (Lee and Chao, 2001), the D1 dopamine receptor (Iwakura et al., 2008), and the β1-adrenergic receptor (Meitzen, 2011). Indeed, estrogen receptors couple to and activate tyrosine kinase receptors in non-neuronal tissue (Stefanova et al., 1991; Kahlert et al., 2000; Song et al., 2007), and similar interactions occur in the nervous system (Etgen et al., 2001; Quesada and Micevych, 2004). ER coupling to G-protein coupled receptors may be more widespread than previously appreciated, and this coupling potentially allows ERs to exert influence over all of the functions typically ascribed to G-protein coupled receptor function.
Conclusions
Here we have discussed recent research into the mechanisms underlying rapid estrogen action in neurons, emphasizing that membrane-associated ERα and ERβ can stimulate mGluRs to initiate signal transduction pathways. Furthermore, which mGluRs are activated are signal transduction pathway and brain region specific, and that functional signaling domains created by caveolin proteins explain some of these effects. These data serve as a potential mechanism by which many rapid estrogen effects could be mediated.
Acknowledgments
The authors acknowledge support from NIH grants NS041302 (PGM), F31 DA07234 (training grant supporting JM), and F32 DA030828 (JM). We thank the participants in the Workshop for Steroid Hormones and Brain Function for stimulating discussion.
Abbreviations
- ER
estrogen receptor
- mER
membrane-associated estrogen receptor
- mGluR
metabotropic glutamate receptor
- pCREB
cAMP response element binding protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham IM, Todman MG, Korach KS, Herbison AE. Critical in vivo roles for classical estrogen receptors in rapid estrogen actions on intracellular signaling in mouse brain. Endocrinology. 2004;145:3055–61. doi: 10.1210/en.2003-1676. Epub 2004 Feb 19. [DOI] [PubMed] [Google Scholar]
- Becker JB, Hu M. Sex differences in drug abuse. Front Neuroendocrinol. 2008;29:36–47. doi: 10.1016/j.yfrne.2007.07.003. Epub 2007 Aug 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthold AA. Transplantation of Testes. English translation by D. P. Quiring. Bull Hist Med. 1944;16:399–401. [Google Scholar]
- Boulware MI, Kordasiewicz H, Mermelstein PG. Caveolin proteins are essential for distinct effects of membrane estrogen receptors in neurons. J Neurosci. 2007;27:9941–50. doi: 10.1523/JNEUROSCI.1647-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulware MI, Weick JP, Becklund BR, Kuo SP, Groth RD, Mermelstein PG. Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. J Neurosci. 2005;25:5066–78. doi: 10.1523/JNEUROSCI.1427-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardona-Gomez GP, DonCarlos L, Garcia-Segura LM. Insulin-like growth factor I receptors and estrogen receptors colocalize in female rat brain. Neuroscience. 2000;99:751–60. doi: 10.1016/s0306-4522(00)00228-1. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Duman RS, Nestler EJ. The many faces of CREB. Trends Neurosci. 2005;28:436–45. doi: 10.1016/j.tins.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Chaban VV, Lakhter AJ, Micevych P. A membrane estrogen receptor mediates intracellular calcium release in astrocytes. Endocrinology. 2004;145:3788–95. doi: 10.1210/en.2004-0149. Epub 2004 May 6. [DOI] [PubMed] [Google Scholar]
- Chaban VV, Li J, McDonald JS, Rapkin A, Micevych P. Estradiol Attenuates ATP-Induced Increase of Intracellular Calcium Through Group II Metabotropic Glutamate Receptors in Rat DRG Neurons. Abstract, Society for Neuroscience Annual Meeting; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaban VV, Mayer EA, Ennes HS, Micevych PE. Estradiol inhibits atp-induced intracellular calcium concentration increase in dorsal root ganglia neurons. Neuroscience. 2003;118:941–8. doi: 10.1016/s0306-4522(02)00915-6. [DOI] [PubMed] [Google Scholar]
- Chaban VV, Micevych PE. Estrogen receptor-alpha mediates estradiol attenuation of ATP-induced Ca2+ signaling in mouse dorsal root ganglion neurons. J Neurosci Res. 2005;81:31–7. doi: 10.1002/jnr.20524. [DOI] [PubMed] [Google Scholar]
- Charlier TD, Cornil CA, Ball GF, Balthazart J. Diversity of mechanisms involved in aromatase regulation and estrogen action in the brain. Biochim Biophys Acta. 2010;1800:1094–105. doi: 10.1016/j.bbagen.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe ES, Wang JQ. Group I metabotropic glutamate receptor activation increases phosphorylation of cAMP response element-binding protein, Elk-1, and extracellular signal-regulated kinases in rat dorsal striatum. Brain Res Mol Brain Res. 2001;94:75–84. doi: 10.1016/s0169-328x(01)00217-0. [DOI] [PubMed] [Google Scholar]
- Dewing P, Boulware MI, Sinchak K, Christensen A, Mermelstein PG, Micevych P. Membrane estrogen receptor-alpha interactions with metabotropic glutamate receptor 1a modulate female sexual receptivity in rats. J Neurosci. 2007;27:9294–300. doi: 10.1523/JNEUROSCI.0592-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etgen AM, Ansonoff MA, Quesada A. Mechanisms of ovarian steroid regulation of norepinephrine receptor-mediated signal transduction in the hypothalamus: implications for female reproductive physiology. Horm Behav. 2001;40:169–77. doi: 10.1006/hbeh.2001.1676. [DOI] [PubMed] [Google Scholar]
- Grove-Strawser D, Boulware MI, Mermelstein PG. Membrane estrogen receptors activate the metabotropic glutamate receptors mGluR5 and mGluR3 to bidirectionally regulate CREB phosphorylation in female rat striatal neurons. Neuroscience. 2010;2010:1045–55. doi: 10.1016/j.neuroscience.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Q, Moss RL. 17 beta-Estradiol potentiates kainate-induced currents via activation of the cAMP cascade. J Neurosci. 1996;16:3620–9. doi: 10.1523/JNEUROSCI.16-11-03620.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwakura Y, Nawa H, Sora I, Chao MV. Dopamine D1 receptor-induced signaling through TrkB receptors in striatal neurons. J Biol Chem. 2008;283:15799–806. doi: 10.1074/jbc.M801553200. Epub 2008 Apr 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joels M. Steroid hormones and excitability in the mammalian brain. Front Neuroendocrinol. 1997;18:2–48. doi: 10.1006/frne.1996.0144. [DOI] [PubMed] [Google Scholar]
- Kahlert S, Nuedling S, van Eickels M, Vetter H, Meyer R, Grohe C. Estrogen receptor alpha rapidly activates the IGF-1 receptor pathway. J Biol Chem. 2000;275:18447–53. doi: 10.1074/jbc.M910345199. [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Moss RL, Dudley CA. Differential sensitivity of preoptic-septal neurons to microelectrophoresed estrogen during the estrous cycle. Brain Res. 1976;114:152–7. doi: 10.1016/0006-8993(76)91017-9. [DOI] [PubMed] [Google Scholar]
- Kuo J, Hamid N, Bondar G, Dewing P, Clarkson J, Micevych P. Sex differences in hypothalamic astrocyte response to estradiol stimulation. Biol. 2010;1:7. doi: 10.1186/2042-6410-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo J, Hamid N, Bondar G, Prossnitz ER, Micevych P. Membrane estrogen receptors stimulate intracellular calcium release and progesterone synthesis in hypothalamic astrocytes. J Neurosci. 2010;30:12950–7. doi: 10.1523/JNEUROSCI.1158-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo J, Hariri OR, Bondar G, Ogi J, Micevych P. Membrane estrogen receptor-alpha interacts with metabotropic glutamate receptor type 1a to mobilize intracellular calcium in hypothalamic astrocytes. Endocrinology. 2009;150:1369–76. doi: 10.1210/en.2008-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DY, Chai YG, Lee EB, Kim KW, Nah SY, Oh TH, Rhim H. 17Beta-estradiol inhibits high-voltage-activated calcium channel currents in rat sensory neurons via a non-genomic mechanism. Life Sci. 2002;70:2047–59. doi: 10.1016/s0024-3205(01)01534-x. [DOI] [PubMed] [Google Scholar]
- Lee FS, Chao MV. Activation of Trk neurotrophin receptors in the absence of neurotrophins. Proc Natl Acad Sci U S A. 2001;98:3555–60. doi: 10.1073/pnas.061020198. Epub 2001 Mar 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Campomanes CR, Sikat PT, Greenfield AT, Allen PB, McEwen BS. Estrogen induces phosphorylation of cyclic AMP response element binding (pCREB) in primary hippocampal cells in a time-dependent manner. Neuroscience. 2004;124:549–60. doi: 10.1016/j.neuroscience.2003.11.035. [DOI] [PubMed] [Google Scholar]
- Levin ER. Integration of the extranuclear and nuclear actions of estrogen. Mol Endocrinol. 2005;19:1951–9. doi: 10.1210/me.2004-0390. Epub 2005 Feb 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonze BE, Ginty DD. Function and regulation of CREB family transcription factors in the nervous system. Neuron. 2002;35:605–23. doi: 10.1016/s0896-6273(02)00828-0. [DOI] [PubMed] [Google Scholar]
- Luoma JI, Boulware MI, Mermelstein PG. Caveolin proteins and estrogen signaling in the brain. Mol Cell Endocrinol. 2008;290:8–13. doi: 10.1016/j.mce.2008.04.005. Epub 2008 Apr 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meitzen J, Luoma J, Stern C, Mermelsten PG. β1-adrenergic receptors activate two distinct signaling pathways in striatal neurons. Journal of Neurochemistry. 2011 doi: 10.1111/j.1471-4159.2010.07137.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mermelstein PG, Becker JB, Surmeier DJ. Estradiol reduces calcium currents in rat neostriatal neurons via a membrane receptor. J Neurosci. 1996;16:595–604. doi: 10.1523/JNEUROSCI.16-02-00595.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micevych P, Bondar G, Kuo J. Estrogen actions on neuroendocrine glia. Neuroendocrinology. 2010;91:211–22. doi: 10.1159/000289568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micevych P, Sinchak K. Synthesis and function of hypothalamic neuroprogesterone in reproduction. Endocrinology. 2008;149:2739–42. doi: 10.1210/en.2008-0011. Epub 2008 Feb 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micevych PE, Mermelstein PG. Membrane estrogen receptors acting through metabotropic glutamate receptors: an emerging mechanism of estrogen action in brain. Mol Neurobiol. 2008;38:66–77. doi: 10.1007/s12035-008-8034-z. Epub 2008 Aug 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niswender CM, Conn PJ. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol. 2010;50:295–322. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel HH, Murray F, Insel PA. Caveolae as organizers of pharmacologically relevant signal transduction molecules. Annu Rev Pharmacol Toxicol. 2008;48:359–91. doi: 10.1146/annurev.pharmtox.48.121506.124841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Kim JK, O’Mahony F, Lee EY, Luderer U, Levin ER. Developmental phenotype of a membrane only estrogen receptor alpha (MOER) mouse. J Biol Chem. 2009;284:3488–95. doi: 10.1074/jbc.M806249200. Epub 2008 Dec 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Bosch MA, Tobias SC, Grandy DK, Scanlan TS, Ronnekleiv OK, Kelly MJ. Rapid signaling of estrogen in hypothalamic neurons involves a novel G-protein-coupled estrogen receptor that activates protein kinase C. J Neurosci. 2003;23:9529–40. doi: 10.1523/JNEUROSCI.23-29-09529.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Bosch MA, Tobias SC, Krust A, Graham SM, Murphy SJ, Korach KS, Chambon P, Scanlan TS, Ronnekleiv OK, Kelly MJ. A G-protein-coupled estrogen receptor is involved in hypothalamic control of energy homeostasis. J Neurosci. 2006;26:5649–55. doi: 10.1523/JNEUROSCI.0327-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesada A, Micevych PE. Estrogen interacts with the IGF-1 system to protect nigrostriatal dopamine and maintain motoric behavior after 6-hydroxdopamine lesions. J Neurosci Res. 2004;75:107–16. doi: 10.1002/jnr.10833. [DOI] [PubMed] [Google Scholar]
- Ramirez CM, Gonzalez M, Diaz M, Alonso R, Ferrer I, Santpere G, Puig B, Meyer G, Marin R. VDAC and ERalpha interaction in caveolae from human cortex is altered in Alzheimer’s disease. Mol Cell Neurosci. 2009;42:172–83. doi: 10.1016/j.mcn.2009.07.001. Epub 2009 Jul 10. [DOI] [PubMed] [Google Scholar]
- Razandi M, Pedram A, Greene GL, Levin ER. Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: studies of ERalpha and ERbeta expressed in Chinese hamster ovary cells. Mol Endocrinol. 1999;13:307–19. doi: 10.1210/mend.13.2.0239. [DOI] [PubMed] [Google Scholar]
- Razandi M, Pedram A, Park ST, Levin ER. Proximal events in signaling by plasma membrane estrogen receptors. J Biol Chem. 2003;278:2701–12. doi: 10.1074/jbc.M205692200. Epub 2002 Nov 5. [DOI] [PubMed] [Google Scholar]
- Remage-Healey L, London SE, Schlinger BA. Birdsong and the neural production of steroids. J Chem Neuroanat. 2009;39:72–81. doi: 10.1016/j.jchemneu.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roepke TA, Qiu J, Bosch MA, Ronnekleiv OK, Kelly MJ. Cross-talk between membrane-initiated and nuclear-initiated oestrogen signalling in the hypothalamus. J Neuroendocrinol. 2009;21:263–70. doi: 10.1111/j.1365-2826.2009.01846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler SE, Bower MA, Maller JL. Studies of a plasma membrane steroid receptor in Xenopus oocytes using the synthetic progestin RU 486. J Steroid Biochem. 1985;22:419–26. doi: 10.1016/0022-4731(85)90448-0. [DOI] [PubMed] [Google Scholar]
- Sadler SE, Maller JL. Identification of a steroid receptor on the surface of Xenopus oocytes by photoaffinity labeling. J Biol Chem. 1982;257:355–61. [PubMed] [Google Scholar]
- Sinchak K, Mills RH, Tao L, LaPolt P, Lu JK, Micevych P. Estrogen induces de novo progesterone synthesis in astrocytes. Dev Neurosci. 2003;25:343–8. doi: 10.1159/000073511. [DOI] [PubMed] [Google Scholar]
- Song RX, Zhang Z, Chen Y, Bao Y, Santen RJ. Estrogen signaling via a linear pathway involving insulin-like growth factor I receptor, matrix metalloproteinases, and epidermal growth factor receptor to activate mitogen-activated protein kinase in MCF-7 breast cancer cells. Endocrinology. 2007;148:4091–101. doi: 10.1210/en.2007-0240. Epub 2007 May 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanova I, Horejsi V, Ansotegui IJ, Knapp W, Stockinger H. GPI-anchored cell-surface molecules complexed to protein tyrosine kinases. Science. 1991;254:1016–9. doi: 10.1126/science.1719635. [DOI] [PubMed] [Google Scholar]
- Stern CM, Mermelstein PG. Caveolin regulation of neuronal intracellular signaling. Cell. 2010;67:3785–95. doi: 10.1007/s00018-010-0447-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szego CM, Davis JS. Adenosine 3′,5′-monophosphate in rat uterus: acute elevation by estrogen. Proc Natl Acad Sci U S A. 1967;58:1711–8. doi: 10.1073/pnas.58.4.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasawa E, Noel SD, Keen KL. Rapid action of oestrogen in luteinising hormone-releasing hormone neurones: the role of GPR30. J Neuroendocrinol. 2009;21:316–21. doi: 10.1111/j.1365-2826.2009.01839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toran-Allerand CD, Guan X, MacLusky NJ, Horvath TL, Diano S, Singh M, Connolly ES, Jr, Nethrapalli IS, Tinnikov AA. ER-X: a novel, plasma membrane-associated, putative estrogen receptor that is regulated during development and after ischemic brain injury. J Neurosci. 2002;22:8391–401. doi: 10.1523/JNEUROSCI.22-19-08391.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudevan N, Pfaff DW. Membrane-initiated actions of estrogens in neuroendocrinology: emerging principles. Endocr Rev. 2007;28:1–19. doi: 10.1210/er.2005-0021. Epub 2006 Oct 3. [DOI] [PubMed] [Google Scholar]
- Wade CB, Dorsa DM. Estrogen activation of cyclic adenosine 5′-monophosphate response element-mediated transcription requires the extracellularly regulated kinase/mitogen-activated protein kinase pathway. Endocrinology. 2003;144:832–8. doi: 10.1210/en.2002-220899. [DOI] [PubMed] [Google Scholar]
- Warwick HK, Nahorski SR, Challiss RA. Group I metabotropic glutamate receptors, mGlu1a and mGlu5a, couple to cyclic AMP response element binding protein (CREB) through a common Ca2+ - and protein kinase C-dependent pathway. J Neurochem. 2005;93:232–45. doi: 10.1111/j.1471-4159.2005.03012.x. [DOI] [PubMed] [Google Scholar]
- Woolley CS. Acute effects of estrogen on neuronal physiology. Annu Rev Pharmacol Toxicol. 2007;47:657–80. doi: 10.1146/annurev.pharmtox.47.120505.105219. [DOI] [PubMed] [Google Scholar]
- Wright CL, McCarthy MM. Prostaglandin E2-induced masculinization of brain and behavior requires protein kinase A, AMPA/kainate, and metabotropic glutamate receptor signaling. J Neurosci. 2009;29:13274–82. doi: 10.1523/JNEUROSCI.3603-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyckoff MH, Chambliss KL, Mineo C, Yuhanna IS, Mendelsohn ME, Mumby SM, Shaul PW. Plasma membrane estrogen receptors are coupled to endothelial nitric-oxide synthase through Galpha(i) J Biol Chem. 2001;276:27071–6. doi: 10.1074/jbc.M100312200. Epub 2001 May 21. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Watters JJ, Dorsa DM. Estrogen rapidly induces the phosphorylation of the cAMP response element binding protein in rat brain. Endocrinology. 1996;137:2163–6. doi: 10.1210/endo.137.5.8612562. [DOI] [PubMed] [Google Scholar]