Abstract
Objective
Adverse changes in lipoprotein particle number and size are common with insulin resistance and are associated with increased cardiovascular risk. Comprehensive information regarding lipoprotein particle number and size, and how these parameters relate to body weight, insulin resistance and hyperandrogenemia is lacking in PCOS. We tested the hypothesis that PCOS is associated with atherogenic changes in lipoprotein profile independent of body weight and examined the role of insulin resistance and androgens in these atherogenic changes.
Design
Case-control study performed at Clinical Research Center at an Academic Medical Center in United States.
Patients and Measurements
Fasting Blood was obtained from 25 PCOS and 25 control women of similar age and BMI. Lipoprotein particle number and size was determined by nuclear magnetic resonance and compared between the groups.
Results
The mean BMI for both groups was less than 30 kg/m2 (P=0.33). Women with PCOS had an increase in VLDL particle number (P=0.005), LDL particle number (P=0.02) and a decrease in HDL size (P=0.04). LDL size was borderline decreased (P=0.09). These differences persisted after adjustment for ethnicity, alcohol and tobacco intake and exercise. In stepwise regression models, bioavailable testosterone was the only predictor of LDL cholesterol, triglyceride, VLDL and LDL particle number. SHBG was the only predictor of LDL and HDL size.
Conclusions
Independent of body weight, PCOS was associated with changes in lipoprotein profile that increases risk for cardiovascular disease. These changes were present in a mostly non-obese group of women and were more closely related to androgens than fasting insulin.
Key Terms: LDL particle number and size, HDL particle number and size, Dyslipidemia, Androgens, Insulin Resistance, Lipoprotein Particle Number and Size
Introduction
Dyslipidemia is one of the most frequent metabolic abnormalities in women with polycystic ovary syndrome (PCOS). Conventional lipid assays have revealed an increase in triglyceride and LDL cholesterol and a decrease in HDL cholesterol in women with PCOS compared to weight-matched control women.1–4 These abnormalities have usually been attributed to presence of insulin resistance in PCOS. The role of androgen elevation in the pathogenesis of dyslipidemia in PCOS has not been examined. Most experts agree that hyperandrogenism is the primary abnormality in PCOS5 and there are various pathways by which androgens can lead to development of dyslipidemia such as by exacerbating insulin resistance, altering body composition and even directly affecting adipose tissue.6
Not much is known about lipoprotein particle number and size in PCOS. Alterations in lipoprotein particle number and size have a stronger association with atherosclerosis than alterations in LDL and HDL cholesterol7, 8 and can not be detected on conventional lipid panel. For example, insulin resistance is characterized by high triglyceride and low HDL cholesterol with no alterations in LDL cholesterol on conventional lipid panel.9 However, studies that have employed euglycemic hyperinsulinemic clamp the gold standard for determination of insulin sensitivity have demonstrated that as insulin resistance becomes more severe, there is an increase in total number of LDL particles as well as a reduction in LDL particle size.9 This is important because an increase in LDL particle number is associated with significant increase in risk for coronary artery disease that is independent of LDL cholesterol concentration.10, 11
A few studies have revealed that PCOS is associated with a decrease in LDL size.12–14 However, these studies have utilized gradient gel electrophoresis, and do not provide information on LDL particle number and mostly lack simultaneous measurement for VLDL and HDL particle number and size. Nuclear magnetic resonance (NMR) is a validated method that allows for detailed simultaneous analyses of particle number and size for all three major lipoproteins. This technique has demonstrated alterations in all 3 lipoprotein subclass size and concentration in insulin resistant states such as type 2 diabetes. To our knowledge this technique has never been utilized in women with PCOS. In this study, we examined whether PCOS independent of body weight is associated with alterations in lipoprotein particle number and size that are associated with increased risk for cardiovascular disease. We also examined the relationship of body composition, androgens and insulin resistance, independent of overall body weight to the atherogenic lipoprotein profile in these women.
Subjects and Methods
Subjects
Fifty premenopausal women with PCOS (n=25) and without PCOS (n=25) were recruited for the study. Women with PCOS were recruited from endocrinology or reproductive endocrinology clinics at University of Illinois (n=10) or from local advertisements (n=15). Eligible women were between 18 to 40 years of age who were free of chronic disease including diabetes and hypertension and reported a history of menstrual irregularity and androgen excess such as hirsutism, acne or androgenic alopecia. The diagnosis of PCOS was confirmed based on the NIH criteria and defined by presence of oligomenorrhea (<6 menses per year) and biochemical hyperandrogenism based on elevated total or bioavailable testosterone levels (greater than two standard deviations above the mean value for the assay).15 Thyroid hormone abnormalities, hyperprolactinemia and non-classical congenital hyperplasia due to 21 hydroxylase deficiency were excluded by appropriate laboratory testing in all women with PCOS. All women with PCOS underwent a history and physical exam by a physician investigator that included detailed questions regarding their reproductive function. Women were considered to have oligomenorrhea if they had <6 menstrual cycles per year since menarche. We did not report hirsutism scores such as Ferriman Gallwey scoring system since it has been demonstrated that these scores have too much variation to be clinically useful and furthermore cosmetic removal of hair is common amongst women with hirsutism.16 However, all of the women with PCOS had to have elevated androgen levels to qualify for participation in the study. None of the women with PCOS had received any oral contraceptive, other forms of hormonal contraception or fertility treatments for at least 3 months prior to their participation nor had they received progesterone for at least one month prior to their participation in the study. None of the women had ever received any insulin sensitizing agents or metformin.
All of the control women were recruited from local advertisements. The control women were recruited to be of comparable weight to women with PCOS. The selection criteria for control women were: 1) regular 27–35 days menstrual cycles throughout their reproductive life, 2) no clinical or biochemical evidence of hyperandrogenism and 3) no history of hypertension or personal or family history of diabetes mellitus. All control women underwent a history and physical exam by a physician investigator that included detailed reproductive history as well as an assessment for hirsutism and other evidence for androgen excess. All control women had to have normal total and bioavailable testosterone levels and no clinical evidence for androgen excess. None of the control women have taken any hormonal contraception for at least 3 months prior to their participation in the study.
Women (both PCOS and control) were excluded from participation if they were pregnant or lactating, had any chronic disease including diabetes, hypertension, psychiatric disorder or any surgical procedure on their ovaries and uterus. None of the subjects were receiving any cholesterol, diabetes or anti-hypertension medications. The study was approved by the institutional review board at University of Illinois and all subjects signed written informed consent prior to their participation in the study.
Data Collection
All women were studied at the clinical research center at University of Illinois and underwent a history and physical exam by a physician investigator that included detailed menstrual and medical history as well as assessment for hirsutism and other signs of hyperandrogenism. Standardized forms were used to obtain medical history including information on exercise habits, alcohol and tobacco use. Height, weight and waist measurements were determined on all subjects. Blood pressure was determined in the seated position in the right arm as the average of 3 separate readings obtained 2 minutes apart after a 5 minute rest. A morning blood sample was obtained after an overnight fast from all subjects that included measurements of total and bioavailable testosterone, sex hormone binding globulin, lipid, lipoprotein profile and glucose and insulin levels. Metabolic syndrome was defined according to the updated National Cholesterol Education Program Adult Treatment Panel (NCEP ATP) III guidelines.17
Laboratory Methods
All laboratory evaluations with the exception of lipoprotein profile and insulin were performed at Quest Diagnostics. Total testosterone was measured by turbulent flow liquid chromatography mass spectrometry that has an assay sensitivity of 0.034 nmol/L and no cross reactivity with 30 testosterone related compounds. Bioavailable testosterone was calculated based on constants for the binding of testosterone to SHBG and albumin. SHBG was measured by extraction, chromatography and radioimmunoassay and albumin was measured by spectrophotometry. Total and HDL cholesterol and triglyceride levels were determined by spectrophotometry. The intra- and inter-assay coefficients of variation were 1.1 and 1.8% for total cholesterol respectively, 2.1 and 2.9% for HDL, 1.1 and 1.9% for triglyceride. The LDL cholesterol was calculated using the Freidewald equation.18 Plasma glucose was collected in a fluoride/oxalate tube and analyzed using spectrophotometry. The intra- and inter-assay coefficient of variation for this assay was 1.1 and 1.5%. Insulin was measured by a chemiluminescent sandwich immunoassay measuring to as low as 14 pmol/L. The inter- and intra-assay coefficient of variation for this assay was 4 and 5%. Lipoproteins were analyzed using NMR technology by LipoScience (Raleigh, NC). The intra- and inter-assay coefficient of variation were 1.4 and 3.1% for VLDL particle number, 2.4 and 2.1% for LDL particle number, 1.2 and 1.5% for HDL particle number, 0.8 and 1.8% for VLDL size, 0.5 and 0.4% for LDL size and 0.5 and 0.6% for HDL size.19
Statistical Analyses
We designed this study to detect at least a 25 percent difference in mean lipoprotein particle number for VLDL and LDL levels between PCOS and control women by a two-sample independent t-test. We expected at least 25% difference in VLDL and LDL particle numbers based on previous studies that have shown a difference of ~25% in LDL levels between non-obese PCOS and control women.2 A sample of 25 women in each group would provide the study with a statistical power of 80 percent to detect this difference with a two-sided significance level of 0.05. The homeostatic index of insulin resistance (HOMA IR) was calculated according to the following formula: [fasting glucose (mmol/L) × fasting insulin (μU/mL)] ÷ 22.5].20 Mean and standard deviations were used to summarize continuous data. Bioavailable testosterone, fasting insulin, HOMA IR and triglyceride were log- transformed (log10) prior to all analyses because of skewed distributions. All other variables were normally distributed based on histogram. Continuous variables were compared by independent t-test. Analyses were repeated after adjustment for ethnicity, smoking, alcohol and exercise history using general linear model. Categorical variables were compared using chi-square statistics. Correlations were performed using Pearson’s correlation coefficients. We then attempted to identify the independent predictors of lipids and lipoprotein parameters. In particular we were interested to examine the impact of insulin resistance and hyperandrogenism on triglyceride and LDL cholesterol as well as VLDL and LDL particle number and LDL and HDL size, parameters that differed between PCOS and control women. To accomplish this goal, stepwise multivariate linear regression models were used. The independent variables for these analyses included age, waist circumference, bioavailable testosterone, SHBG and fasting insulin levels. Bioavailable testosterone and fasting insulin levels were chosen as surrogate measures for hyperandrogenism and insulin resistance respectively. SHBG levels reflect a combined effect of both androgens and insulin resistance. Age and waist circumference were included as independent variables since both can impact lipids and lipoprotein profile. BMI was not chosen as an independent variable since the two groups were matched for BMI by design. In separate stepwise analyses, we included triglycerides and HDL cholesterol as additional predictors in the models for lipoprotein parameters since these measures impact lipoprotein particle number and size. Analyses were performed using the 18.0 PC package of SPSS statistical software (SPSS, Inc., Chicago, IL). A P ≤ 0.05 was considered significant.
Results
Baseline clinical and laboratory characteristics of women with PCOS and control women is summarized in Table 1. The mean age for women with PCOS was 26 ± 4 years that was similar to control women at 27 ± 6 years (P=0.24, Table 1). There was no difference in distribution of race/ethnicity between the two groups (P=0.53, Table 1). Very few women in the study smoke or drank alcohol in either group and there were no statistical differences between the two groups in regards to these two factors (Table 1). The mean BMI was 28.1 ± 4.0 kg/m2 for women with PCOS and 26.6 ± 6.4 kg/m2 for control women; differences that were not significant (P=0.33, Table 1). The mean waist circumference was 82 ± 9 cm for women with PCOS and 77 ± 13 cm for control women (p=0.20, Table 1). There was no difference in blood pressure between the two groups (Table 1). As expected women with PCOS had significantly higher bioavailable testosterone compared to control women (P<0.001, Table 1) and significantly lower SHBG compared to control women (P=0.003, Table 1). Fasting glucose levels were similar (P=0.55, Table 1) but fasting insulin levels (P=.02, Table 1) and HOMA IR (P=0.03, Table 1) were significantly higher in women with PCOS compared to control women. Only one woman in the PCOS group had metabolic syndrome and none of the women in the control group had metabolic syndrome based on the updated ATP III guidelines (data not shown).17
Table 1.
Baseline clinical and laboratory characteristics
| Variable | PCOS (n=25) | Control (n=25) | P-value |
|---|---|---|---|
| Age, y | 26 ± 4 | 27 ± 6 | 0.24 |
| Race/Ethnicity | 0.53 | ||
| Non-Hispanic White, (n) | 8 | 6 | |
| Non-Hispanic Black | 7 | 7 | |
| Hispanic | 5 | 3 | |
| Asian | 3 | 8 | |
| Other/mixed | 2 | 1 | |
| Smokers | 0.72 | ||
| Never | 22 | 22 | |
| Ex-smokers | 1 | 1 | |
| Current | 1 | 2 | |
| Alcohol | 0.58 | ||
| Never | 15 | 18 | |
| Social (≤2 drinks/week) | 5 | 5 | |
| Moderate (3 to 7 drinks/week) | 4 | 2 | |
| Heavy (>7 per week) | 0 | 0 | |
| Exercise History | |||
| ≥3 times/week | 10 | 10 | >0.99 |
| BMI, kg/m2 | 28.1 ± 4.0 | 26.6 ± 6.4 | 0.33 |
| Waist, cm | 82 ± 9 | 77 ± 13 | 0.20 |
| Blood Pressure, mmHg | |||
| Systolic | 112 ± 12 | 110 ± 9 | 0.70 |
| Diastolic | 70 ± 7 | 68 ± 9 | 0.33 |
| Bioavailable Testosterone, nmol/L | 0.69 ± 0.47 | 0.21 ± 0.06 | <0.001 |
| SHBG, nmol/L | 28 ± 13 | 42 ± 17 | 0.003 |
| Fasting Glucose, mmol/L | 4.4 ± 0.3 | 4.5 ± 0.4 | 0.55 |
| Fasting Insulin, pmol/L | 74 ± 78 | 35 ± 24 | 0.02 |
| HOMA IR | 2.1 ± 2.1 | 1.1 ± 0.8 | 0.03 |
| Cholesterol, mmol/L | 4.8 ± 0.9 | 4.3 ± 0.7 | 0.08 |
| LDL, mmol/L | 2.9 ± 0.9 | 2.4 ± 0.6 | 0.04 |
| HDL, mmol/L | 1.4 ± 0.3 | 1.6 ± 0.4 | 0.09 |
| Triglyceride, mmol/L | 1.2 ± 0.5 | 0.8 ± 0.2 | 0.002 |
Continuous data are presented as mean ± SD. Continuous variables are compared by independent t-test. Bioavailable testosterone, fasting insulin, HOMA IR and Triglycerides were log-transformed prior to the analyses. Categorical variables are compared using chi-square statistics.
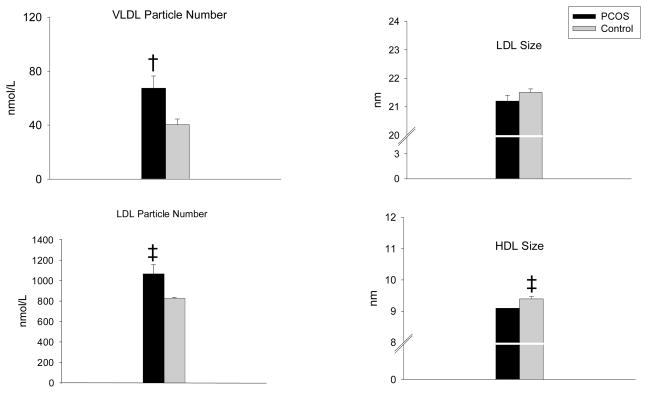
Women with PCOS had significantly higher LDL (P=0.04, Table 1) and triglyceride levels (P=0.002, Table 1) compared to control women. HDL cholesterol was borderline higher in control women compared to PCOS (P=0.09, Table 2). NMR analysis of lipoprotein particle number and size demonstrated that women with PCOS had significantly higher VLDL particle number (67 ± 40 nmol/L vs. 40 ± 22 nmol/L; P=0.005) (Figure 1) and LDL particle number (1066 ± 443 nmol/L vs. 829 ± 203 nmol/L; P=0.02) (Figure 1) compared to weight-matched control women. There was no difference in HDL particle number (30 ± 5 nmol/L vs. 30 ± 6 nmol/L; P=0.76) (data not shown). HDL size was significantly smaller (9.1 ± 0.4 nm vs. 9.4 ± 0.4 nm; P=0.04) (Figure 1) and LDL size was borderline smaller (21.2 ± 0.9 nm vs. 21.5 ± 0.6 nm; P=0.09) (Figure 1) in women with PCOS compared to control women. There was no difference in VLDL particle size between the two groups (46.2 ± 7.1 nm vs. 46.7 ± 9.3 nm; p=0.81) (data not shown). All of the above differences remained significant after adjustments for race/ethnicity, alcohol and tobacco intake and exercise history.
Table 2.
Pearson’s correlation coefficients between lipid, lipoprotein particle number and size and Age, anthropometric measurements, bioavailable testosterone, SHBG and fasting insulin in women with PCOS (n=25) and control women (n=25)
| Variable | LDL | Triglyceride | VLDL Particle Number | LDL Particle Number | LDL Size | HDL size |
|---|---|---|---|---|---|---|
| Age | 0.09 | −0.08 | −0.12 | 0.16 | −0.14 | 0.06 |
| BMI | 0.21 | 0.22 | −0.08 | 0.36‡ | −0.32‡ | −0.25 |
| Waist | 0.27 | 0.13 | 0.10 | 0.40† | −0.41† | −0.40† |
| Bioavailable Testosterone | 0.29‡ | 0.42† | 0.46† | 0.34‡ | −0.27 | −0.36‡ |
| SHBG | −0.21 | −0.40† | −0.32‡ | −0.40† | 0.49* | 0.61* |
| Fasting Insulin | 0.16 | 0.22 | 0.19 | 0.14 | −0.31‡ | −0.35‡ |
Bioavailable testosterone, triglyceride and fasting insulin were log-transformed prior to analyses.
P<0.001,
P<0.01,
P<0.05.
Figure 1.
Women with PCOS (n=25) had significantly higher VLDL particle number (†P=0.005) and LDL particle number (‡P=0.02) compared to control women. HDL size was significantly smaller (‡P=0.04) and LDL size was borderline smaller (P=0.09) in women with PCOS compared to control women. All variables were compared by independent t- tests. Women with PCOS, black bars; Control women, white bars.
Table 2 represents relations of the lipids and lipoprotein parameters that significantly differed between control and PCOS women (Table 2 and Figure 1) to age, BMI, waist circumference, bioavailable testosterone, SHBG and fasting insulin levels using Pearson’s correlation. These analyses included the entire group consisting of both control and women with PCOS. A significant negative association of SHBG with triglyceride (P<0.01, Table 2), VLDL (P< 0.05, Table 2) and LDL particle numbers (P<0.01, Table 2) was found. There was positive association of SHBG with LDL size (P<0.001, Table 2) and HDL size (P<0.001, Table 2). Bioavailable testosterone was positively correlated with LDL cholesterol (P<0.05, Table 2), triglyceride (P<0.01, Table 2), VLDL particle number (P<0.01, Table 2) and LDL particle number (P<0.05, Table 2) and negatively correlated with HDL size (P<0.05, Table 2). Fasting insulin was negatively correlated with LDL size (P<0.05, Table 2) and HDL size (P<0.05, Table 2). Waist circumference was more strongly correlated to LDL particle number and size compared to BMI (Table 2).
Stepwise multivariable regression was used to identify the predictors of LDL, triglyceride and lipoprotein subclass particle number and size that were different between the two groups. The predictors for each model included age, waist circumference, bioavailable testosterone, SHBG and fasting insulin. The results of these analyses are summarized in Table 3. Bioavailable testosterone was the only independent predictor of LDL (P=0.005, Table 3), log triglyceride (P=0.005), VLDL particle number (P<0.001, Table 3) and LDL particle number (P=0.003, Table 3). SHBG was the only independent predictor of LDL size (P<0.001, Table 3) and HDL size (P<0.001, Table 3). Stepwise multivariable models were repeated for VLDL and LDL particle number and LDL and HDL size with additional inclusion of triglycerides and HDL in the models. Bioavailable testosterone was still a predictor for VLDL particle number (P=0.006, data not shown) and SHBG was still a predictor for both LDL and HDL size (P=0.02 for both, data not shown). Additionally, triglyceride was an independent predictor of VLDL and LDL particle numbers (P<0.001 both, data not shown) and LDL and HDL size (P<0.001 and P=0.02 respectively, data not shown). HDL cholesterol was an independent predictor of HDL size (p=0.003, data not shown).
Table 3.
Predictors of lipids and lipoprotein particle number and size in women with PCOS (n=25) and control women (n=25), based on stepwise multivariable regression
| Variable | R2 Entire Model | Significant Predictors | Effect Size (CI) | P-values |
|---|---|---|---|---|
| LDL | 0.20 | Bioavailable Testosterone* | 0.09 (0.03) mmol/L | 0.005 |
| Log TG | 0.31 | Bioavailable Testosterone* | 0.02(0.006) log units | 0.005 |
| VLDL Particle Number | 0.43 | Bioavailable Testosterone* | 5 (1) nmol/L | <0.001 |
| LDL Particle Number | 0.21 | Bioavailable Testosterone* | 41 (13) nmol/L | 0.003 |
| LDL Size | 0.28 | SHBG† | 0.25 (0.07) nm | <0.001 |
| HDL Size | 0.38 | SHBG† | 0.16 (0.03) nm | <0.001 |
The models included age, waist circumference, bioavailable testosterone, SHBG and fasting insulin levels as predictors.
For every 0.1 nmol/L increase in bioavailable testosterone,
for every 10 nmol/L increase in SHBG.
Discussion
Our Results show that women with PCOS had significantly higher LDL and triglyceride levels on conventional lipid panel consistent with previous reports.1, 2 Our new findings indicate that women with PCOS had significantly higher VLDL and LDL particle number and significantly lower HDL size and borderline lower LDL size compared to weight-matched control women. Our findings persisted after adjustment for several factors such as ethnicity, exercise, smoking and alcohol use. To our knowledge this is the first report of alterations in all three major lipoprotein subclass in women with PCOS independent of body weight using a well validated technique. A decrease in HDL size has been associated with metabolic disorders21 and with increased risk for atherosclerosis.22–24 An increase in small dense LDL may carry greater risk for cardiovascular disease than an increase in LDL cholesterol.7, 8, 10, 25 These data indicate that the risk for cardiovascular disease in women with PCOS due to dyslipidemia maybe greater than presumed based on abnormalities on conventional lipid panel. Another important aspect of our study is that these adverse alterations in lipoprotein profile were present in a mostly non-obese group of women as the average BMI in our women was less than 30 kg/m2 in both groups.
Lower HDL cholesterol levels have been reported in women with PCOS1, 3, 4 but there is no information regarding composition of HDL particles in these women. Our study is the first to report a significant reduction in HDL particle size in women with PCOS compared to control women of similar weight. HDL cholesterol is thought to be atheroprotective primarily by its role in reverse cholesterol transport involving removal of cholesterol from macrophages in the vessel wall back to the liver.26 HDL particles are heterogeneous in regards to their ability to facilitate cholesterol transport with smaller HDL particles being less effective in reverse cholesterol transport and hence less atheroprotective.9, 21 Individuals with similar HDL cholesterol level can differ substantially in terms of HDL size distribution and particle concentration. Decreased HDL size has been associated with an increase in atherosclerotic measures such as carotid intima media thickness, a surrogate marker for cardiovascular disease.24 A decrease in HDL particle size in women with PCOS could contribute to higher risk for cardiovascular disease.
In our study we also demonstrate an increase in LDL particle number and a borderline decrease in LDL size. Previous studies in women with PCOS using techniques such as gel gradient electrophoresis have shown that LDL particle size is reduced in women with PCOS12, 14 but have not provided information regarding LDL particle number. Our findings indicate adverse alteration in both LDL particle number and size. In a prospective study of healthy middle-aged women, both LDL particle number and size measured by NMR technique were strong predictors of development of cardiovascular disease independent of LDL concentrations.27 This finding has been replicated in other epidemiologic studies that have included both men and women.7 Our study is also the first to report an increase in VLDL particle number in women with PCOS independent of body weight. Again this abnormality has been strongly associated with increased risk for cardiovascular disease.28
Another novel aspect of our study relates to examination of the association of androgens, body composition and insulin resistance to lipids and lipoprotein abnormalities in PCOS. Traditionally, the lipid abnormalities in PCOS have been attributed to the presence of insulin resistance.2, 3, 12 However, our findings suggest that androgens may play a more significant role in pathogenesis of lipid abnormalities in PCOS. In our study, bioavailable testosterone was the only independent predictor of the increase in LDL and triglyceride levels as well as the increase in VLDL and LDL particle number. Fasting insulin levels, BMI, waist circumference and SHBG were not independent predictors of any of the above parameters. SHBG was an independent predictor of LDL and HDL size indicating perhaps the combined effect of insulin resistance and hyperandrogenism as manifested by the reduction in SHBG levels in women with PCOS.
The mechanism by which hyperandrogenism may contribute to development of lipid abnormalities in PCOS is not clear. Since our study is cross-sectional in nature, it cannot determine cause and effect in these associations or provide any possible mechanisms. Hyperandrogenism may lead to the abnormalities in lipoprotein profile by working directly at the liver or it may alter body composition by favoring central adiposity. We have shown that in subjects with type 2 diabetes, the lipoprotein abnormalities such as increase in VLDL and LDL particle number and reduction in LDL and HDL size correlate best with visceral fat rather than overall adiposity or waist circumference.29 An increase in visceral fat depot has been demonstrated by administration of androgens in female to male transsexuals30 as well as after exogenous administration of androgens to postmenopausal women.31 It is possible that prolonged exposure to higher androgen levels in women with PCOS can lead to an increase in visceral fat depot and subsequently to the lipoprotein abnormalities noted in our study.
Similar to previous studies, we also noted that women with PCOS are more insulin resistant.32 Our population consisted of mostly non-obese women, our findings therefore reaffirm that PCOS is associated with insulin resistance independent of body weight. In summary, our results indicate that in addition to lipid abnormalities commonly observed on conventional lipid panel, PCOS is associated with adverse alterations in all 3 major lipoprotein subtypes independent of obesity. These abnormalities were present in a mostly non-obese group of women and correlated strongly with hyperandrogenism. These abnormalities could contribute to increased risk for cardiovascular disease in these women.
Acknowledgments
This project was supported by the following National Institutes of Health grants: K23 DK080988-01A1 to Susan Sam and UL1RR029879 to CTSA at University of Illinois.
We thank all the women for participating in this study. We also thank the nursing staff at University of Illinois Clinical Research Center for their invaluable help with all the studies.
Footnotes
Disclosure Summery: None of the authors report any conflict of interest.
References
- 1.Talbott E, Clerici A, Berga SL, Kuller L, Guzick D, Detre K, Daniels T, Engberg RA. Adverse lipid and coronary heart disease risk profiles in young women with polycystic ovary syndrome: results of a case-control study. Journal of Clinical Epidemiology. 1998;51:415–422. doi: 10.1016/s0895-4356(98)00010-9. [DOI] [PubMed] [Google Scholar]
- 2.Legro RS, Kunselman AR, Dunaif A. Prevalence and predictors of dyslipidemia in women with polycystic ovary syndrome. The American Journal of Medicine. 2001;111:607–613. doi: 10.1016/s0002-9343(01)00948-2. [DOI] [PubMed] [Google Scholar]
- 3.Conway GS, Agrawal R, Betteridge DJ, Jacobs HS. Risk factors for coronary artery disease in lean and obese women with the polycystic ovary syndrome. Clinical Endocrinology. 1992;37:119–125. doi: 10.1111/j.1365-2265.1992.tb02295.x. [DOI] [PubMed] [Google Scholar]
- 4.Wild RA, Painter PC, Coulson PB, Carruth KB, Ranney GB. Lipoprotein lipid concentrations and cardiovascular risk in women with polycystic ovary syndrome. The Journal of Clinical Endocrinology and Metabolism. 1985;61:946–951. doi: 10.1210/jcem-61-5-946. [DOI] [PubMed] [Google Scholar]
- 5.Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, Janssen OE, Legro RS, Norman RJ, Taylor AE, Witchel SF. Positions statement: criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: an Androgen Excess Society guideline. The Journal of Clinical Endocrinology and Metabolism. 2006;91:4237–4245. doi: 10.1210/jc.2006-0178. [DOI] [PubMed] [Google Scholar]
- 6.Diamanti-Kandarakis E, Papavassiliou AG, Kandarakis SA, Chrousos GP. Pathophysiology and types of dyslipidemia in PCOS. Trends in Endocrinology and Metabolism. 2007;18:280–285. doi: 10.1016/j.tem.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Gardner CD, Fortmann SP, Krauss RM. Association of small low-density lipoprotein particles with the incidence of coronary artery disease in men and women. The Journal of American Medical Association. 1996;276:875–881. [PubMed] [Google Scholar]
- 8.Lamarche B, Tchernof A, Moorjani S, Cantin B, Dagenais GR, Lupien PJ, Despres JP. Small, dense low-density lipoprotein particles as a predictor of the risk of ischemic heart disease in men. Prospective results from the Quebec Cardiovascular Study. Circulation. 1997;95:69–75. doi: 10.1161/01.cir.95.1.69. [DOI] [PubMed] [Google Scholar]
- 9.Garvey WT, Kwon S, Zheng D, Shaughnessy S, Wallace P, Hutto A, Pugh K, Jenkins AJ, Klein RL, Liao Y. Effects of insulin resistance and type 2 diabetes on lipoprotein subclass particle size and concentration determined by nuclear magnetic resonance. Diabetes. 2003;52:453–462. doi: 10.2337/diabetes.52.2.453. [DOI] [PubMed] [Google Scholar]
- 10.Rizzo M, Pernice V, Frasheri A, Di Lorenzo G, Rini GB, Spinas GA, Berneis K. Small, dense low-density lipoproteins (LDL) are predictors of cardio- and cerebro-vascular events in subjects with the metabolic syndrome. Clinical Endocrinology. 2009;70:870–875. doi: 10.1111/j.1365-2265.2008.03407.x. [DOI] [PubMed] [Google Scholar]
- 11.Griffin BA, Freeman DJ, Tait GW, Thomson J, Caslake MJ, Packard CJ, Shepherd J. Role of plasma triglyceride in the regulation of plasma low density lipoprotein (LDL) subfractions: relative contribution of small, dense LDL to coronary heart disease risk. Atherosclerosis. 1994;106:241–253. doi: 10.1016/0021-9150(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 12.Pirwany IR, Fleming R, Greer IA, Packard CJ, Sattar N. Lipids and lipoprotein subfractions in women with PCOS: relationship to metabolic and endocrine parameters. Clinical Endocrinology. 2001;54:447–453. doi: 10.1046/j.1365-2265.2001.01228.x. [DOI] [PubMed] [Google Scholar]
- 13.Dejager S, Pichard C, Giral P, Bruckert E, Federspield MC, Beucler I, Turpin G. Smaller LDL particle size in women with polycystic ovary syndrome compared to controls. Clinical Endocrinology. 2001;54:455–462. doi: 10.1046/j.1365-2265.2001.01245.x. [DOI] [PubMed] [Google Scholar]
- 14.Berneis K, Rizzo M, Lazzarini V, Fruzzetti F, Carmina E. Atherogenic lipoprotein phenotype and low-density lipoproteins size and subclasses in women with polycystic ovary syndrome. The Journal of Clinical Endocrinology and Metabolism. 2007;92:186–189. doi: 10.1210/jc.2006-1705. [DOI] [PubMed] [Google Scholar]
- 15.Zawadzki J, Dunaif A. Diagnostic criteria for polycystic ovary syndrome towards a rational approach. In: Dunaif A, Givens JR, Haseltine FP, Merriam GR, editors. Polycystic ovary syndrome. Blackwell Scientific Publications; Boston: 1992. pp. 377–384.pp. 377–384. [Google Scholar]
- 16.Wild RA, Vesely S, Beebe L, Whitsett T, Owen W. Ferriman Gallwey self-scoring I: performance assessment in women with polycystic ovary syndrome. The Journal of Clinical Endocrinology and Metabolism. 2005;90:4112–4114. doi: 10.1210/jc.2004-2243. [DOI] [PubMed] [Google Scholar]
- 17.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr, Spertus JA, Costa F. Diagnosis and management of the metabolic syndrome. An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Executive summary. Cardiology in Review. 2005;13:322–327. [PubMed] [Google Scholar]
- 18.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clinical Chemistry. 1972;18:499–502. [PubMed] [Google Scholar]
- 19.Jeyarajah EJ, Cromwell WC, Otvos JD. Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clinics in Laboratory Medicine. 2006;26:847–870. doi: 10.1016/j.cll.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 20.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 21.El Harchaoui K, Arsenault BJ, Franssen R, Despres JP, Hovingh GK, Stroes ES, Otvos JD, Wareham NJ, Kastelein JJ, Khaw KT, Boekholdt SM. High-density lipoprotein particle size and concentration and coronary risk. Annals of Internal Medicine. 2009;150:84–93. doi: 10.7326/0003-4819-150-2-200901200-00006. [DOI] [PubMed] [Google Scholar]
- 22.Cheung MC, Brown BG, Wolf AC, Albers JJ. Altered particle size distribution of apolipoprotein A-I-containing lipoproteins in subjects with coronary artery disease. Journal of Lipid Research. 1991;32:383–394. [PubMed] [Google Scholar]
- 23.Freedman DS, Otvos JD, Jeyarajah EJ, Barboriak JJ, Anderson AJ, Walker JA. Relation of lipoprotein subclasses as measured by proton nuclear magnetic resonance spectroscopy to coronary artery disease. Arteriosclerosis, Thrombosis, and Vascular Biology. 1998;18:1046–1053. doi: 10.1161/01.atv.18.7.1046. [DOI] [PubMed] [Google Scholar]
- 24.Watanabe H, Soderlund S, Soro-Paavonen A, Hiukka A, Leinonen E, Alagona C, Salonen R, Tuomainen TP, Ehnholm C, Jauhiainen M, Taskinen MR. Decreased high-density lipoprotein (HDL) particle size, prebeta-, and large HDL subspecies concentration in Finnish low-HDL families: relationship with intima-media thickness. Arteriosclerosis, Thrombosis, and Vascular Biology. 2006;26:897–902. doi: 10.1161/01.ATV.0000209577.04246.c0. [DOI] [PubMed] [Google Scholar]
- 25.Koba S, Yokota Y, Hirano T, Ito Y, Ban Y, Tsunoda F, Sato T, Shoji M, Suzuki H, Geshi E, Kobayashi Y, Katagiri T. Small LDL-cholesterol is superior to LDL-cholesterol for determining severe coronary atherosclerosis. Journal of Atherosclerosis and Thrombosis. 2008;15:250–260. doi: 10.5551/jat.e572. [DOI] [PubMed] [Google Scholar]
- 26.Rader DJ. Molecular regulation of HDL metabolism and function: implications for novel therapies. The Journal of Clinical Investigation. 2006;116:3090–3100. doi: 10.1172/JCI30163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blake GJ, Otvos JD, Rifai N, Ridker PM. Low-density lipoprotein particle concentration and size as determined by nuclear magnetic resonance spectroscopy as predictors of cardiovascular disease in women. Circulation. 2002;106:1930–1937. doi: 10.1161/01.cir.0000033222.75187.b9. [DOI] [PubMed] [Google Scholar]
- 28.Kuller L, Arnold A, Tracy R, Otvos J, Burke G, Psaty B, Siscovick D, Freedman DS, Kronmal R. Nuclear magnetic resonance spectroscopy of lipoproteins and risk of coronary heart disease in the cardiovascular health study. Arteriosclerosis, Thrombosis, and Vascular Biology. 2002;22:1175–1180. doi: 10.1161/01.atv.0000022015.97341.3a. [DOI] [PubMed] [Google Scholar]
- 29.Sam S, Haffner S, Davidson MH, D’Agostino RB, Feinstein S, Kondos G, Perez A, Mazzone T. Relationship of Abdominal Visceral and Subcutaneous Adipose Tissue to Lipoprotein Particle Number and Size in Type 2 Diabetes. Diabetes. 2008;57:2022–2027. doi: 10.2337/db08-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elbers JM, Asscheman H, Seidell JC, Megens JA, Gooren LJ. Long-term testosterone administration increases visceral fat in female to male transsexuals. The Journal of Clinical Endocrinology and Metabolism. 1997;82:2044–2047. doi: 10.1210/jcem.82.7.4078. [DOI] [PubMed] [Google Scholar]
- 31.Lovejoy JC, Bray GA, Bourgeois MO, Macchiavelli R, Rood JC, Greeson C, Partington C. Exogenous androgens influence body composition and regional body fat distribution in obese postmenopausal women--a clinical research center study. The Journal of Clinical Endocrinology and Metabolism. 1996;81:2198–2203. doi: 10.1210/jcem.81.6.8964851. [DOI] [PubMed] [Google Scholar]
- 32.Ehrmann DA. Polycystic ovary syndrome. The New England Journal of Medicine. 2005;352:1223–1236. doi: 10.1056/NEJMra041536. [DOI] [PubMed] [Google Scholar]