Abstract
Ghrelin increases food intake and decreases energy expenditure, promoting a positive energy balance. We observed a single case of serious hypothermia during sustained ghrelin treatment in a male subject, suggesting that ghrelin may play a role in the regulation of body temperature. We therefore investigated the effect of ghrelin treatment on body temperature in rodents and humans under controlled conditions. Intriguingly, we could demonstrate ghrelin binding in axon terminals of the medial preoptic area of the hypothalamus located in the vicinity of cold-sensitive neurons. This localization of ghrelin receptors provides a potential anatomical basis for the regulation of body temperature by ghrelin. However, our follow-up studies also indicated that neither a chronic i.c.v. application of ghrelin in rats, nor a single s.c. injection under cold exposure in mice resulted in a relevant decrease in body core temperature. In addition, a four-hour intravenous ghrelin infusion did not decrease body surface temperature in healthy humans. We concluded that while there is a theoretical molecular basis for ghrelin to modify body temperature in mammals, its magnitude is irrelevant under physiologic circumstances. Hypothermia is not likely to represent a serious risk associated with this agent and pathway.
Keywords: ghrelin, hypothermia, medial preoptic area, thermogenesis, body temperature, cold exposure
1 Introduction
Energy balance is achieved by constant adjustment of energy* intake and energy expenditure as required by changing metabolic demands of the mammalian organism. While energy supply is provided by food intake, total energy expenditure results from the cumulative effects of resting metabolic rate (resting thermogenesis), thermic effect of food, spontaneous locomotor activity (SPA, or non-exercise activity thermogenesis, NEAT) and exercise as well as facultative thermogenesis (in case of demand e.g. during cold exposure). While obesity researchers have studied these individual components intensely during recent decades to understand how chronic energy balance and, consequently, body fat mass are regulated, the exact molecular underpinnings of these control processes remain to be elucidated.
The gastrointestinal peptide hormone, ghrelin, has been reported to increase food intake [1–3] and to impair nutrient partitioning [3], thereby leading to positive energy balance and increased body fat mass [1, 3]. However, it remains unclear to which extent ghrelin directly acts to decrease total energy expenditure in rodents [1, 3–5]. Obviously, single components of energy expenditure appear to be affected by ghrelin action. A single i.c.v. injection of ghrelin can decrease spontaneous locomotor activity (SPA) in rats for duration of 24 h [6, 7]. In addition, single i.c.v. injections of ghrelin or growth hormone secretagogues in rats were shown to transiently decrease body core temperature for the first 1–2 h after administration [2] and to decrease temperature and sympathetic activity in brown adipose tissue [8]. However, it is still unclear whether ghrelin affects thermogenesis in humans and via which specific pathways in the central nervous system ghrelin might induce these changes.
We observed a single case of severe hypothermia in a patient with cancer cachexia while receiving a pharmacological dose of ghrelin treatment. We subsequently investigated if there was an obvious neuroanatomical basis for such an event and whether exogenous ghrelin administration could induce hypothermia, performing a series of studies in rodents and in healthy humans.
2 Materials and Methods
2.1 Human Studies
2.1.1 Case report of hypothermia during sustained ghrelin treatment
A single case of hypothermia was observed during performing the study “Individual Dose-Escalated bi-Daily Subcutaneously Ghrelin in Cancer Cachexia: a Phase I/II Study”, (ClinicalTrials.gov Identifier: NCT00933361). The primary objective of the study was to investigate the dose effect of natural ghrelin (manufactured by Bachem, Weil am Rhein, Germany; filled by Clinalfa, Läufelingen, Switzerland; [9]) on nutritional intake (the minimal dose for maximal nutritional intake; MD-MANI) and to identify the maximally tolerable dose (MTD) in patients with cancer cachexia. The starting dose of 32 µg/kg was found to be safe to use in subjects with cancer cachexia from our previously published study [9]. The dose escalation was 50% between doses for the first 4 dose levels (32, 48, 72, 108 µg/kg) and 25% between the 4th and the 5th dose level (135, 169µg/kg). The volume of ≥5 ml (20 mg ghrelin) was defined as one criterion for MTD. Plasma levels for total and active ghrelin during this study were determined by Lincoplex RIA (Millipore, GHRT-89HK and GHRA-88HK, respectively)
2.1.2 Continuous ghrelin infusion in humans
Subjects
Healthy volunteers between the ages of 18 and 55 years with a BMI between 18 and 29 kg/m2 were recruited from the greater Cincinnati area. Subjects with a history or clinical evidence of impaired fasting glucose or diabetes mellitus, recent myocardial infarction, congestive heart failure, active liver or kidney disease, growth hormone deficiency or excess, neuroendocrine tumor, anemia or who were on medications known to alter insulin sensitivity were excluded.
All study procedures were conducted at the Cincinnati Children’s Medical Center Clinical Translation Research Center (CTRC). All study participants gave informed consent for the study by signing a form approved by University of Cincinnati Institutional Review Board.
Experimental protocol
Subjects arrived at the CTRC between 0700 and 0730 after a 10–12 hour fast for three separate experiments. Intravenous (i.v.) catheters were placed in veins of both forearms for blood sampling and infusion of ghrelin. The arm with the sampling catheter was heated to 55° to arterialize venous blood. A skin temperature sensor (GE Healthcare, WI, U.S.A.) was placed in the axilla of study subjects with transpore tape. The sensor was connected to a bedside vital signs monitor (Dash 4000, GE Healthcare, WI, U.S.A.) for display and data storage. Body surface temperature was measured continuously and was recorded every 5 minutes during the study. Adverse events were assessed and recorded by study staff at the CTRC during the study and by study investigators outside of the study unit. Total immunoreactive ghrelin was measured by radioimmunoassay (Millipore, Billarica, MA, U.S.A.). The lower and upper limits of detection were 27 and 1765 pM respectively, and the intra- and inter-assay coefficients of variation were 6.4 and 16.3%, respectively.
The study was originally designed to study the effect of exogenous ghrelin administration on glucose homeostasis in humans. A frequently sampled i.v. glucose tolerance test (FSIGT) was performed during ghrelin infusion to measure insulin secretion and insulin sensitivity (presented as poster at 46th EASD Meeting 2010). Synthetic human acylated ghrelin was obtained from Bachem AG (Rubendorf, Switzerland). The authenticity of the peptide was verified by mass spectrometry, the purity was > 95%, and reconstituted material was sterile and free of detectable pyrogens. On each morning of the three study days, either saline or synthetic ghrelin dissolved in sterile saline solution was infused at doses of 0.2 or 0.6 nmol/kg/hr (equivalent to 0.5 or 2 µg/kg/hr) for a total of 225 minutes. Specifically, following 45 minutes of ghrelin infusion, previously shown to be sufficient to bring plasma ghrelin levels to a steady state [10] subjects received an i.v. bolus of glucose (11.4 g/m2 body surface area) over 60 seconds at the initiation of the FSIGT (time 0). At time 20 minutes subjects received an i.v. infusion of insulin (0.025 µU/kg) over 5 minutes. Blood samples were collected in EDTA containing tubes at intervals varying between 1 to 20 minutes during the duration of the FSIGT (total of 180 minutes). The order of ghrelin infusions was randomized using a computer generated randomized number set (http://www.randomizer.org), and study visits were separated by at least five days.
2.2 Rodent Studies
2.2.1 Animals
All experiments were performed according to the federal guidelines of the Brandenburg Ministry of Agriculture, Environment Protection and Regional Planning and the Institutional Animal Care and Use Committee of Yale University. Male Wistar rats and male C57BL/6 mice were purchased from Charles River WIGA GmbH (Sulzfeld, Germany) at the age of 5 weeks and 10 weeks, respectively. Animals were maintained singly under standard conditions (25°C, 12h:12hdark:light cycle) receiving standard chow (sniff RIM-H, V1534-000, Soest, Germany) and tab water ad libitum, unless otherwise stated. Three different sets of experiments were performed
2.2.1 Immunohistochemistry and ghrelin binding assay
Immunofluorescence labeling for ghrelin was carried out using the same ghrelin antisera and secondary antisera as described in our earlier studies [11]. Double labeling for ghrelin and c-fos was done in rats exposed to 4°C ambient temperature for 6 hours using avidin-biotin peroxidase and peroxidase anti-peroxidase methods, and, the tissue-bound peroxidase was visualized by a nickel-diaminobenzidine reaction as described in our earlier studies. [12]. Ghrelin binding to hypothalamic preoptic area sections was carried out as described in our earlier studies [11].
2.2.2 Chronic i.c.v. Ghrelin Infusion in Rats
This experiment was designed to evaluate if chronic ghrelin infusion for six days induces a sustained decrease in body core temperature in male Wistar rats. In addition, gross locomotor activity and feeding patterns were constantly monitored. Body temperature and gross locomotor activity were monitored using biotelemetry (see details in 2.2.5). Feeding behavior was measured using the TSE Drinking & Feeding Monitoring System (TSE GmbH, Bad Homburg, Germany). This system permits the measurement of feeding behavior online for several days or weeks. Feeding baskets are attached to a scale, which monitors weight changes due to consumption of food by the laboratory animal during the experiment.
Rats were given two weeks for acclimation to housing conditions and were handled daily. Rats were then adapted to cages for the measurement of food intake for four consecutive days. Two weeks later, all rats received an intraperitoneal implanted transponder for the measurement of body temperature and gross locomotor activity. Rats were allowed to recover for at least 7 days until regaining their pre-surgical body weight. Then rats were subjected to intracerebral implantation (right ventricle: AP: −0.080, L: −0.140 D: −0.350, [13]) of a permanent cannula (Osmotic Pump single Connector Cannula 322OP/Spc, gauge 22, length below pedestal 5 mm, Plastics One Inc., Roanoke, VA,USA) with their heads securely held in a stereotaxic frame (David Kopf instruments, Tujunga, CA, USA). For implantation, rats were anesthetized with ketamin hydrochloride (100 mg/kg, Ketamin Graeub, A. Albrecht GmbH, Aulendorf, Germany)/xylazine hydrochloride (10 mg/kg, Rompun®, Bayer Vital GmbH, Leverkusen, Germany) given intraperitoneally (i.p.). The cannula was connected via PE tubing to an osmotic minipump (ALZET model 2002, 0.5 µl/h, DURECT Corporation, Cupertino, CA, USA) filled with either saline (Sigma-Aldrich) or rat ghrelin (generated in-house by the DiMarchi laboratory), which was subcutaneously (s.c.) implanted at the same time. The connecting tube was filled with saline and had a length of 21.8 cm corresponding to a pumped volume sufficient for 5 days. This period served to give rats time for recovery from the surgery before ghrelin treatment started. Ghrelin concentration in the pump was adjusted to the pump rate for releasing 2.5 nmol/d for 6 days. This dose was shown to be effective to increase food intake and body weight after a one-week treatment [3]. The cannula was fixed using dental cement (Stoelting Co. Illinois, USA) and the skin was closed using resorbable suture (PGA Resorba, Resorba, Nürnberg, Germany). After surgery rats received a single subcutaneous (s.c.) injection of Buprenorphin (0.05 mg/kg, Temgesic®, Essex Pharma GmbH Munich, Germany) and Carprofen (5 mg/kg, Rimadyl®, Pfizer GmbH, Karlsruhe, Germany) for analgesic treatment. Proper placement of the cannula was confirmed in the end of the experiment at time of sacrifice by injecting 5 µl of bromophenol blue into the cannula. Only animals with proper staining of the lateral ventricle were included in the data analysis. Increase in food intake and body weight in response to ghrelin treatment was used as a second marker for proper cannula placement. Using these two markers, 7 saline-treated and 6 ghrelin-treated rats were identified as having successful cannulation and were included in data analysis.
2.2.3 Acute Peripheral Ghrelin Injection in Mice
This experiment was performed to investigate whether peripherally injected ghrelin affects body core temperature as well as locomotor activity, total energy expenditure (TEE) and respiratory quotient (RQ). To force the thermogenic capacity of body temperature regulation, mice were subjected to acute non-acclimated cold exposure (4°C) for 23 h following rat ghrelin injection (treatment day). For comparison to basal conditions, all mice were injected with saline (100 µl) i.p. and measured for the above mentioned parameters at 21°C ambient temperature 2 days before cold exposure was applied (control day), leaving one resting day between measurements.
Body temperature and gross locomotor activity were monitored using the biotelemetry system as described under 2.2.5 After 2 weeks of recovery from the implantation surgery, mice were then injected with 100 µl of saline i.p. at 3 hours before lights off followed by constant monitoring of body core temperature, gross locomotor activity, total energy expenditure (TEE) and respiratory quotient for 23 h at 21°C ambient temperature, these measurements serving as baseline (control day). After one day of rest mice were i.p. injected with 100 µl of either saline or mouse ghrelin (10 mg/kg, Bachem AG, Rubendorf, Switzerland) at 3 hours before lights off followed by constant monitoring of the above mentioned parameters for 23 h at cold ambient temperature (4°C) which was applied by placing the mouse cages into a cold chamber (treatment day).
Acute peripheral administration of ghrelin can increase food intake [1]. However, food intake and nutrient absorption are accompanied by increased substrate turnover, which results in increased energy expenditure and body temperature (postprandial thermogenesis). To control for the thermogenic effect of food consumption, half of the mice from both treatment groups were kept without food for the first 5 hours following injection. Therefore, 4 treatment groups were used for the measurement at cold ambient temperature: saline treated mice with ad libitum access to food, ghrelin treated mice with ad libitum access to food, saline treated mice without access to food for 5 hours following injection and ghrelin treated mice without access to food for 5 hours following injection. Data shown for measurements at 21°C represent all mice (n=40), while data shown for measurements at 4°C represent 10 mice per treatment group. Most of the effects were only present during the first 5 hours after ghrelin treatment. Therefore, most data presented cover only this time period.
2.2.4 Measurement of energy expenditure
Energy expenditure and respiratory quotient were measured by indirect calorimetry using a self-constructed system equipped with the gas analyzing system Advance Optima from ABB AG (Mannheim, Germany, formerly Hartmann & Braun). The system provides one measurement every six minutes per cage. Mice were adapted to respiratory cages for two days prior to baseline measurement at 21°C. Mice we re kept in respiratory cages until completion of measurements at 4°C.
2.2.5 Implantation of biotelemetry transponders
Body core temperature and gross locomotor activity of mice and rats were measured using biotelemetry (Mini Mitter Co., Inc., Bend, OR, USA). This system requires implantation of transponders into the abdominal cavity and the animal cage to be placed on a receiver. Signal frequency (translating into body core temperature values) and localization of the transponder signal on the receiver (interpreted as movement) were measured every 6 minutes being in line with EE measurements for the cold exposure experiment. Mice and rats were implanted with transponders under ketamin hydrochloride (1 µl/g, Ketamin Graeub, A. Albrecht GmbH, Aulendorf, Germany)/xylazine hydrochloride (0.2 µl/g, Rompun®, Bayer Vital GmbH, Leverkusen, Germany) an aesthesia. The abdominal cavity was closed using resorbable suture (PGA Resorba, Resorba, Nürnberg), the skin was closed with clips that were removed 1 week after surgery. Animals received a single i.p. analgesic treatment of Carprofen (5 mg/kg, Rimadyl®, Pfizer GmbH, Karlsruhe, Germany) following surgery. Animals were allowed to recover for at least one (rats) or two weeks (mice) following transponder implantation before the start of the experiment.
2.3 Statistics
Animal studies
In chronic i.c.v. ghrelin experiments, food intake and body temperature were calculated as hourly means, while locomotor activity was presented as hourly sum. Data for plasma ghrelin concentration was not normally distributed and did not meet variance homogeneity requirement. Therefore, the difference between the saline and ghrelin treated groups was compared using a non-parametric Mann-Whitney-U test.
In acute peripheral ghrelin experiments, the most prominent effects were observed during the 5 hours of food deprivation following injection. Therefore, most parameters were presented only for the first 5 hours following treatment. The indirect calorimetry measurements were presented as group means of values measured every 6 min. Food intake was measured 24 hours after injection. Differences between groups were analyzed using analysis of Variance (ANOVA), and LSD was used as post-hoc test for multiple comparisons. All results are expressed as mean ± SEM. Significance was assumed at P<0.05. Analysis was performed using SPSS 8.0 (SPSS Inc., 1998, Chicago, IL, USA).
Clinical study in humans
Data were analyzed using ANOVA with three treatment levels (control, and ghrelin infusion rates of 0.2 and 0.6 nmol/kg/hr) and time of sampling being the repeated measure. Dependent variable was body surface temperature. Data were analyzed using Graph Pad Prism version 5.0 (Graph Pad Software).
3 Results and Discussion
3.1 A single case of serious hypothermia in response to sustained ghrelin treatment in a male subject (see supplemental material for details)
A 66 year-old male was diagnosed with locally advanced (infiltration of stomach) and metastatic pancreatic adeno-carcinoma one year before ghrelin study started. He was treated with tumor-debulking surgery and additional gastrectomy, splenectomy, and cholecystectomy, followed by nine months chemotherapy with gemcitabine and capecitabine. A break in chemotherapy at month five resulted in tumor progression. He had no relevant co-morbidities. He lost 9.2% weight during the last 6 months before ghrelin treatment. Two weeks prior to study begun, symptoms that could impact nutrition (e.g. constipation, abdominal pain and dietary) were treated with appropriate medications.
At baseline, the patient’s BMI was 18 kg/m2, blood pressure was 92/55 mmHg in supine position, heart rate was 71 beats per minute (bpm), body temperature was 36.6°C (ear), morning fasting level of active ghrelin was 26 pg/ml, while total ghrelin was 432 pg/ml. His renal and hepatic function and electrolytes were normal. He received dose levels 1–4 per protocol (two days per dose level, with two s.c. injections per day). There was a washout period of 1–2 days between doses. The patient tolerated the treatment well. Despite the lack of improvement regarding nutritional intake, he had improved appetite, alertness and mood based on subjective assessment using narratives and qualitative content analysis. Pharmacokinetics of ghrelin were measured on days 1 (dose level 1), 8 (dose level 3), and 15 (dose level 5). Pre-injection levels for active ghrelin and total ghrelin were 18 and 408 pg/ml (day 1), 20 and 464 pg/ml (day 8), and 52 and 897 pg/ml (day 15), respectively. Peak values at 45 minutes were 190 pg/ml and 2685 pg/ml, ≥1800 pg/ml (above upper detection limit) and 2694 pg/ml, and ≥1800 pg/ml and 2706 pg/ml, respectively. Since no MD-MANI and no MTD was achieved, dose level 5 (6750 µg, 1.7 ml) was given on day 15 in the morning at the hospital. The patient was stable and had normal vital signs. The second dose was given by patients’ spouse at home in the evening of the same day. On day 16 the third dose of level 5 was given s.c. at home at 12:45. Fifteen minutes into the ghrelin administration the patient started to experience hot flashes and acute tiredness that lasted for 30 minutes. Then he reported that he felt cold. His heart rate was 68 bpm and at 14:30 his temperature was 33°C. The spouse gave him granulate d sugar to treat suspected hypoglycemia. The patient did not have shivering or chills and remained alert and fully conscious without impaired cognition. At 15:30 patient was seen by the general practitioner and had a low blood pressure of 71/55 mmHg, low body temperature of 32.6°C on examination. No clinical signs of acute cardiac ischemia or heart failure were found. Advice was given to administer 0.9% normal saline intravenously, to warm the patient and to transfer him to the hospital. Upon arrival at the Oncology clinic at 16:25, his blood pressure was 100/70 mmHg, heart rate 48 bpm, temperature 33.6°C (auricular), oxygen saturation 88%, and blood glucose 6.9 mmol/l. There was no biochemical evidence of organ failure or acute infection. The patient was tired, but able to communicate. A blood sample was drawn one hour later at the emergency room for analysis of ghrelin levels. Blood sample was stored at −80°C after collection but was not treated with protease inhibitors as for other samples due to the unforeseen unfolding of events. Under these circumstances, active ghrelin was 86 pg/ml; total ghrelin level was 4027 pg/ml.
The patient received continuous normal saline infusion, a warming blanket, and 8 mg of dexamethas one (intravenously). The patient felt hungry at 17:15 and felt better after eating soup. His temperature and other vital signs continued to improve with time. At 19:45 the patient felt significantly better. His vital signs slowly normalized. He was later transferred to the inpatient Oncology ward for observation and went home the day after.
To summarize, in this male subject with pancreatic adeno-carcinoma associated cachexia, hypothermia occurred in the late stage of sustained ghrelin treatment with escalating doses. After administering the third injection of highest ghrelin dose (level 5 at 6,750 µg) on day 15, the patient’sbody temperature dropped from a subjectively normal range down to 33°C within two hours after ghrelin injection was given. The patient remained hypothermic (33 – 34°C) for a total of three hours before his temperature started to rise again. The decrease in temperature was accompanied by only moderate changes in blood pressure, and the patient showed no signs of organ failure or other associated pathology. Intriguingly, the low body temperature was associated with a high plasma concentration of total ghrelin that was about 150% above the level at 45 min after ghrelin injection was given.
Based on these observations we revisited the question, if ghrelin may be a potent and direct regulator of body temperature. Evidence exists that that ghrelin may decrease body temperature in rodents. When ghrelin was acutely injected into the brain, body core temperature decreases transiently for the first 1–2 h after administration [2]. Furthermore, third ventricular administration of ghrelin transiently decreased the temperature of brown adipose tissue (BAT) and suppressed BAT sympathetic nerve activity in rats [8]. The latter phenomenon indicates diminished energy expenditure due to decreased rate of facultative thermogenesis. However, reports regarding a relevant ghrelin induced decrease in energy expenditure are inconsistent [1, 3–5]. Based on our observation of a case of severe hypothermia following high-dose ghrelin administration, we investigated the role of ghrelin in body temperature regulation. We first examined whether there was a theoretical neuroanatomical basis for ghrelin receptor activation to affect the central nervous system (CNS) circuitry known to control body temperature.
3.2 Ghrelin binds to GHS-R in the medial preoptic area (MPOA)
The MPOA of the hypothalamus is believed to represent the key area for the central regulation of autonomic thermo-effect or responses [14]. When stimulated, warm-sensitive MPOA neurons trigger heat defense mechanisms, whereas when inhibited, these neurons trigger cold defense mechanisms [14]. Thereby the MPOA is a central element to the control of body core temperature.
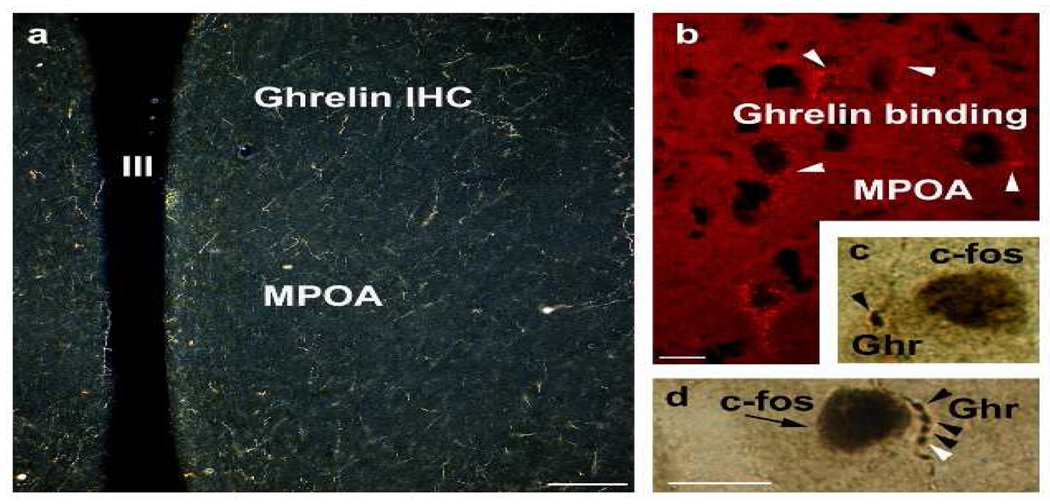
We detected ghrelin binding and ghrelin-immunoreactivity in the rat medial preoptic area (Figure 1). In addition, ghrelin-immunolabeled axon terminals contacted neurons that expressed c-fos in response to cold exposure. These data suggest that hypothalamic neurons expressing ghrelin immunoreactivity are in anatomical position to participate in the regulation of core body temperature by the MPOA.
Fig. 1.
Ghrelin-imunoreactivity (a) and ghrelin binding (b) in rat medial preoptic area. Axon terminal containing ghrelin immunolabelinglay in close apposition to neurons that express c-fos in response to cold exposure (c and d).
The precise mechanism of temperature regulation has not been fully elucidated. It is assumed that the MPOA in the hypothalamus is a major site for integrating and controlling body core temperature regulation [14, 15]. Efferent neuronal projections form the MPOA to the periphery are sympathetic nerves extending to skin blood vessels, skeletal muscle and BAT [14]. Ghrelin has been shown to influence sympathetic out flow to BAT by decreased sympathetic nerve activity in BAT [8] and chronic i.c.v. ghrelin infusion decreased expression of UCP1 mRNA in BAT of rats that were either fed ad libitum or that were pair-fed to control rats [3]. Our immunohistochemical results however indicate that ghrelin may also regulate body temperature at a higher central level by binding to receptors in the MPOA. In addition, the reported ghrelin binding to cell bodies in the MPOA coincide with the expression of the ghrelin receptor GHS-R1 a in this hypothalamic area [16, 17], indicating that ghrelin may play a role in body temperature regulation. However, our data are descriptive in nature, and it is not yet known which specific type of neurons ghrelin is binding to in the MPOA, or if and how ghrelin elicits a change in their activity or thermo regulating output by binding to MPOA neurons lying adjacent to thermo sensitive neurons.
Nevertheless, our data show that there is a theoretical neuroanatomical basis for ghrelin induced activation of neurons in the MPOA - a key CNS area regulating body temperature. To further elucidate the physiologic role of ghrelin for body temperature regulation we next performed in vivo experiments in rodents to study the chronic and acute effects of ghrelin on body temperature regulation in the context of overall changes in energy balance.
3.3 Ghrelin does not induce significant hypothermia in rodents
3.3.1 Chronic central ghrelin infusion is ineffective in decreasing body temperature
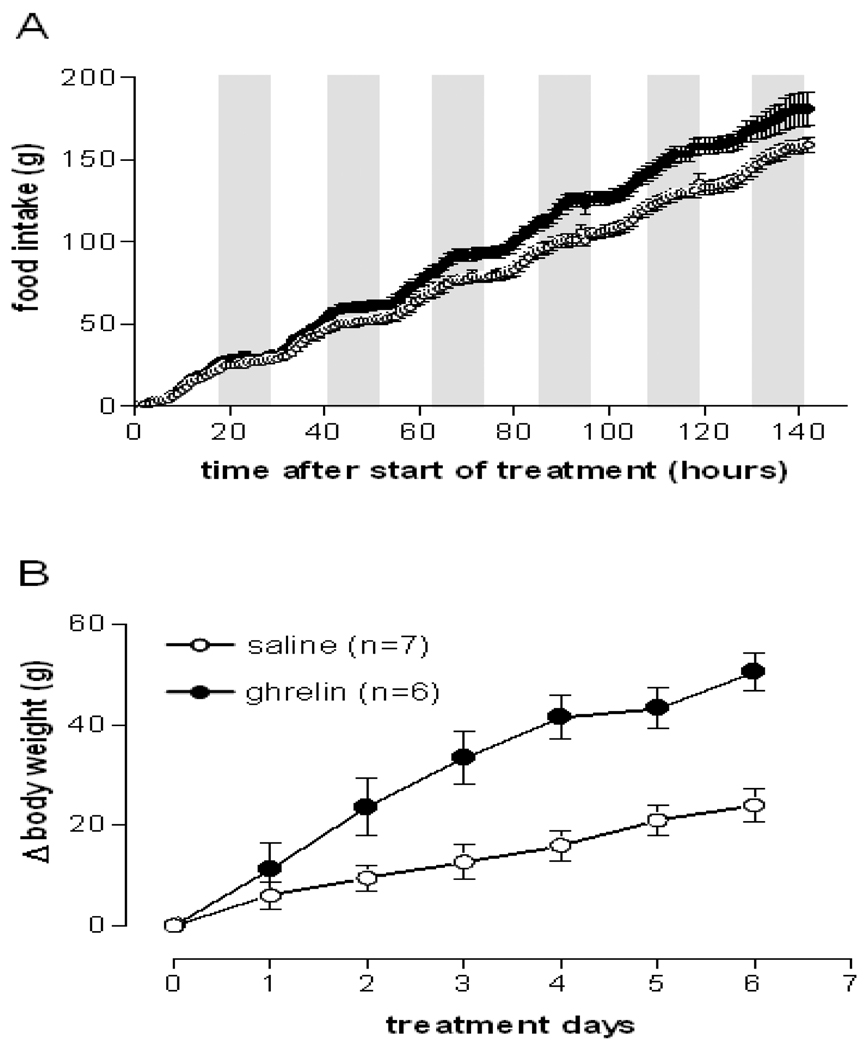
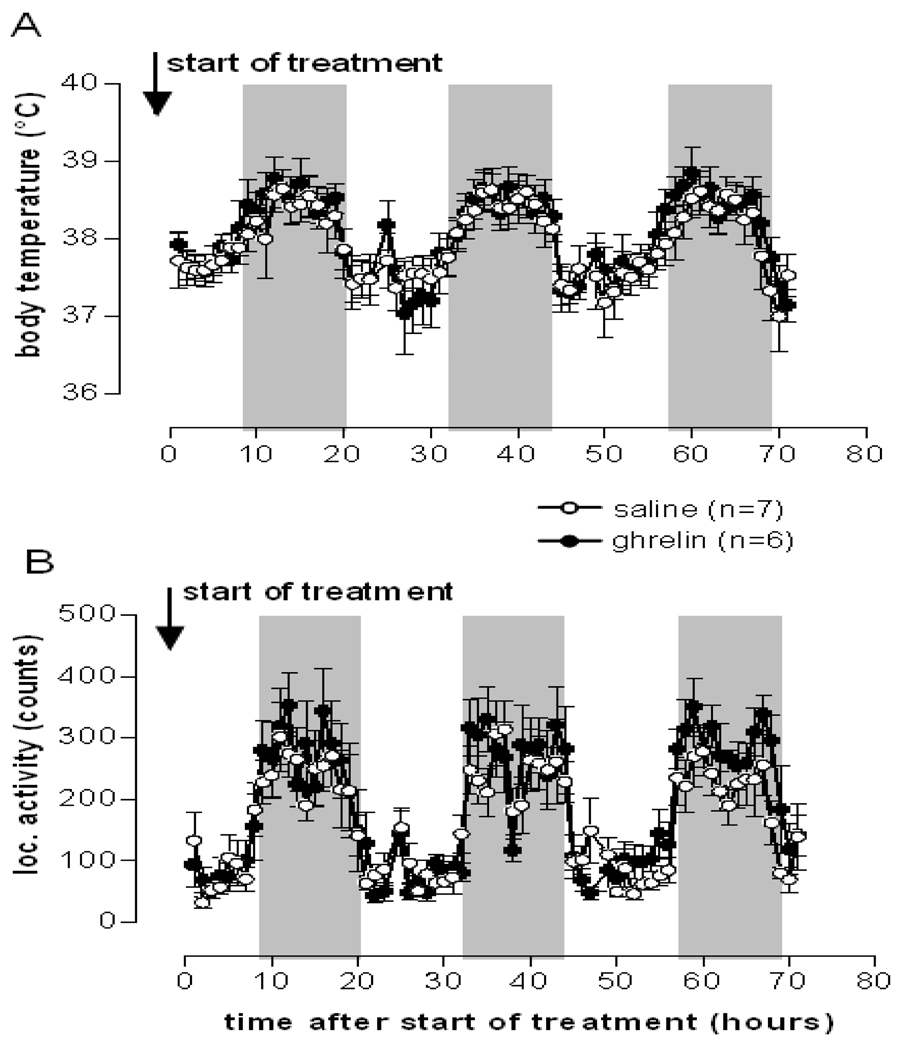
Centrally administered ghrelin was reported to stimulate lipogenesis in white adipose tissue and liver in rats after a one week treatment to facilitate storage of incoming nutrients as fat [3]. Since acute i.c.v. ghrelin injection transiently decreased body temperature in rats [2], we intended to investigate whether a chronically increased ghrelin level in the hypothalamus would induce a persistent decrease in body core temperature thereby contributing to energy storage. To test this hypothesis, we chronically infused rats with ghrelin (2.5 nmol/day) for 6 days via osmotic minipumps into the left lateral ventricle. The ghrelin dose used was reported to promote food intake and adiposity in rats during a 6-day treatment period [3]. The ghrelin infusion significantly increased food intake and body weight starting from the second day of treatment (Figures 2A and B) confirming that the ghrelin preparation was bioactive. However, this treatment did not affect body core temperature or gross locomotor activity (Figures 3A and B). Taking the lack of effect on body temperature with chronic ghrelin i.c.v. infusion together with the previously reported decrease in body temperature with acute i.c.v. injection [2] it appears that a hypothermic effect of ghrelin may require an acute rise in ghrelin level above a certain threshold. Consistently, lower ghrelin concentrations appear to be insufficient to alter body temperature. This observation makes physiological sense since ghrelin is secreted in a pulsatile manner and peaks before meals [18–20]. A ghrelin induced drop in body temperature prior to a meal may prepare the organism better for an efficient absorption and storage of incoming nutrients and energy. Also, ghrelin-induced hypothermia may be more easily inducible under conditions of lower environmental temperature. To test these hypotheses we next investigated the potential hypothermic effect of a single peripheral ghrelin injection in mice under challenged conditions such as cold exposure.
Fig. 2.
Ghrelin chronically infused i.c.v. (2.5 nmol/d) increased food intake and body weight. Absolute food intake (A) and body weight change (B) of rats treated i.c.v. with either saline or 2.5 nmol/d ghrelin. Data represent mean±SEM. P<0.001 (time × treatment) for both food intake and body weight, one-way repeated measures ANOVA.
Fig. 3.
Ghrelin chronically infused i.c.v. (2.5 nmol/d) did not change body temperature or locomotor activity. Body core temperature (A) and gross locomotor activity (B) of rats treated i.c.v. with either saline or 2.5 nmol/d ghrelin. Shown are the first 3 days following start of treatment. Data represent mean±SEM.
3.3.2 A single peripheral ghrelin injection is ineffective in decreasing body temperature
We subjected normal male mice to a single peripheral (i.p.) injection of ghrelin (10 mg/kg) and measured body core temperature, energy expenditure, locomotor activity, food intake, and respiratory quotient subsequently for 24 hours. To test the robustness of the hypothermic response to the acute rise in ghrelin level, we challenged the thermoregulatory system by exposing the injected mice to cold ambient temperature (4°C). Changes in the parameters of energy balance were compared to another group of mice treated with saline under the same experimental conditions The same parameters were also measured at normal temperature (21°C) following saline injection in the same mice (basal condition). In order to measure the contribution of the thermic effect of food, we removed food from a group of mice for 5 hours immediately following ghrelin or saline injection. We compared changes in body temperature and energy expenditure in 4 groups of mice that were exposed to cold ambient temperature: saline treated mice with ad libitum access to food, ghrelin treated mice with ad libitum access to food, saline treated mice without access to food for 5 hours following injection and ghrelin treated mice without access to food for 5 hours following injection.
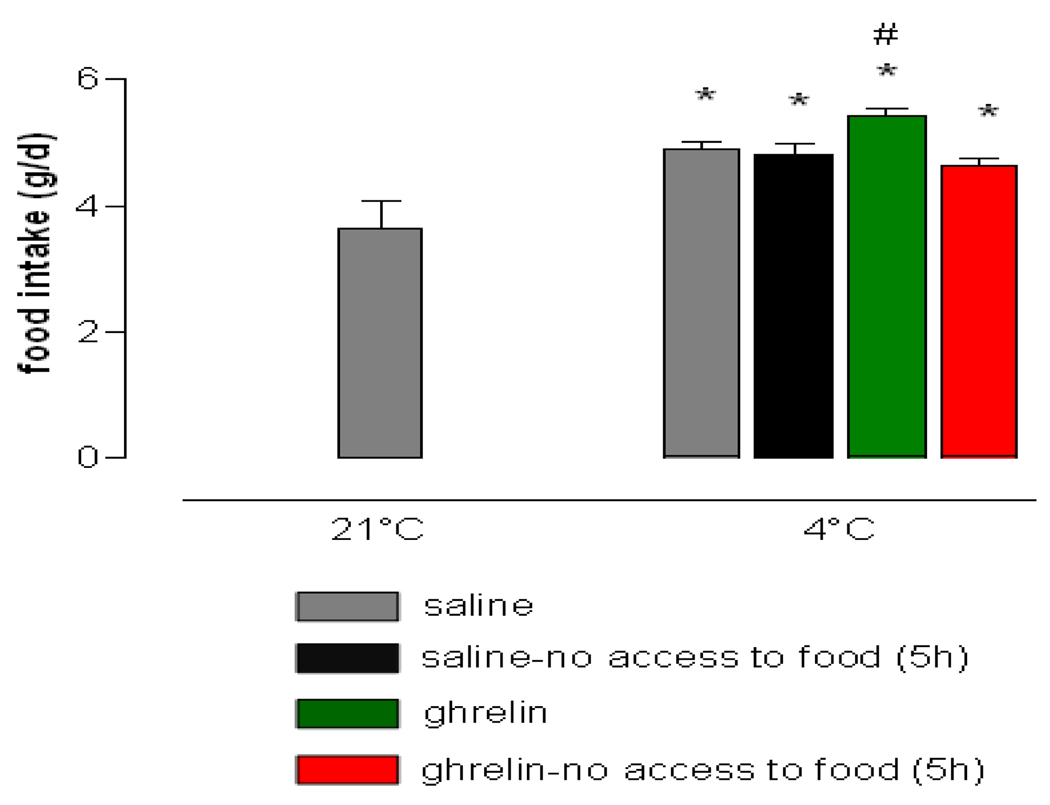
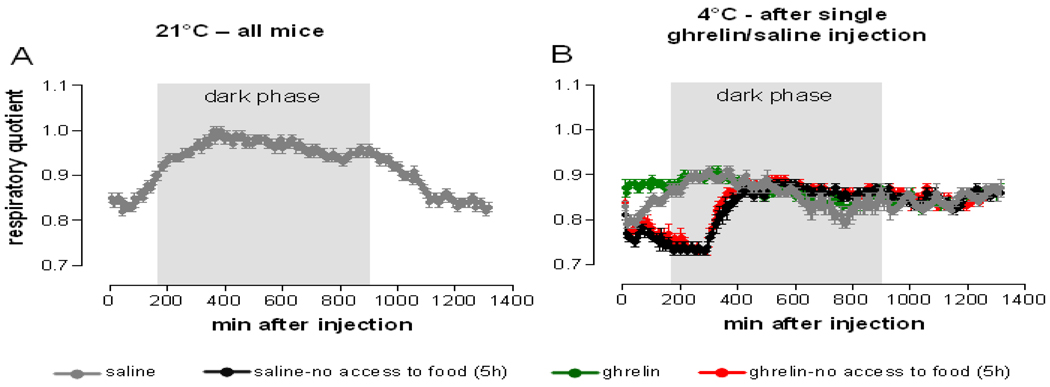
Cold exposure increased 24 h food intake in all treatment groups as compared to normal temperature exposure (Figure 4). Mice receiving ghrelin with ad libitum access to food ingested significantly more calories under cold ambient temperature conditions than the other treatment groups indicating bioactivity of the ghrelin preparation. Ghrelin treatment failed to increase food intake when food was supplied beginning at 5 h after injection, possibly due to the short half-life of the ghrelin peptide [20]. Peripheral ghrelin administration transiently increased respiratory quotient when mice had access to food (Figure 6B), indicating immediate start of food uptake. Saline injected mice with ad libitum access to food increased respiratory quotient with the beginning of the dark phase, when, according to normal circadian rhythm, the majority of the food is usually consumed. Food deprivation for the duration of 5 h following injection decreased respiratory quotient to a similar extent in saline and ghrelin treated mice.
Fig. 4.
Ghrelin further increased cold induced food intake. Cumulative Food intake (24 h) after single i.p. saline injection at 21°C (all mice) and after single i.p. injection of either saline or ghrelin (10 mg/kg) at 4°C ambient temperature (n=10 /group). Data represent mean±SEM. * P<0.001 vs. saline 21°C, # P<0.05 vs. saline, saline no food access (5 h) and ghrelin no food access (5 h) at 4°C; (ANOVA, Post-hoc LSD)
Fig. 6.
Ghrelin induced increase of respiratory quotient depends on food availability. Respiratory quotient was measured at 21°C following single i.p. saline injection (A) and during acute cold exposure following single i.p. ghrelin (10 mg/kg) or saline injection (B). The whole measurement period of 23 h following injection is shown. Data represent mean±SEM, n=10/group.
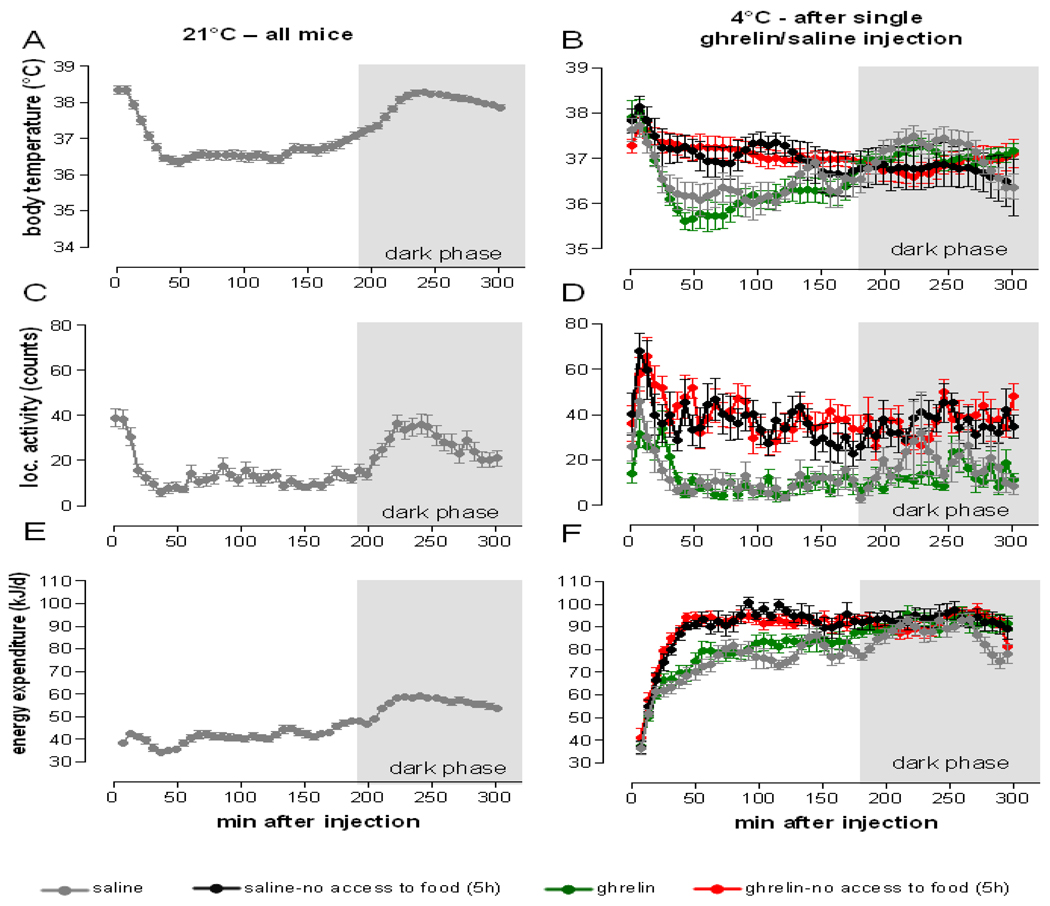
Our data suggest that changes in body core temperature were much more significantly influenced by the presence or absence of food than by ghrelin treatment (Figure 5B). Body core temperature decreased transiently for 2.5 hours after exposure to 4°C when mice had ad libitum access to food, regardless of the type of treatment. Of note, ghrelin treated mice had slightly, but insignificantly lower body temperature after 1 hour. Interestingly, the cold induced decrease in body temperature was fully blocked by food deprivation. When mice were food deprived for 5 h following saline or ghrelin treatment, body temperature increased in concordance with increased locomotor activity similarly in saline and ghrelin treated mice (Figure 5D). As a result, this led to an increase in total energy expenditure in both food restricted groups (Figure 5F). Increased locomotor activity was likely an indicator for increased food seeking behavior due to cold induced increase in energy demands. Since ghrelin and saline injected animals responded similarly, it can be assumed that the drive to eat was increased in both food restricted groups due to cold exposure. Furthermore, food deprivation for 5 h following injection decreased respiratory quotient to a similar extent in saline and ghrelin treated mice. This likely indicates a shift toward preferential fat oxidation to ensure sufficient energy supply when food was not available and to meet the increased demand for thermogenesis when ambient temperature was low. Therefore, we speculate that the potential hypothermic effect of ghrelin is dependent on food availability and may be influenced by ambient temperature and food restriction. While there is evidence that ghrelin decreases sympathetic nerve activity [8], possibly via neuropeptide Y and agouti-related protein [8, 21–26], we were unable to show that this effect is associated with lowering body temperature in rodents. It is still possible that ghrelin may have lowered temperature in certain tissues or organs like BAT, liver or skin, which was not significant enough to be detected by changes in core temperature. In this context, the decrease in body core or BAT temperature after i.c.v. injection of ghrelin reported elsewhere [2, 8] may just represent a result or “by-product” of decreased sympathetic outflow to BAT. The extent to which temperature is reduced may depend on the ability of ghrelin to reach the central receptors, the timing of ghrelin injection, and the initial capacity for non-shivering thermogenesis at a given point in time and condition. Such details in experimental design may explain the discrepancy between reported temperature decreases and our results [2, 8].
Fig. 5.
Cold induced changes in body core temperature, locomotor activity and energy expenditure depend on food availability, but not on ghrelin action. Body temperature, gross locomotor activity and total energy expenditure at 21°C (A, C and E, respectively) after single i.p. saline injection and during acute cold exposure after single i.p. ghrelin (10 mg/kg) or saline injection (B, D and F, respectively). The first 5 h following injection are shown. Data represent mean±SEM, n=10/group.
3.1 Peripheral ghrelin infusion does not decrease body surface temperature in humans
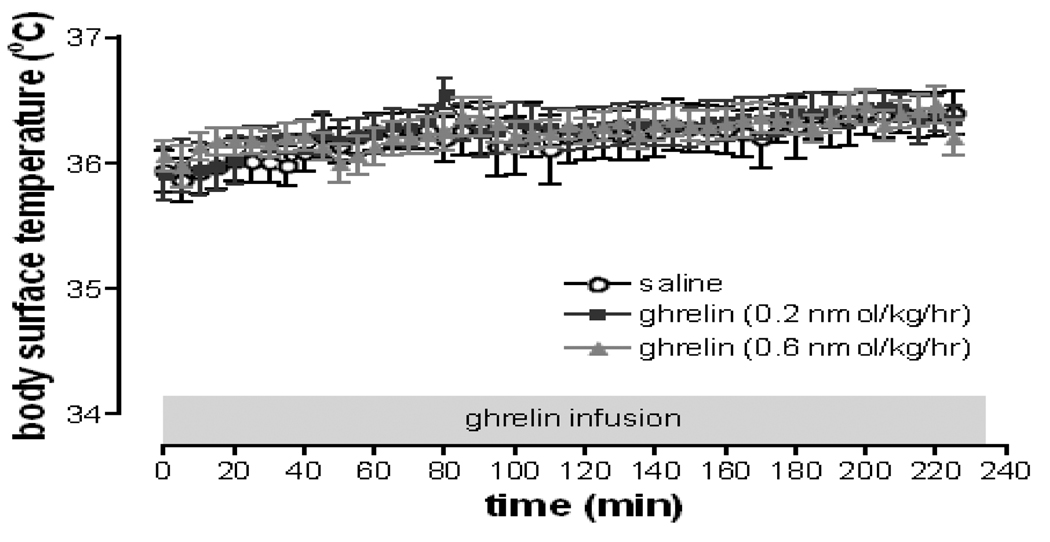
We subjected healthy subjects to a 225 min continuous intravenous (i.v.) infusion of either saline or ghrelin in doses of either 0.2 or 0.6 nmol/kg/hr on three separate days and measured body surface temperature for the whole infusion period. Sixteen healthy subjects (8 male and 8 females) aged 28.3 ± 6.0 years with a BMI of 27.7 ± 2.7 kg/m2 completed the study. Steady-state levels of ghrelin in the plasma were reached after approximately 45 minutes after the ghrelin infusion began. Ghrelin infusions at 0.2 and 0.6 pmol/kg/hr (equivalent to 0.5 and 2 µg/kg/h respectively) raised steady-state plasma total ghrelin levels to 2.4 and 6.7-fold above fasting concentrations, respectively. Continuous acyl ghrelin infusion at 0.2 and 0.6 nmol/kg/hr had no effect on body surface temperature (p = 0.15) (Figure 7). The administration of glucose and insulin during the frequently sampled i.v. glucose tolerance test did not appear to alter body surface temperature either. Ghrelin infusion was well tolerated. The most common complaints during the infusion of ghrelin were hunger and warm sensation. No unexpected or serious adverse event occurred during the study.
Fig. 7.
Ghrelin has no effect on body surface temperature in humans. Synthetic human ghrelin (0.2 and 0.6 nmol/kg/hr) or saline were infused on three separate days for a total of 225 minutes each in 16 healthy volunteers. No difference in body surface temperature between ghrelin treatment and control was found (p = 0.15, two-way repeated measures ANOVA). Data represent mean±SEM.
It is worth noting that the dose of ghrelin used in this study was much lower than the one used in the case report (< 800 µg vs. 6750 µg, respectively). The route of administration also differed between the two studies (i.v. infusion vs. s.c. injection as in the case report). The study subjects in this study were healthy, while the patient who suffered from hypothermia had terminal cancer of the digestive system. To our knowledge, data on chronic ghrelin treatment on body temperature regulation in humans is lacking.
With the exception of the here reported single case of hypothermia that occurred following high dose, repeated, ghrelin injections, changes in body temperature related to ghrelin administration in humans have not been reported elsewhere. Despite the existing evidence in rodents that ghrelin may lower body temperature following acute administration [2], decrease sympathetic outflow to BAT and decrease BAT temperature [8], a clear, direct and clinically relevant effect of ghrelin on body temperature regulation cannot be established based on our studies.
4 Conclusions
We have shown here that intriguingly there is ongoing ghrelin binding to GHSR-1 a in the MPOA, a critical region for temperature regulation. This implies that there is a theoretical basis for potential direct regulation of body core temperature by ghrelin in mammals. However, under the several specific conditions studied here (e.g. ad libitum feeding, normal and cold ambient temperature exposure), ghrelin does not alter body temperature in rodents or humans. The here reported single case of hypothermia in a cancer patient might involve several coincided influencing factors. One of these factors could be the loss of vagal connection to the gut as a result of total gastrectomy and vagotomy that could result in disturbed ghrelin action on the autonomic nervous system. It is known that the ghrelin receptors are expressed in the no dose ganglion [27] indicating an important pathway connecting the central and peripheral (gastrointestinal tract) nervous system. While abdominal vagal afferents are not required for the acute appetite-stimulatory effect of peripheral ghrelin [28], there is evidence that genetic blockage of ghrelin receptors in autonomic nerve endings leads to increased body temperature and energy expenditure as well as brown adipose tissue activity [29]. This implies that peripheral actions of ghrelin or its receptor in autonomic nerve endings may play a role in body temperature regulation.
Even though the exact cause for the isolated case of hypothermia during ghrelin administration has not been identified, it appears to be an isolated occurrence in a highly selected population (terminally ill cancer patient with cachexia). Potentially, a yet unknown constellation of sensitizing factors in that particular individual may have induced this severe drop in temperature. A similar phenomenon was not observed in our subsequent series of studies of ghrelin treatment in populations of mice, rats or humans.
Based on our and all other available data we would still recommend careful monitoring of body temperature and other vital signs in clinical studies involving ghrelin administration. Further studies are needed to better understand the potential relevance of ghrelin action at receptors in the MPOA and the specific conditions under which acute and chronic ghrelin treatment may have a pharmacologically or clinically meaningful effect on central temperature control or cell autonomous thermogenic processes.
Acknowledgements
The skillful technical assistance of Susanne deWolf-Linder (Clinical Research Nurse Oncological Palliative Medicine), the staff of the Clinical Research Unit Oncology of Cantonal Hospital St.Gallen and of CarolaPlaue (Department of Experimental Diabetology, German Institute of Human Nutrition) is gratefully acknowledged. Funding for J.T. is provided by NIH/NIDDK (5K23DK80081).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- 2.Lawrence CB, Snape AC, Baudoin FM, Luckman SM. Acute central ghrelin and GH secretagogues induce feeding and activate brain appetite centers. Endocrinology. 2002;143:155–162. doi: 10.1210/endo.143.1.8561. [DOI] [PubMed] [Google Scholar]
- 3.Theander-Carrillo C, Wiedmer P, Cettour-Rose P, Nogueiras R, Perez-Tilve D, Pfluger P, et al. Ghrelin action in the brain controls adipocyte metabolism. J Clin Invest. 2006;116:1983–1993. doi: 10.1172/JCI25811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asakawa A, Inui A, Kaga T, Yuzuriha H, Nagata T, Ueno N, et al. Ghrelin is an appetite-stimulatory signal from stomach with structural resemblance to motilin. Gastroenterology. 2001;120:337–345. doi: 10.1053/gast.2001.22158. [DOI] [PubMed] [Google Scholar]
- 5.Tschop M, Statnick MA, Suter TM, Heiman ML. GH-releasing peptide-2 increases fat mass in mice lacking NPY: indication for a crucial mediating role of hypothalamic agouti-related protein. Endocrinology. 2002;143:558–568. doi: 10.1210/endo.143.2.8633. [DOI] [PubMed] [Google Scholar]
- 6.Tang-Christensen M, Vrang N, Ortmann S, Larsen PJ, Horvath T, Tschoep M. Ghrelin induces long lasting changes in food intake and locomotor behaviour. Proceedings of the 85th Annual Meeting of the Endocrine Society; Philadelphia. 2003. pp. P3–P94. 497 (abs) [Google Scholar]
- 7.Tang-Christensen M, Vrang N, Ortmann S, Bidlingmaier M, Horvath TL, Tschop M. Central administration of ghrelin and agouti-related protein (83–132) increases food intake and decreases spontaneous locomotor activity in rats. Endocrinology. 2004;145:4645–4652. doi: 10.1210/en.2004-0529. [DOI] [PubMed] [Google Scholar]
- 8.Yasuda T, Masaki T, Kakuma T, Yoshimatsu H. Centrally administered ghrelin suppresses sympathetic nerve activity in brown adipose tissue of rats. Neurosci Lett. 2003;349:75–78. doi: 10.1016/s0304-3940(03)00789-4. [DOI] [PubMed] [Google Scholar]
- 9.Strasser F, Lutz TA, Maeder MT, Thuerlimann B, Bueche D, Tschop M, et al. Safety, tolerability and pharmacokinetics of intravenous ghrelin for cancer-related anorexia/cachexia: a randomised, placebo-controlled, double-blind, double-crossover study. Br J Cancer. 2008;98:300–308. doi: 10.1038/sj.bjc.6604148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tong J, Prigeon RL, Davis HW, Bidlingmaier M, Kahn SE, Cummings DE, et al. Ghrelin Suppresses Glucose-Stimulated Insulin Secretion and Deteriorates Glucose Tolerance in Healthy Humans. Diabetes. 2010;59:2145–2151. doi: 10.2337/db10-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cowley MA, Smith RG, Diano S, Tschop M, Pronchuk N, Grove KL, et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37:649–661. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- 12.Diano S, Horvath B, Urbanski HF, Sotonyi P, Horvath TL. Fasting Activates the Nonhuman Primate Hypocretin (Orexin) System and Its Postsynaptic Targets. Endocrinology. 2003;144:3774–3778. doi: 10.1210/en.2003-0274. [DOI] [PubMed] [Google Scholar]
- 13.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4 ed. New York: Academic Press; 1998. [Google Scholar]
- 14.Romanovsky AA. Thermoregulation: some concepts have changed. Functional architecture of the thermoregulatory system. Am J Physiol Regul Integr Comp Physiol. 2007;292:R37–R46. doi: 10.1152/ajpregu.00668.2006. [DOI] [PubMed] [Google Scholar]
- 15.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 16.Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK. Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol. 2006;494:528–548. doi: 10.1002/cne.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guan XM, Yu H, Palyha OC, McKee KK, Feighner SD, Sirinathsinghji DJ, et al. Distribution of mRNA encoding the growth hormone secretagogue receptor in brain and peripheral tissues. Brain Res Mol Brain Res. 1997;48:23–29. doi: 10.1016/s0169-328x(97)00071-5. [DOI] [PubMed] [Google Scholar]
- 18.Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–1719. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- 19.Yildiz BO, Suchard MA, Wong ML, McCann SM, Licinio J. Alterations in the dynamics of circulating ghrelin, adiponectin, and leptin in human obesity. Proc Natl Acad Sci U S A. 2004;101:10434–10439. doi: 10.1073/pnas.0403465101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tolle V, Bassant MH, Zizzari P, Poindessous-Jazat F, Tomasetto C, Epelbaum J, et al. Ultradian rhythmicity of ghrelin secretion in relation with GH, feeding behavior, and sleep-wake patterns in rats. Endocrinology. 2002;143:1353–1361. doi: 10.1210/endo.143.4.8712. [DOI] [PubMed] [Google Scholar]
- 21.Gluck EF, Stephens N, Swoap SJ. Peripheral ghrelin deepens torpor bouts in mice through the arcuate nucleus neuropeptide Y signaling pathway. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1303–R1309. doi: 10.1152/ajpregu.00232.2006. [DOI] [PubMed] [Google Scholar]
- 22.Billington CJ, Briggs JE, Grace M, Levine AS. Effects of intracerebroventricular injection of neuropeptide Y on energy metabolism. Am J Physiol. 1991;260:R321–R327. doi: 10.1152/ajpregu.1991.260.2.R321. [DOI] [PubMed] [Google Scholar]
- 23.Billington CJ, Briggs JE, Harker S, Grace M, Levine AS. Neuropeptide Y in hypothalamic paraventricular nucleus: a center coordinating energy metabolism. Am J Physiol. 1994;266:R1765–R1770. doi: 10.1152/ajpregu.1994.266.6.R1765. [DOI] [PubMed] [Google Scholar]
- 24.Egawa M, Yoshimatsu H, Bray GA. Neuropeptide Y suppresses sympathetic activity to interscapular brown adipose tissue in rats. Am J Physiol. 1991;260:R328–R334. doi: 10.1152/ajpregu.1991.260.2.R328. [DOI] [PubMed] [Google Scholar]
- 25.Wortley KE, Anderson KD, Yasenchak J, Murphy A, Valenzuela D, Diano S, et al. Agouti-related protein-deficient mice display an age-related lean phenotype. Cell Metab. 2005;2:421–427. doi: 10.1016/j.cmet.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Brito MN, Brito NA, Baro DJ, Song CK, Bartness TJ. Differential Activation of the Sympathetic Innervation of Adipose Tissues by Melanocortin Receptor Stimulation. Endocrinology. 2007;148:5339–5347. doi: 10.1210/en.2007-0621. [DOI] [PubMed] [Google Scholar]
- 27.Burdyga G, Varro A, Dimaline R, Thompson DG, Dockray GJ. Ghrelin receptors in rat and human nodose ganglia: putative role in regulating CB-1 and MCH receptor abundance. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1289–G1297. doi: 10.1152/ajpgi.00543.2005. [DOI] [PubMed] [Google Scholar]
- 28.Arnold M, Mura A, Langhans W, Geary N. Gut vagal afferents are not necessary for the eating-stimulatory effect of intraperitoneally injected ghrelin in the rat. J Neurosci. 2006;26:11052–11060. doi: 10.1523/JNEUROSCI.2606-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mano-Otagiri A, Iwasaki-Sekino A, Nemoto T, Ohata H, Shuto Y, Nakabayashi H, et al. Genetic suppression of ghrelin receptors activates brown adipocyte function and decreases fat storage in rats. Regul Pept. 2010;160:81–90. doi: 10.1016/j.regpep.2009.11.010. [DOI] [PubMed] [Google Scholar]