Abstract
Study Design
Biomechanics of human intervertebral discs before and after nucleotomy.
Objective
To noninvasively quantify the effect of nucleotomy on internal strains under axial compression in flexion, neutral, and extension positions, and to determine whether the change in strains depended on degeneration.
Summary of Background Data
Herniation and discectomy may accelerate the progression of disc degeneration. Removal of NP tissue has resulted in altered disc mechanics in vitro, including in a decrease in internal pressure and an increase in the deformations at physiologically relevant strains. We recently presented a technique to quantify internal disc strains using magnetic resonance imaging.
Methods
Degeneration was quantitatively assessed by the T1ρ relaxation in the nucleus pulposus (NP). Samples were prepared from human levels L3-L4 and/or L4-L5. A 1000N compressive load was applied while in the MR scanner. Nucleotomy was performed by removing 2g of NP through the posterior-lateral AF. The discs were rehydrated, reimaged and retested. The analyzed parameters include axial deformation, AF radial bulge and strains.
Results
The axial deformation was more compressive following nucleotomy. In the neutral position, the axial deformation following nucleotomy correlated with degeneration (as quantified by T1ρ in the NP), with minimal alteration in nondegenerated discs. Nucleotomy altered the radial displacements and strains in the neutral position, such that the inner AF radial bulge decreased and the radial strains were more tensile in the lateral AF and less tensile in the posterior AF. In the bending loading positions the radial strains were not affected by nucleotomy.
Conclusions
Nucleotomy alters the internal radial and axial AF strains in the neutral position, which may leave the AF vulnerable to damage and microfractures. In bending, the effects of nucleotomy were minimal; likely due to more of the applied load being directed over the AF. Some of the nucleotomy effects are modulated by degeneration, where the mechanical effect of nucleotomy was magnified in degenerated discs and may further induce mechanical damage and degeneration.
Introduction
Herniation is the expulsion of nucleus pulposus (NP) material through a tear in the annulus fibrosus (AF), and discectomy is a surgical procedure to remove NP fragments following herniation. Discectomy has increased steadily over the past decade.1 Short-term benefits of discectomy have been shown, but long-term benefits and reherniation rates remain controversial.2–4 Furthermore, discectomy may accelerate the progression of disc degeneration by damaging the annulus fibrosus (AF), decreasing the NP pressure, decreasing the disc height, impairing the disc’s ability to rehydrate, and increasing the AF stresses and strains.1,5–10 Nucleotomy, removing a clinically relevant amount of NP material through an AF incision in a laboratory setting, has shown altered disc mechanics, including in a decrease in the internal pressure and endplate strain and an increase in the neutral zone.11–19 The radial bulging of the inner AF changes from outward in intact discs to inward following nucleotomy.16,17,20 In spite of these observations, the internal AF strains following nucleotomy remain unknown because the internal tissue deformation could not be quantified without altering the tissue by placing markers.16,17 Furthermore, it is unknown whether the effect of nucleotomy depends on degenerative state of the disc.
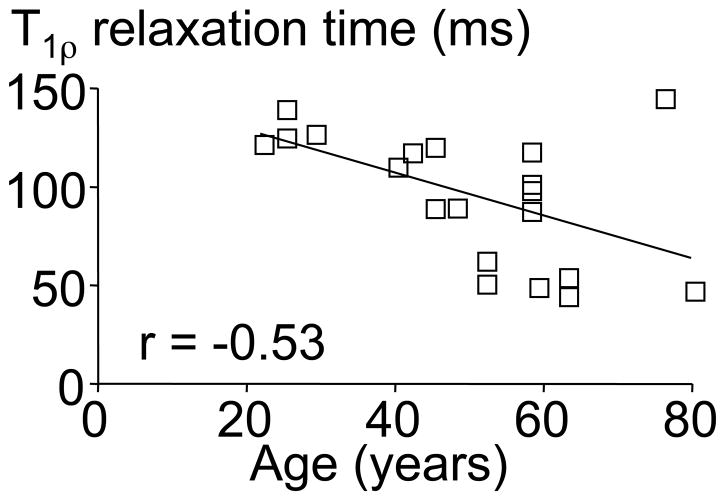
We recently presented a technique to quantify internal disc strains under load by using magnetic resonance imaging (MRI) and image correllation.21,22 This technique was applied to quantify the effect of degeneration on the internal disc deformations and strains under axial compression.22 The AF of degenerated discs had higher tensile radial and compressive axial strains, which was likely due to decreased NP pressure placing more of the applied load directly onto the AF. This analysis was made possible by using MRI-based T1ρ relaxation time as a quantitative measure of degeneration to perform statistical correlations with measured parameters.22,23 The T1ρ relaxation time has a strong positive correlation to the NP proteoglycan content (r = 0.70) and pressure, and a negative correlation to the MRI-based Pfirrmann grade (r = −0.75) and age (r = −0.53; Figure 1).23–26 Although T1ρ relaxation time does not incorporate other known degenerative changes (e.g., disc height narrowing and bulging), it does provide a continuous measure of degeneration without intra- and inter-observer variability, which provides an important advantage over the more traditional grading methods for statistical analyses.
Figure 1.
The nucleus pulposus T1ρ relaxation time was significantly correlated with the donor age (p<0.05). The equation for the correlation line is T1ρ = −1.1*years + 150.
We hypothesize that partial NP removal will increase AF internal strains and that the effect of nucleotomy will be greater in degenerated discs. The objectives of this study were to noninvasively quantify the effect of nucleotomy on human AF strains away from the AF incision site under axial compression in three loading positions (flexion, neutral, extension) and to determine whether the effect of nucleotomy depended on the state of degeneration.
Materials and Methods
Thirteen human spine sections were obtained from an IRB approved tissue source (NDRI, Philadelphia, Pennsylvania). A series of T1ρ-weighted images were acquired to determine the T1ρ relaxation time as a quantitative measure of degeneration.22,23,25 Bone-disc-bone segments were prepared by removing the muscles and facet joints from L3–L4 and/or L4–L5 levels (n = 19; 22– 76 years old; Figure 1) and potted in polymethylmethacrylate bone cement such that the mid-disc height transverse plane was horizontal. The samples were wrapped in gauze, hydrated in a refrigerated phosphate buffered saline (PBS) bath, and allowed to equilibrate to room temperature prior to testing. The PBS-soaked gauze was kept wrapped around the disc during imaging to prevent dehydration.
A loading device was constructed of non-magnetic materials to apply axial compressive loads to the disc while in a 3T MR scanner (Trio, Siemens Medical Solutions), as previously described.21 Samples were constrained in the fixture to prevent shear, bending or torsion. Each disc was tested under axial compression in flexion, neutral, and extension positions (applied in a random order) before and after nucleotomy. (Note that the data for the intact condition have been previously published.22) The disc recovered unloaded for at least 8 hours in a refrigerated PBS bath between tests. Flexion and extension loading positions were achieved by placing a 5° wedge into the loading device for both the undeformed and deformed image, thus this condition represents to effects of lifting from a bending posture, not bending from a neutral position. A high-resolution T2-weighted turbo spin-echo sequence was used to acquire mid-sagittal MR images with a custom-built 80 mm square surface coil (512 × 512 matrix size, TR = 3000 ms, TE = 113 ms, slice thickness = 3 mm, 10 averages, total scan time 12.5 min, SNR 13; resolution = 0.234 mm/pixel). A mid-coronal image was also acquired when loading in the neutral positionto calculate strains in the lateral AF.
The disc was preconditioned with 5 cycles from 0 to 20N and a nominal 20N preload applied for 5 minutes to ensure contact with the loading platen. A reference (undeformed) image was acquired while the disc was under the nominal 20N compressive load. A 1000N compressive load was applied rapidly (~3 sec) and maintained for 20 minutes, to allow for creep deformation, before repeating the imaging sequence to acquire a deformed image.
Following imaging and mechanical loading of the intact disc, each disc was rehydrated for 8 hours. A nucleotomy was performed by making a cruciform incision with a #11 scalpel blade through the posterior-lateral AF consistent with clinical procedures.27 Preliminary studies showed no significant effect of the annulotomy alone (i.e., a cruciform incision through the AF without removal of NP tissue) on the strains. NP material (approximately 20% of NP volume)21 was removed with pituitary ronguers from the posterior-lateral and central regions of the NP. Two grams of tissue was removed, based on the amount of removed NP material reported by Fountas et al.2 The disc was rehydrated and loaded as described above.
A custom program was used to calculate the average disc height of the reference and deformed images (Matlab Inc. 7.0.1).21,22,28 The axial deformation was calculated as the change in disc height between the reference and deformed image, normalized by the undeformed height. The reference and deformed MR images were used to calculate the internal tissue displacements and 2D Lagrangian strains using a commercial texture correlation algorithm (resolution = 1/20th of a pixel = 0.01 mm, Vic 2D, Correlated Solutions, Inc.). Two-dimensional strain analysis was performed in three disc regions: the anterior AF, posterior AF, and the left and right lateral AF (note that all of these regions are away from the posterior-lateral AF incision site). The Cartesian coordinate system was defined by the spine geometry such that the x-direction corresponded to radial strains oriented across lamellae and the y-direction corresponded to axial strains along the spinal axis (Figure 2A). Strains were reported as a percent and the shear strains were reported as an absolute value. The radial bulge for the inner and outer AF was calculated as the average radial displacement of the node at the mid-disc height and an outward radial bulge from the NP was defined as positive.22
Figure 2.
Midsagittal magnetic resonance images after nucleotomy for a representative A) nondegenerate and B) degenerated disc. In most discs the NP swelled to fill the void caused by nucleotomy, however in four of the degenerated discs the void remained. NP = nucleus pulposus, AAF = anterior annulus fibrosus, PAF = posterior annulus fibrosus, VB = vertebral body.
Since the data did not follow a Gaussian distribution, nonparametric statistical analyses were used, and the data are presented as median (interquartile range). To evaluate the effect of nucleotomy, a Wilcoxon matched pairs test was performed comparing parameters from intact and following nucleotomy. The analyzed parameters include the initial disc height, the axial deformation (actual and normalized to initial disc height), the inner and outer radial bulge, and the average strains (radial, axial, and shear components) in each AF region. Evaluation was undertaken to determine if the impact of nucleotomy was correlated with degeneration. A change in the parameter before and after nucleotomy was calculated (Δx = xnucleotomy – xintact, where ‘x’ represents the parameter), and a Spearman’s correlation was performed with the T1ρ relaxation time. Significance was set at p ≤ 0.05.
Results
The wet weight of the removed NP material was 1.96 g (interquartile range = 1.79 to 2.01g). In 15 of 19 samples, the remaining NP material is presumed to have swelled and redistributed to fill the void from the nucleotomy (Figure 2A). However, a void was observed in the NP of four degenerated discs (T1ρ relaxation times = 46 – 64 msec; Figure 2B). Therefore, NP strain analysis was not performed in this study. The initial disc height was 11.8 mm (9.9 to 12.5 mm) and was not affected by nucleotomy (p > 0.7). The qualitative strain pattern following nucleotomy was similar to the intact case, such that axial strains had horizontal bands of tension and compression (Figure 3 – Left), radial strains had vertical bands (Figure 3 – Right), and shear strains were highest at the endplates.
Figure 3.

Representative axial (left) and radial (right) strain maps of an intact disc and the same disc after nucleotomy. The three regions of interests, from left to right, are the anterior AF, NP and the posterior AF. While the NP strain map is shown for this representative sample, the values were not included in the statistical analysis, due to four samples having a large void in the NP (see Figure 2). The strain patterns are similar but magnified after nucleotomy compared to intact. Note that the scale bar and 0% strain position is different for the axial and radial strain component.
Neutral loading position
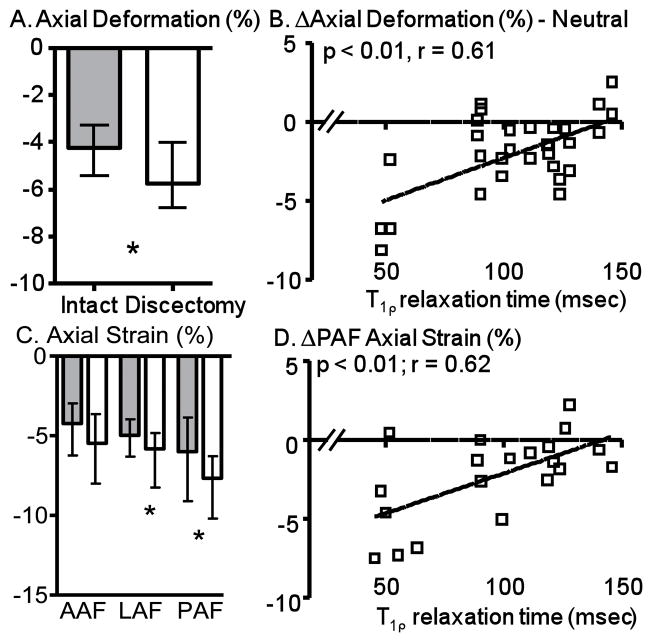
In the neutral position, the axial displacement under compression increased from 0.63 mm (0.46 to 0.84 mm) for intact discs to 0.78 mm (0.63 to 1.11 mm) following nucleotomy (p < 0.01). Similarly, the axial deformation (axial displacement normalized by the initial disc height) was more compressive following nucleotomy (p < 0.01; Figure 4A). The effect of nucleotomy on the axial deformation correlated with degeneration (p = 0.04, r = 0.61, Figure 4B), where the effect of nucleotomy was minimal in nondegenerated discs (e.g., at T1ρ = 150 msec).
Figure 4.
A) The axial deformation was more compressive following nucleotomy compared to intact. B) The effect of nucleotomy correlated with degeneration for axial strain. Note that a larger T1ρ relaxation time (e.g., at 150 ms) represents a less degenerated disc and a smaller T1ρ (at 50 ms) represents a more degenerated disc. C) Average axial strain was more compressive following nucleotomy for LAF and PAF locations. D) The effect of nucleotomy correlated with degeneration for axial strain in the posterior AF. *p < 0.05. Grey bars = intact, White bars = nucleotomy, AAF = anterior AF, LAF = lateral AF, PAF = posterior AF.
As one might expect, the effect of nucleotomy on the AF axial strain was similar to the disc axial deformation. The AF axial strain was more compressive with nucleotomy, reaching significance in the lateral and posterior AF (Figure 4C). The anterior AF axial strain followed a similar pattern, but was not significant (p = 0.06; Figure 4C). Similar to the axial deformation, the effect of nucleotomy on the AF axial strain was dependent on degeneration in the lateral and posterior AF (Figure 4D), where nondegenerate discs were relatively unaltered (Figure 4D).
Following nucleotomy, the inner AF radial bulge in the mid-coronal plane was 65% less outward (i.e. less bulging; p < 0.01; Figure 5A). The outward radial bulge of the inner AF in the mid-sagittal plane also decreased with nucleotomy; however, the effect was not significant (p = 0.09; Figure 5A). The decrease in the inner AF radial bulge followed nucleotomy was not dependent on degeneration in either the mid-coronal or mid-sagittal plane (p > 0.4). Therefore, the inner AF radial bulge decreased for both nondegenerate and degenerate discs. The outer AF radial bulge was 0.50 (0.41 to 0.60) mm (pooled for mid-coronal and mid-sagittal) and was not affected by nucleotomy (p > 0.6).
Figure 5.
A) Inner AF radial bulge decreased in the mid-coronal and mid-sagittal plane following nucleotomy compared to intact. B) Average radial strain following nucleotomy increased in the LAF and decreased in the PAF. C) The effect of nucleotomy on the radial strain in the posterior AF did not correlate with degeneration. *p < 0.05. Grey bars = intact, White bars = nucleotomy, AAF = anterior AF, LAF = lateral AF, PAF = posterior AF.
The radial strain increased in the lateral AF (p < 0.01), decreased in the posterior AF (p < 0.01), and was not significantly altered in the anterior AF following nucleotomy (p = 0.6; Figure 5B). The effect of nucleotomy on the radial strain was not correlated with degeneration in any AF region (p > 0.1). Therefore, the observed change in the lateral AF and posterior AF radial strains were similar across all states of degeneration (Figure 5C – shown for posterior AF).
Flexion and Extension Loading Positions
Like the neutral loading position, in the flexion and extension loading position, the axial deformation was more compressive following nucleotomy (p ≤ 0.02; Figure 6A). In contrast to the neutral loading position, the effect of nucleotomy was not dependent on degeneration in flexion and extension (p ≥ 0.2). Therefore, the increase in axial deformation was similar for both nondegenerate and degenerate discs. In flexion and extension, the axial strain in the anterior and posterior AF increased with nucleotomy (Figure 6B), and the effects of nucleotomy were not correlated with degeneration (p > 0.1). One exception to these findings was the posterior AF axial strain in extension, which was not significantly altered with nucleotomy (Figure 6B) and was correlated with degeneration. The posterior AF axial strains in extension were more compressive in degenerated discs (T1ρ ~ 50 msec) following nucletomy (p = 0.04, r = 0.46).
Figure 6.
A) The axial deformation was more compressive following nucleotomy for flexion and extension loading positions. B) Axial strain increased following nucleotomy for flexion and extension loading positions except for the PAF in extension. * p ≤ 0.05. Grey bars = intact, White bars = nucleotomy, AAF = anterior AF, PAF = posterior AF.
Consistent with neutral loading, the inner and outer AF radial bulge in flexion and extension positions were not affected by nucleotomy (p > 0.1). In the flexion position, the effect of nucleotomy on the inner AF radial bulge was dependent on degeneration (p = 0.01, Figure 7A), where the outward radial bulge decreased more in degenerated discs. Overall, the AF radial strains were not significantly altered by nucleotomy in flexion or extension (Figure 7B). Following nucleotomy, in the flexion position the anterior AF radial strain was dependent on degeneration and was more tensile in degenerated discs (p = 0.02, r = −0.52). No other correlations were observed with degeneration (p > 0.1).
Figure 7.
A) Effect of nucleotomy on the inner AF radial bulge correlated with degeneration for the flexion loading position. B) No significant effects of nucleotomy were observed for radial strain in the flexion and extension loading position. Grey bars = intact, White bars = nucleotomy, AAF = anterior AF, PAF = posterior AF.
The shear strain was 3.0% (2.1 to 4.1%) across all AF regions and loading positions, and was not altered by nucleotomy in the anterior AF or lateral AF (p ≥ 0.2). The posterior AF shear strain in the neutral position decreased from 3.5% in intact discs to 2.6% with nucleotomy (p = 0.03) and was not altered in bending.
Discussion
In this study, the effect of nucleotomy on internal disc displacement and AF strain in axial compression was investigated, as was the dependence of these parameters on the degree of degeneration. While several studies have measured deformation and NP pressure in response to axial compression in models of nucleotomy,10,13,29 internal strains were neither measured nor correlated with degeneration. The use of T1ρ relaxation time, which has a strong correlation to the NP proteoglycan content and the MRI-based Pfirrmann grade,22,23,25 as a quantitative measure of disc degeneration, represents a significant advancement in the analysis of the role of degeneration on disc mechanics (although T1ρ does not incorporate other degeneration effects such as los of disc height and bulging). The axial deformation prior to nucleotomy was between 4 and 6% and increased to a range of 6 to 7% after nucleotomy (Figures 4 and 6), which is consistent with previous studies that reported a 10–20% increase in deformation following nucleotomy.12,15,17 The axial strains also significantly increased with nucleotomy (Figures 4 and 6) and had similar values to the normalized axial deformation (which were measured independently). The consistency of the average axial strains derived by texture correlation with the dimensionally derived axial deformation results supports the validity of the MRI technique as previously reported.21
The advantage of examining the internal disc strains are highlighted in the flexion and extension loading positions, where the axial deformation is widely bracketed by the posterior and anterior AF axial strains (Figure 6). That is, in the anterior and posterior AF, a nominal 5% axial strain in the neutral position is increased to a range of 7.5 to 10% when the compressive load is more directly applied in flexion and extension positions (i.e. anterior AF in flexion). This represents a 50 to 100% increase in the axial strain within large regions of the disc. While the bone-disc-bone segments examined here are not an in vivo functional spinal unit, these findings support the clinical edict to lift in a balanced position to minimize injury.
The outer AF bulge was not altered by nucleotomy. The inner AF bulge remained outward but was significantly decreased in the neutral position following nucleotomy, consistent with previous studies.16,17 While this effect was seen to a lesser degree in the flexion and extension positions, it was not significant. Similarly, the radial strains, which were significant altered by nucleotomy in the neutral position, were not altered in the flexion or extension positions. The difference in response to nucleotomy for flexion and extension loading versus the neutral loading position may be due to a greater portion of the applied load being carried by the AF in bending (e.g., the anterior AF in flexion and the posterior AF in extension). A decrease in the NP loading with bending resulted in a decreased effect with nucleotomy, which obviously alters the NP most directly. Restated, the state of the NP pressure may not be as critical in the flexion and extension loading positions, and the AF radial strains in these positions are not as dependent on nucleotomy or degenerative state.22
Some of the nucleotomy effects are modulated by the state of degeneration, and when that was true the mechanical effect of nucleotomy was minimized in nondegenerated discs (examples Figure 4B and 4D). This is likely due to the observed ability of the NP in nondegenerated and moderately degenerated discs to swell and redistribute to fill the void left from the nucleotomy. Of the 19 discectomies, only four had voids after re-hydration, and those four had T1ρ values consistent with advanced degeneration. The results suggest that as long as the amount of NP removed is not too large the residual NP tissue may fill the void and may maintain mechanical function in inverse proportion to its state of degeneration.15,18,30 Conversely, changes observed in this study induced by nucleotomy have the greatest effect on degenerated discs.
Inhomogeneity between the anterior, lateral, and posterior AF strains was observed. Anterior AF radial strain was not affected by nucleotomy while the posterior AF radial strain became less tensile (Figure 5B). This inhomogenity in strain has previously been observed,31,32 and is not surprising given the spatial variability in AF geometry, fiber structure, biochemistry, and material properties. The decreased radial strain in the posterior AF with nucleotomy was surprising and may be due to shifting of the NP towards the posterior region under load.16,31–33 One possible explanation is that the remaining NP moved towards the posterior under load, placing more radial compression loading on that region. While such shift of the NP would have the positive effect of decreased radial tensile strains, posterior NP shift may also make it more susceptible to re-herniation in the clinical case of herniation and/or discecotmy.
Study limitations include the long imaging time, which permits study of the steady-state response but not the dynamic response, and the two-dimensional imaging sequence, which permits calculation of deformation and strain only for cases in which the tissue remains in the same imaging plane during loading (e.g., mid-sagittal and mid-coronal).21 Future studies will expand this method to three-dimensional imaging and strain analysis. This advance will permit measurement of strain at the posterior-lateral annulotomy site, it is likely that the annulotomy site experiences high strains due to disruption in AF structural integrity.34 It is currently unknown how the incision site responds to applied load and knowledge of this response will be helpful to design and evaluate new AF closures to potentially decrease the risk for re-herniation. Finally, the acute mechanical effects of nucleotomy presented here do not reflect the potential biological remodeling and progressive degeneration that may occur over time following herniation and nucleotomy.
In conclusion, nucleotomy alters the magnitude of radial and axial AF strains. Increased strains may make the AF vulnerable to fatigue damage and microfractures that develop into circumferential or radial tears following herniation and/or nucleotomy. Some of the nucleotomy effects are modulated by the state of degeneration, where nondegenerated discs had minimal changes for some strain components following nucleotomy. In these cases the NP swelled and redistributed to support the AF. The combined mechanical effect of nucleotomy in already degenerated discs is magnified and may further induce mechanical damage and incite progressive the degeneration. In future work these techniques can be applied to evaluate the mechanical efficacy of NP replacements and other interventions.35–39 While induced mechanical damage and progressive degeneration as a consequence of strain alterations following nucleotomy may be hypothesized, long-term in vivo studies would be necessary to determine the clinical outcomes following discectomy that are suggested by this study.
Acknowledgments
This study supported by grants from the NIH (EB86292; AR50052), and the National Football League Charities.
Footnotes
The authors have no disclosures.
References
- 1.Weinstein JN, Lurie JD, Tosteson TD, et al. Surgical vs nonoperative treatment for lumbar disk herniation: the Spine Patient Outcomes Research Trial (SPORT) observational cohort. Jama. 2006;296(20):2451–2459. doi: 10.1001/jama.296.20.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fountas KN, Kapsalaki EZ, Feltes CH, et al. Correlation of the amount of disc removed in a lumbar micronucleotomy with long-term outcome. Spine. 2004;29(22):2521–2524. doi: 10.1097/01.brs.0000145413.79277.d0. discussion 2525–2526. [DOI] [PubMed] [Google Scholar]
- 3.Thome C, Barth M, Scharf J, Schmiedek P. Outcome after lumbar sequestrectomy compared with micronucleotomy: a prospective randomized study. J Neurosurg Spine. 2005;2(3):271–278. doi: 10.3171/spi.2005.2.3.0271. [DOI] [PubMed] [Google Scholar]
- 4.Carragee EJ, Spinnickie AO, Alamin TF, Paragioudakis S. A prospective controlled study of limited versus subtotal posterior nucleotomy: short-term outcomes in patients with herniated lumbar intervertebral discs and large posterior anular defect. Spine (Phila Pa 1976) 2006;31(6):653–657. doi: 10.1097/01.brs.0000203714.76250.68. [DOI] [PubMed] [Google Scholar]
- 5.Hanley EN, Jr, Shapiro DE. The development of low-back pain after excision of a lumbar disc. J Bone Joint Surg Am. 1989;71(5):719–721. [PubMed] [Google Scholar]
- 6.Kambin P, Cohen LF, Brooks M, Schaffer JL. Development of degenerative spondylosis of the lumbar spine after partial nucleotomy. Comparison of laminotomy, nucleotomy, and posterolateral nucleotomy. Spine (Phila Pa 1976) 1995;20(5):599–607. doi: 10.1097/00007632-199503010-00018. [DOI] [PubMed] [Google Scholar]
- 7.Mariconda M, Galasso O, Attingenti P, Federico G, Milano C. Frequency and clinical meaning of long-term degenerative changes after lumbar nucleotomy visualized on imaging tests. Eur Spine J. 2009 doi: 10.1007/s00586-009-1201-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGirt MJ, Eustacchio S, Varga P, et al. A prospective cohort study of close interval computed tomography and magnetic resonance imaging after primary lumbar nucleotomy: factors associated with recurrent disc herniation and disc height loss. Spine (Phila Pa 1976) 2009;34(19):2044–2051. doi: 10.1097/BRS.0b013e3181b34a9a. [DOI] [PubMed] [Google Scholar]
- 9.Yorimitsu E, Chiba K, Toyama Y, Hirabayashi K. Long-term outcomes of standard nucleotomy for lumbar disc herniation: a follow-up study of more than 10 years. Spine (Phila Pa 1976) 2001;26(6):652–657. doi: 10.1097/00007632-200103150-00019. [DOI] [PubMed] [Google Scholar]
- 10.Brinckmann P, Grootenboer H. Change of disc height, radial disc bulge, and intradiscal pressure from nucleotomy. An in vitro investigation on human lumbar discs. Spine (Phila Pa 1976) 1991;16(6):641–646. doi: 10.1097/00007632-199106000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Broc GG, Crawford NR, Sonntag VK, Dickman CA. Biomechanical effects of transthoracic micronucleotomy. Spine (Phila Pa 1976) 1997;22(6):605–612. doi: 10.1097/00007632-199703150-00005. [DOI] [PubMed] [Google Scholar]
- 12.Cannella M, Arthur A, Allen S, et al. The role of the nucleus pulposus in neutral zone human lumbar intervertebral disc mechanics. J Biomech. 2008;41(10):2104–2111. doi: 10.1016/j.jbiomech.2008.04.037. [DOI] [PubMed] [Google Scholar]
- 13.Frei H, Oxland TR, Rathonyi GC, Nolte LP. The effect of nucleotomy on lumbar spine mechanics in compression and shear loading. Spine. 2001;26(19):2080–2089. doi: 10.1097/00007632-200110010-00007. [DOI] [PubMed] [Google Scholar]
- 14.Heuer F, Schmidt H, Wilke HJ. Stepwise reduction of functional spinal structures increase disc bulge and surface strains. J Biomech. 2008;41(9):1953–1960. doi: 10.1016/j.jbiomech.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 15.Johannessen W, Cloyd JM, O’Connell GD, Vresilovic EJ, Elliott DM. Trans-endplate nucleotomy increases deformation and creep response in axial loading. Annals of biomedical engineering. 2006;34(4):687–696. doi: 10.1007/s10439-005-9070-8. [DOI] [PubMed] [Google Scholar]
- 16.Meakin JR, Redpath TW, Hukins DW. The effect of partial removal of the nucleus pulposus from the intervertebral disc on the response of the human annulus fibrosus to compression. Clinical biomechanics (Bristol, Avon) 2001;16(2):121–128. doi: 10.1016/s0268-0033(00)00075-9. [DOI] [PubMed] [Google Scholar]
- 17.Seroussi RE, Krag MH, Muller DL, Pope MH. Internal deformations of intact and denucleated human lumbar discs subjected to compression, flexion, and extension loads. J Orthop Res. 1989;7(1):122–131. doi: 10.1002/jor.1100070117. [DOI] [PubMed] [Google Scholar]
- 18.Vresilovic EJ, Johannessen W, Elliott DM. Disc mechanics with trans-endplate partial nucleotomy are not fully restored following cyclic compressive loading and unloaded recovery. J Biomech Eng. 2006;128(6):823–829. doi: 10.1115/1.2354210. [DOI] [PubMed] [Google Scholar]
- 19.Kuroki H, Goel VK, Holekamp SA, Ebraheim NA, Kubo S, Tajima N. Contributions of flexion-extension cyclic loads to the lumbar spinal segment stability following different nucleotomy procedures. Spine (Phila Pa 1976) 2004;29(3):E39–46. doi: 10.1097/01.brs.0000106683.84600.e5. [DOI] [PubMed] [Google Scholar]
- 20.Meakin JR, Hukins DW. Effect of removing the nucleus pulposus on the deformation of the annulus fibrosus during compression of the intervertebral disc. J Biomech. 2000;33(5):575–580. doi: 10.1016/s0021-9290(99)00215-8. [DOI] [PubMed] [Google Scholar]
- 21.O’Connell GD, Johannessen W, Vresilovic EJ, Elliott DM. Human Internal Disc Strains in Axial Compression Measured Non-Invasively Using Magnetic Resonance Imaging. Spine. 2007 doi: 10.1097/BRS.0b013e31815b75fb. In Press. [DOI] [PubMed] [Google Scholar]
- 22.O’Connell GD, Vresilovic E, Elliott DM. Human Intervertebral Disc Internal Strain In Compression Loading: The Effect of Disc Region, Loading Position and Degeneration. J Orthop Res. 2010 doi: 10.1002/jor.21232. In Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johannessen W, Auerbach JD, Wheaton AJ, et al. Assessment of human disc degeneration and proteoglycan content using T1rho-weighted magnetic resonance imaging. Spine (Phila Pa 1976) 2006;31(11):1253–1257. doi: 10.1097/01.brs.0000217708.54880.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Auerbach JD, Johannessen W, Borthakur A, et al. In vivo quantification of human lumbar disc degeneration using T(1rho)-weighted magnetic resonance imaging. Eur Spine J. 2006;15 (Suppl 3):S338–344. doi: 10.1007/s00586-006-0083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nguyen AM, Johannessen W, Yoder JH, et al. Noninvasive quantification of human nucleus pulposus pressure with use of T1rho-weighted magnetic resonance imaging. J Bone Joint Surg Am. 2008;90(4):796–802. doi: 10.2106/JBJS.G.00667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pfirrmann CW, Metzdorf A, Zanetti M, Hodler J, Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine (Phila Pa 1976) 2001;26(17):1873–1878. doi: 10.1097/00007632-200109010-00011. [DOI] [PubMed] [Google Scholar]
- 27.Watkins RG. In: Lumbar Nucleotomy and Laminectomy. Collis JS, editor. 1987. [Google Scholar]
- 28.O’Connell GD, Vresilovic EJ, Elliott DM. Comparison of animals used in disc research to human lumbar disc geometry. Spine (Phila Pa 1976) 2007;32(3):328–333. doi: 10.1097/01.brs.0000253961.40910.c1. [DOI] [PubMed] [Google Scholar]
- 29.Adams MA, McNally DS, Dolan P. ‘Stress’ distributions inside intervertebral discs. The effects of age and degeneration. The Journal of bone and joint surgery. 1996;78(6):965–972. doi: 10.1302/0301-620x78b6.1287. [DOI] [PubMed] [Google Scholar]
- 30.Johannessen W, Elliott DM. Effects of degeneration on the biphasic material properties of human nucleus pulposus in confined compression. Spine. 2005;30(24):E724–729. doi: 10.1097/01.brs.0000192236.92867.15. [DOI] [PubMed] [Google Scholar]
- 31.Costi JJ, Stokes IA, Gardner-Morse M, Laible JP, Scoffone HM, Iatridis JC. Direct measurement of intervertebral disc maximum shear strain in six degrees of freedom: motions that place disc tissue at risk of injury. J Biomech. 2007;40(11):2457–2466. doi: 10.1016/j.jbiomech.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsantrizos A, Ito K, Aebi M, Steffen T. Internal strains in healthy and degenerated lumbar intervertebral discs. Spine. 2005;30(19):2129–2137. doi: 10.1097/01.brs.0000181052.56604.30. [DOI] [PubMed] [Google Scholar]
- 33.Krag MH, Seroussi RE, Wilder DG, Pope MH. Internal displacement distribution from in vitro loading of human thoracic and lumbar spinal motion segments: experimental results and theoretical predictions. Spine (Phila Pa 1976) 1987;12(10):1001–1007. doi: 10.1097/00007632-198712000-00011. [DOI] [PubMed] [Google Scholar]
- 34.Heuer F, Ulrich S, Claes L, Wilke HJ. Biomechanical evaluation of conventional anulus fibrosus closure methods required for nucleus replacement. Laboratory investigation. J Neurosurg Spine. 2008;9(3):307–313. doi: 10.3171/SPI/2008/9/9/307. [DOI] [PubMed] [Google Scholar]
- 35.Cloyd JM, Malhotra NR, Weng L, Chen W, Mauck RL, Elliott DM. Material properties in unconfined compression of human nucleus pulposus, injectable hyaluronic acid-based hydrogels and tissue engineering scaffolds. Eur Spine J. 2007;16(11):1892–1898. doi: 10.1007/s00586-007-0443-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berlemann U, Schwarzenbach O. An injectable nucleus replacement as an adjunct to micronucleotomy: 2 year follow-up in a pilot clinical study. Eur Spine J. 2009;18(11):1706–1712. doi: 10.1007/s00586-009-1136-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bron JL, Koenderink GH, Everts V, Smit TH. Rheological characterization of the nucleus pulposus and dense collagen scaffolds intended for functional replacement. J Orthop Res. 2009;27(5):620–626. doi: 10.1002/jor.20789. [DOI] [PubMed] [Google Scholar]
- 38.Rundell SA, Guerin HL, Auerbach JD, Kurtz SM. Effect of nucleus replacement device properties on lumbar spine mechanics. Spine (Phila Pa 1976) 2009;34(19):2022–2032. doi: 10.1097/BRS.0b013e3181af1d5a. [DOI] [PubMed] [Google Scholar]
- 39.Boyd LM, Carter AJ. Injectable biomaterials and vertebral endplate treatment for repair and regeneration of the intervertebral disc. Eur Spine J. 2006;15 (Suppl 3):S414–421. doi: 10.1007/s00586-006-0172-2. [DOI] [PMC free article] [PubMed] [Google Scholar]