Abstract
Differentiated thyroid cancer (DTC) is the second most common cancer in pregnancy. Its management is a challenge for both doctors and patients, and the best timing for surgery is unclear. A systematic review evaluating the prognosis of DTC in pregnant patients was conducted. After reviewing 401 unique citations and 54 full texts, 4 studies that compared the prognosis of patients with DTC related to pregnancy (DTC diagnosed during pregnancy or within 12 months after childbirth) or not were included. In two studies the primary outcome was overall survival, in one study the primary outcomes were recurrent disease and death related to thyroid cancer, and in one study the primary outcome was recurrent or persistent disease. In the first two studies, there was no difference in overall survival in patients with pregnancy-related DTC, when compared with matched controls; in one study, there was no difference in death caused by DTC nor recurrence in DTC related to pregnancy. Nevertheless, in a recent retrospective study, a higher rate of recurrent or persistent DTC was observed in patients with DTC related to pregnancy. There are not many studies on which to base treatment decisions in pregnant patients with DTC.
1. Introduction
Thyroid cancer currently ranks tenth in incidence among solid organ malignancies. The majority of thyroid cancers are classified as papillary (88%) or follicular (9%); together these two histological types are grouped as differentiated thyroid cancers (DTC) [5]. While the annual rate of cancer incidence is decreasing, there has been a 2.4-fold increase in thyroid cancer from 1973 to 2002 [6].
DTC occurs more commonly in women of child-bearing age with an incidence of 14 per 100,000 live births and represents the second most frequent tumor diagnosed during pregnancy only behind breast cancer [7, 8]. As DTC is commonly found during pregnancy or in the early postpartum period [9], it is possible that physiological changes associated with it, as high levels of estrogen, human chorionic gonadotropin (hCG) and/or others, could create a favorable environment to tumor development and growth. Maternal thyroid gland secretes more thyroid hormone during early pregnancy in response to the thyrotropic activity of hCG that overrides the operation of the hypothalamic-pituitary-thyroid feedback system. This could partially explain an increase in the size of preexisting thyroid nodules as well as new thyroid nodule formation in pregnancy [10–15].
The best treatment option for thyroid cancer in pregnant women or in the early postpartum period should be based on evidence, so the aim of this paper was to evaluate whether the prognosis of DTC associated with pregnancy is similar or not to DTC in nonpregnant women.
2. Methods
2.1. Search Strategy
This literature search was conducted in the PubMed, Cochrane, and Scopus databases, combining the MESH terms: thyroid neoplasms and pregnancy. All studies in English until February 2011 were included.
2.2. Inclusion and Exclusion Criteria for Studies
To be included in this review the original article should describe the comparison between the outcomes in patients diagnosed with DTC during pregnancy or in the first 12 months postpartum (DTC related to pregnancy), with a control group comprising women of child-bearing age, diagnosed with DTC when nonpregnant or at least 12 months after delivery.
Patients should have no prior exposure to radiation or previous malignancies.
2.3. Selection of Studies for Inclusion
All citations and abstracts identified by the electronic search were reviewed by two independent reviewers. Any abstract identified as relevant was analyzed as a full text. Other sources of obtaining papers were used, as cross-referencing texts reviewed.
3. Results and Discussion
After reviewing 398 abstracts from the electronic search and 3 summaries obtained by manual search, 54 articles were reviewed in full-text form. However, after analysis of these studies, several were excluded, for the following reasons: DTC did not occur during pregnancy, there was no control group, there was no reporting of outcomes, or they described small case series, so only four studies were included in this paper, as shown in Figure 1 [1–4]. The characteristics of the studies and the outcomes are described, respectively, in Tables 1 and 2.
Figure 1.
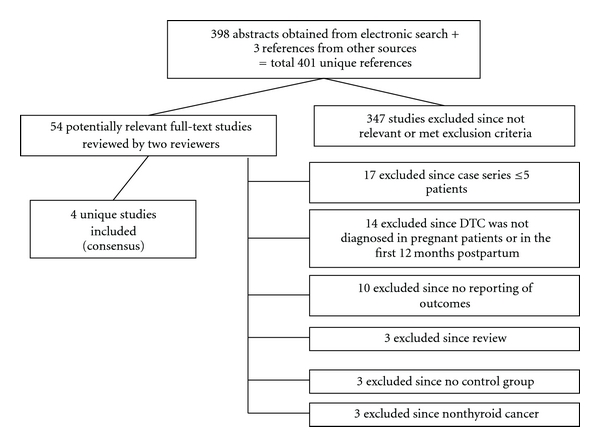
Process of study selection for the systematic review.
Table 1.
Characteristics of the included studies.
| Study | Study design | Study population | Control group description | Followup (median) |
|---|---|---|---|---|
| Vannucchi et al., 2010 [1] | Retrospective cohort in a single institution—1995–2006 | 14 women (one affected twice), age 32.2 ± 6.4 yr, DTC diagnosed during pregnancy (G2) | 47 women, age 36.1 ± 3.6 yr, DTC diagnosed at least one year after delivery (G1), and 61 nulliparous women when diagnosed with DTC, age 34.1 ± 6.2 yr, (G3) | G1: 68.2 months G2: 60.1 months G3: 64.7 months |
| Yasmeen et al., 2005 [2] | Population-based case control study—California 1991–1999 | 595 pregnant women with DTC, 129 diagnosed during pregnancy and 466 diagnosed within 12 months postpartum | 2270 age-matched nonpregnant women with DTC | Not reported |
| Moosa and Mazzaferri, 1997 [3] | Case control study—United States Air Force registry | 61 pregnant women with DTC, age 26.0 ± 5.9 yr | 528 age-matched nonpregnant women with DTC, age 26.3 ± 5.9 yr | 22.4 yr in study population and 19.5 yr in control group |
| Herzon et al., 1994 [4] | Case control study—New Mexico Registry 1970–1991 | 22 pregnant women with DTC, age 18–46 yr | 465 women with DTC in the same database, age 18–46 yr | 6 months to 20 years reported for study population |
Age (years) is expressed as mean + SD; DTC: differentiated thyroid cancer; G: group.
Table 2.
Main outcomes in patients with differentiated thyroid cancer (DTC) diagnosed during pregnancy or within 12 months of childbirth.
| Study | Timing of surgery (study group) | Outcomes | Main study results | Comments and others outcomes |
|---|---|---|---|---|
| Vannucchi et al., 2010 [1] | (i) 11 patients operated during pregnancy and (ii) 4 patients operated after deliveryd |
(i) Persistent or recurrent disease detected by highly sensitive Tg and rhTSH (ii) ERα tumor expression by IHC |
(i) ↑Persistent/recurrent disease in G2 versus G1 and G3 (60% versus 4.2% and 13.1%)a (ii) ↑ERα tumor expression in G2 versus G1 and G3 (87.5% versus 31% and 0%)b |
(i) PTC more frequent in G1 and G3 versus G2 (97.8% and 98.3% versus 80%)c (ii) DTC was an incidental finding more frequently in G1 and G3 (iii) More sensitive methods for detecting recurrence were used in this study when compared with others (iv) Conclusion: pregnancy has a negative impact on the outcome of DTC |
| Yasmeen et al., 2005 [2] | (i) 96 patients operated during pregnancy and (ii) 27 patients operated after deliverye |
Overall survival | No difference in survival between pregnancy-associated thyroid cancer and aged-matched nonpregnant women with DTC | Persistent/recurrent disease was not evaluated |
| Moosa and Mazzaferri, 1997 [3] | (i) 14 patients operated during pregnancy and (ii) 47 patients operated after delivery |
(i) Death (ii) Recurrence diagnosed by biopsy, or by 131I uptake in distant site |
No difference in cancer recurrence and death in study and control groups | (i) Outcomes similar in patients operated after delivery and during pregnancy (ii) Fewer pregnant patients showed symptoms associated with thyroid nodules when compared with nonpregnant (74% versus 43%)b |
| Herzon et al., 1994 [4] | (i) 6 patients operated during pregnancy and (ii) 16 patients operated after delivery |
Overall survival | No difference in survival between pregnancy-associated thyroid cancer and aged-matched nonpregnant women with DTC | |
PTC: papillary histotype; Tg: serum thyroglobulin; rhTSH: recombinant human TSH; ERα: estrogen receptor alfa; G: group; G1: DTC diagnosed at least one year after pregnancy, G2: DTC diagnosed during pregnancy, G3: DTC diagnosed in nulliparous women or before pregnancy; aP < 0.0001; bP = 0.01; cP < 0.0001; d1 patient was considered twice: she had two tumors in two different pregnancies; eIn patients with DTC diagnosed during pregnancy.
There are only a few studies about outcomes of DTC related to pregnancy. As DTC has a good prognosis, the number of patients studied should be large, and the followup should be very long to detect any difference in survival or even recurrence. Most recurrences of DTC occur within the first five years after initial treatment, but recurrences may occur many years or even decades later, particularly in patients with papillary cancer [16, 17]. In addition to this, decisions about cancer treatment during pregnancy are associated with ethical conflicts between the best option for the mother and for the fetus [18, 19]. The management of pregnant women with cancer should consider the maternal-fetal risk related to treatment, as well as the possibility of tumor progression for postponing treatment or for the tumor being related to pregnancy. During this study we could observe that there is little published data comparing outcomes in patients with DTC related to pregnancy or not. No randomized controlled trial was available.
In the study of Moosa and Mazzaferri there was no impact from pregnancy in DTC-related death [3]; in the studies of Yasmeen et al. and Herzon et al. overall survival was not affected by DTC [2, 4]; when evaluating if the timing of surgical treatment, during pregnancy or after birth, affected the prognosis of patients with DTC detected during pregnancy, Moosa and Mazzaferri and Yasmeen et al. [2, 3] have not shown differences in recurrence rates and, respectively, in DTC-related death and overall survival. Such findings, however, are not in agreement with the study published by Vannucchi et al. They found a strong association of DTC in pregnant women with recurrence or persistence of cancer (60% in pregnant women (group 2) versus 4.2% in women with DTC diagnosed 1 year after delivery (group 1) versus 13.1% in nulliparous patients when diagnosed with DTC (group 3)). After a stepwise logistic regression analysis entering the following variables: extrathyroidal extension, lymph-node metastases, radioiodine treatment, pregnant or not pregnant status at diagnosis, histotype, and tumor size ≤2 or >2 cm, pregnancy was found to be the most significant predictor for disease recurrence or persistence [1]. As the outcomes and the methodology employed in each study were different, it was not possible to compare their results and to combine the data in a meta-analysis. In the studies of Yasmeen et al. and Herzon et al., the overall survival was the main outcome [2, 4], while the study of Moosa and Mazzaferri had death related to DTC and recurrence, evaluated by biopsy or by 131I uptake in distant sites, as primary outcomes [3], and the more recent study of Vannucchi et al. evaluated persistent/recurrent DTC through more sensible tests such as Tg basal levels and Tg response to rhTSH [1]. Such methods were not used in the study of Moosa and Mazzaferri [3], which could explain some of the discrepancies among them.
In the study of Vannucchi et al. more patients from the pregnant women with DTC had follicular histotype. However, this factor does not seem to explain the worse outcome which has been found on the group of patients with DTC associated to pregnancy, since two of three patients with follicular histology remained in remission [1].
Two studies reported differences among clinical presentation between pregnant and nonpregnant women [1, 3]. In the study of Moosa and Mazzaferri, fewer pregnant patients showed symptoms associated to thyroid nodules (74% versus 43%, P < 0.01) [3]. On the other hand, in the study of Vannucchi et al. DTC was less commonly an incidental finding in pregnant patients, probably indicating that these patients had more aggressive disease [1].
A very interesting molecular datum was described by Vannucchi et al. The expression of the estrogen receptor alfa through immunohistochemical analysis was higher in group 2 patients, as compared to groups 1 and 3 [1]. As estrogen probably increases proliferative activity of thyroid follicular cells [20], this hormone could be implicated in a more aggressive pattern of DTC diagnosed in pregnancy.
Other important factor found in this paper refers to the time of followup of the studies. The mean median followup ranged from four to twenty-three years. Considering that DTC is a disease with low lethality and the followup was not very long, it is possible that the full impact of DTC on the patients survival was not evident.
The treatment of choice for both pregnant and nonpregnant patients was thyroidectomy. The central lymphadenectomy (VI-VII levels) was performed on all patients in the cohort described by Vannucchi et al. [1], and, according to clinical judgment, in the other studies [2–4]. There seemed to be no difference on the outcome of DTC during pregnancy whether the surgery took place at the second trimester of pregnancy or after childbirth [2–4]. In contrast to these findings, Kuy et al. compared the risk of thyroid and parathyroid surgery complications in pregnant and nonpregnant women, paired by age, in a retrospective cross-sectional study. A total of 201 pregnant women and 31155 nonpregnant women were included; among the 201 pregnant women, 45.8% have undergone surgery due to thyroid cancer, the others had benign thyroid and parathyroid diseases. Thyroidectomy during pregnancy was associated with an increased surgical complication rate in both malignant (21% to 8%) and benign diseases (27% to 14%), as well as higher endocrine complication rates (15.9% to 8.2%) and treatment costs ($6873 versus $5963) [21].
In summary, there are few studies which give base to the policies about pregnant patients with DTC. Up to present time, data obtained through systematic review show conflicting results when it comes to observed outcomes in this population. There seem to be a higher disease recurrence and persistence rates in this population when current treatment response evaluation methods are employed. However, the impact on overall survival in the long time appears to be unaltered. There is no evidence to support termination of pregnancy when the diagnosis of DTC is performed. The guidelines of the endocrine society for pregnancy-related DTC recommend thyroidectomy after delivery for patients with no evidence of advanced disease or without rapid progression, and thyroidectomy in the second trimester of pregnancy for the others (USPSTF recommendation level B). Radioactive iodine should only be given after delivery and the ending of breastfeeding [22].
Prospective studies should be done to compare the prognosis of DTC diagnosed during pregnancy or not, as well as the effects of postponing surgery after childbirth in DTC diagnosed during pregnancy.
Conflict of Interests
The authors declare that there is no conflict of interests.
References
- 1.Vannucchi G, Perrino M, Rossi S, et al. Clinical and molecular features of differentiated thyroid cancer diagnosed during pregnancy. European Journal of Endocrinology. 2010;162(1):145–151. doi: 10.1530/EJE-09-0761. [DOI] [PubMed] [Google Scholar]
- 2.Yasmeen S, Cress R, Romano PS, et al. Thyroid cancer in pregnancy. International Journal of Gynecology and Obstetrics. 2005;91(1):15–20. doi: 10.1016/j.ijgo.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 3.Moosa M, Mazzaferri EL. Outcome of differentiated thyroid cancer diagnosed in pregnant women. Journal of Clinical Endocrinology and Metabolism. 1997;82(9):2862–2866. doi: 10.1210/jcem.82.9.4247. [DOI] [PubMed] [Google Scholar]
- 4.Herzon FS, Morris DM, Segal MN, Rauch G, Parnell T. Coexistent thyroid cancer and pregnancy. Archives of Otolaryngology—Head and Neck Surgery. 1994;120(11):1191–1193. doi: 10.1001/archotol.1994.01880350009002. [DOI] [PubMed] [Google Scholar]
- 5.Burns WR, Zeiger MA. Differentiated thyroid cancer. Seminars in Oncology. 2010;37(6):557–566. doi: 10.1053/j.seminoncol.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 6.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. Journal of the American Medical Association. 2006;295(18):2164–2167. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 7.Smith LH, Dalrymple JL, Leiserowitz GS, Danielsen B, Gilbert WM. Obstetrical deliveries associated with maternal malignancy in California, 1992 through 1997. American Journal of Obstetrics and Gynecology. 2001;184(7):1504–1513. doi: 10.1067/mob.2001.114867. [DOI] [PubMed] [Google Scholar]
- 8.Smith LH, Danielsen B, Allen ME, Cress R. Cancer associated with obstetric delivery: results of linkage with the California cancer registry. American Journal of Obstetrics and Gynecology. 2003;189(4):1128–1135. doi: 10.1067/s0002-9378(03)00537-4. [DOI] [PubMed] [Google Scholar]
- 9.Lambe M, Ekbom A. Cancers coinciding with childbearing: delayed diagnosis during pregnancy? British Medical Journal. 1995;311(7020):1607–1608. doi: 10.1136/bmj.311.7020.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akslen LA, Nilssen S, Kvale G. Reproductive factors and risk of thyroid cancer. A prospective study of 63,090 women from Norway. British Journal of Cancer. 1992;65(5):772–774. doi: 10.1038/bjc.1992.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galanti MR, Lambe M, Ekbom A, Sparen P, Pettersson B. Parity and risk of thyroid cancer: a nested case-control study of a nationwide Swedish cohort. Cancer Causes and Control. 1995;6(1):37–44. doi: 10.1007/BF00051679. [DOI] [PubMed] [Google Scholar]
- 12.Yoshimura M, Hershman JM. Thyrotropic action of human chorionic gonadotropin. Thyroid. 1995;5(5):425–434. doi: 10.1089/thy.1995.5.425. [DOI] [PubMed] [Google Scholar]
- 13.Rosen IB, Walfish PG. Pregnancy as a predisposing factor in thyroid neoplasia. Archives of Surgery. 1986;121(11):1287–1290. doi: 10.1001/archsurg.121.11.1287. [DOI] [PubMed] [Google Scholar]
- 14.Hod M, Sharony R, Friedman S, Ovadia J. Pregnancy and thyroid carcinoma: a review of incidence, course and prognosis. Obstetrical and Gynecological Survey. 1989;44(11):774–779. [PubMed] [Google Scholar]
- 15.Neale RE, Darlington S, Murphy MFG, Silcocks PBS, Purdie DM, Talbäck M. The effects of twins, parity and age at first birth on cancer risk in Swedish women. Twin Research and Human Genetics. 2005;8(2):156–162. doi: 10.1375/1832427053738809. [DOI] [PubMed] [Google Scholar]
- 16.Shaha AR, Loree TR, Shah JP, et al. Prognostic factors and risk group analysis in follicular carcinoma of the thyroid. Surgery. 1995;118(6):1131–1138. doi: 10.1016/s0039-6060(05)80124-2. [DOI] [PubMed] [Google Scholar]
- 17.Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. American Journal of Medicine. 1994;97(5):418–428. doi: 10.1016/0002-9343(94)90321-2. [DOI] [PubMed] [Google Scholar]
- 18.Oduncu FS, Kimmig R, Hepp H, Emmerich B. Cancer in pregnancy: maternal-fetal conflict. Journal of Cancer Research and Clinical Oncology. 2003;129(3):133–146. doi: 10.1007/s00432-002-0406-6. [DOI] [PubMed] [Google Scholar]
- 19.Mazzaferri EL. Approach to the pregnant patient with thyroid cancer. Journal of Clinical Endocrinology and Metabolism. 2011;96(2):265–272. doi: 10.1210/jc.2010-1624. [DOI] [PubMed] [Google Scholar]
- 20.Santin AP, Furlanetto TW. Role of estrogen on thyroid function and growth regulation. Journal of Thyroid Research. 2011;2011:7 pages. doi: 10.4061/2011/875125. Article ID 875125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuy S, Roman SA, Desai R, Sosa JA. Outcomes following thyroid and parathyroid surgery in pregnant women. Archives of Surgery. 2009;144(5):399–406. doi: 10.1001/archsurg.2009.48. [DOI] [PubMed] [Google Scholar]
- 22.Abalovich M, Amino N, Barbour LA, et al. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society Clinical Practice Guideline. The Journal of Clinical Endocrinology and Metabolism. 2007;92(8 , supplement):S1–S47. doi: 10.1210/jc.2007-0141. [DOI] [PubMed] [Google Scholar]


