Abstract
Objective
A PET method is developed for non-invasive measurement of regional metabolic liver function using the galactose analog 2-[18F]fluoro-2-deoxy-D-galactose, FDGal. The aim of the present study was to determine the reproducibility of the method in pigs before translating it to human studies.
Material and methods
Five anesthetized pigs were studied twice within an interval of three days. A dynamic PET recording was performed with an injection of 100 MBq FDGal. Non-radioactive galactose was administered throughout the PET recordings to achieve near-saturated elimination kinetics. Arterial blood samples were collected for determination of blood concentrations of FDGal and galactose (cgal). Net metabolic clearance of FDGal, KFDGal, was calculated from linear representation of data. The approximate maximal hepatic removal rate, Vmax, of galactose (mmol/l tissue/min) was calculated as KFDGal cgal. The estimates from Day 1 and Day 2 were compared and the coefficient of variation, COV, of the estimates calculated. Functional heterogeneity in normal pig liver was evaluated as COV of the tissue concentration of radioactivity during quasi steady-state metabolism.
Results
There was no significant difference between Vmax from Day 1 and Day 2 (p = 0.38), and the reproducibility was good with a COV of 14% for the whole liver. In normal pig liver tissue, mean COV after an injection of FDGal was on average 15.6% with no day-to-day variation (p = 0.7).
Conclusions
The novel FDGal PET method for determination of hepatic metabolic function has a good reproducibility and is promising for future human studies of regional liver function.
Keywords: Galactose elimination capacity, hepatobiliary, liver metabolism, nuclear hepatology, physiology
Introduction
The galactose elimination capacity, GEC, is a clinical liver test that gives an approximate measure of the liver’s maximum removal rate of galactose, Vmax, and is interpreted as a measure of metabolic liver function [1–4]. Galactose is converted to galactose-1-phosphate by galactokinase, an enzyme found almost exclusively in the cytosol of hepatocytes. The GEC is performed as a single intravenous injection of galactose to a blood concentration of galactose high enough to ensure near-saturation of the galactokinase enzyme, followed by measurements of the declining blood concentration of galactose in arterialized, capillary blood samples. When corrected for the amount of galactose excreted in urine, an approximate estimate of the hepatic Vmax for galactose is achieved from the blood disappearance curve using blood concentrations ensuring near-saturation of the enzymatic system [1–4]. The GEC accurately assessed survival prognosis of patients with acute liver failure [5], chronic liver disease [6,7], and patients undergoing liver resection [8]. More interestingly, it has been shown that quantitative liver function tests such as the GEC are far more predictive of the remaining liver function following partial hepatectomy than measurements of liver volumes [9–11].
The GEC is, however, biased by a minor but variable extra-hepatic galactose metabolism which cannot be corrected for [12,13]. Also, the test gives an estimate of metabolic function of the whole liver but does not provide information on potential regional differences which in some cases could be of particular interest, for example, in clinical evaluation of patients with liver cirrhosis or in patients undergoing local treatment of diseases of the liver such as stereotactic body radiation therapy.
We recently developed an in vivo method for measuring local hepatic GEC in pigs using PET and the galactose-analog 2-[18F]fluoro-2-deoxy-D-galactose, FDGal [14]. The method is based on Michaelis-Menten saturation kinetics of the hepatic galactose elimination:
| (1) |
where v is the removal rate of galactose and Km is the concentration (mM) at which v = ½Vmax. When the blood concentration of galactose, c, is >10 Km, then v approximates Vmax with more than 90% [15]. The mean hepatic Km for galactose in pig in vivo is 0.25 mM [16] and the hepatic galactose metabolism may hence be considered at near-saturation at c >3 mM. In this case, the approximate Vmax can be calculated from PET data using the equation [14]:
| (2) |
where cGal is the arterial concentration of galactose and KFDGal is the net metabolic clearance of FDGal calculated from the PET data. The number 6.92 in Equation (2) is the reciprocal of the lumped constant for FDGal, which corrects for the different affinity by galactokinase for galactose and FDGal [14].
As the PET method provides 3-dimensional images of the galactose metabolism, we expect that this novel method will make it possible to measure potential regional variations in metabolic liver function in patients with diseases of the liver and to evaluate regional metabolic effects of treatment such as radiation therapy. In addition, because the PET camera records the concentration of radioactivity in liver tissue, the FDGal PET-GEC estimate is unaffected by any extrahepatic distribution and metabolism of FDGal and galactose. For the novel PET method to have clinical potential it must, however, be precise and reproducible within reasonable limits; the classic GEC had a reproducibility of 10% [4]. Therefore, the aim of the present study was to study the reproducibility of the FDGal PET-GEC in pigs. This was done by studying the same animal twice on two different days and comparing the estimated values. Normal functional heterogeneity in pig liver was also assessed from the PET data.
Methods
Animals and preparation
Five female pigs (Yorkshire and Danish Landrace crossbreed; body weight 37–39 kg; mean 38 kg) underwent duplicate FDGal PET/CT recordings on two separate days, three days apart. The animals were fasted for 16 h before each experiment but had free access to water. On Day 1, the animal was given an intramuscular (i.m.) injection of 600 mg amoxicillin on arrival to the PET center in order to prevent infections from the procedures. The pig was sedated with an i.m. injection of 50 mg midazolam + 250 mg S-ketamine. A cannula was placed in an ear vein and anesthesia induced by intravenous (i.v.) injection of 50 mg midazolam + 125 mg S-ketamine. The animal was then intubated and ventilated mechanically (Hallowell EMC model 2000 respirator). Anesthesia was maintained by isoflurane administered through the respirator (Hallowell EMC Model 200 Respirator). Using sterile procedures, an open incision was made in the left femoral region and catheters (Cordis, Waterloo, Belgium) were placed into the left femoral artery and the femoral vein.
At the end of the PET study, anesthesia was terminated and the femoral catheters were removed. The blood vessels were compressed until complete hemostasis was obtained and the incision in the femoral region was closed by single sutures using sterile procedures. The pig was extubated when spontaneous respiration was observed. An analgesic (75 mg flunixin) was injected i.m. and the animal observed closely for the rest of the day. The next day, the animal received an i.m. injection of 75 mg flunixin and 600,000 IE benzylpenicillinprocain.
On Day 2, the animals were prepared as on Day 1 except that the catheters were placed in the right femoral artery and vein and no antibiotics were given. At the end of the PET study, the anesthetized animals were euthanized by an i.v. injection of a pentobarbital overdose. The liver was removed and the liver tissue density was measured (mean 1.05 g/ml; range 1.02–1.07 g/ml).
Physiological parameters (body temperature, arterial pO2, pCO2, pH, and blood glucose) were monitored throughout each study and kept within normal reference values by proper correction, if necessary [14].
Infusion of unlabeled galactose
In order to achieve steady-state blood concentrations of galactose high enough to ensure near-saturation of the hepatic galactose metabolism, non-radioactive galactose (Kabi, Sweden) dissolved in 0.9% saline was administered by means of a constant i.v. infusion (2.77 mmol/ml; infusion rate 27–30 ml/h) preceded by a priming dose of 70 mmol galactose in 50 ml 0.9% saline. Doses were based on experiences from the in vivo PET study in pigs [14]. The infusion was started 1 h before (mean 69 min, range 58–80) and continued throughout the PET experiment. On Day 2, the time from infusion start to the start of the PET recording was kept as similar as possible to that on Day 1.
PET/CT protocol
The pig was placed in a supine position on the scanner bed of a combined PET/CT camera (40-slice Siemens Biograph TruePoint PET/CT; Siemens AG, Erlangen, Germany). Before each PET-study, a CT scan of the liver was performed 35 s after i.v. injection of 60 ml contrast media (Visipaque 270 mg/ml; Nycomed Amersham). A 60 min dynamic PET recording was performed with an i.v. injection of 100 MBq FDGal given over the initial 15 s. PET data were recorded in list-mode and reconstructed in a Gaussian filtered back-projection mode using the CT scan for attenuation correction of emission data and corrected for radioactive decay back to start of the scan. This yielded pictures with a resolution of 6.7 mm full width at half maximum and a voxel size of 2.4 × 2.4 × 3.1 mm3. Data were reconstructed using a time-frame structure of 18 × 5 s, 15 × 10 s, 4 × 30 s, 4 × 60 s, and 6 × 300 s, and 2 × 600 s (total 60 min). During each PET recording, arterial blood samples (0.5 ml) were collected manually (18 × 5 s, 6 × 10 s, 3 × 20 s, 3 × 60 s, 1 × 120 s, 1 × 240 s, 1 × 360 s, and 4 × 600 s) for determination of arterial blood concentration of FDGal. The samples were counted in a Packard well-counter (Packard Instruments, Meridin, CT) and corrected for radioactive decay back to start of the scan, generating an arterial blood time-activity-curve (TACartery; kBq/ml blood vs. minutes after tracer injection). Additional blood samples were collected at time 0, 20, 40, and 60 min for determination of arterial blood concentration of galactose [17].
Data analysis
Using the fused FDGal PET and contrast enhanced CT images, volumes of interest (VOIs) were drawn in the liver avoiding large blood vessels and the very top of the liver which is subject to respiratory movements. Two types of VOIs were used for analysis; a whole liver VOI encircling as much liver tissue as possible and five minor VOIs placed in different regions of the liver. Larger hepatic blood vessels were avoided using the contrast-enhanced CT image. For the whole liver VOI, the mean VOI size was 740 ml (range 690–1020) on Day 1 and 800 ml (ranges 670–910) on Day 2, the difference being statistically insignificant (p = 0.7). The mean size of the small VOIs was 1.92 ml (range 1.17–3.96) on Day 1 and 2.05 ml (range 1.10–4.43) on Day 2. For each pair of the small VOIs, the difference in volume between Day 1 and Day 2 was statistically insignificant (p = 0.38).
The full time course of the tissue concentration of radioactivity in each VOI was generated from the dynamic PET data (TACliver; kBq/ml tissue vs. minutes after tracer injection). The net metabolic clearance of FDGal, KFDGal, was calculated from the data during quasi steady-state metabolism (17.5–40 min after injection of the tracer, [14]) according to the Gjedde-Patlak representation of data [18,19]. KFDGal was used to calculate the maximum hepatic galactose elimination, Vmax, according to Equation (2) [14]. The estimated Vmax has the unit mmol/l tissue/min and was calculated for Day 1 (Vmax–1) and Day 2 (Vmax–2). For comparison with estimates from other studies, a Vmax corrected for tissue density (1.05 kg/l tissue) was also calculated. Additionally, an estimate of the total liver function, Vmax–total (mmol/min), was calculated by multiplying the Vmax with the corresponding volume of the VOI for the whole liver.
Day-to-day differences in measured parameters were assessed using the paired t-test. The reproducibility of the method was evaluated by the coefficient of variation (SD/mean), COVestimate, for the duplicate estimates of Vmax.
Functional heterogeneity within the liver tissue was evaluated as COVtissue calculated for the tissue radioactivity concentration measured by PET during quasi steady-state metabolism. COVtissue was calculated as the standard deviation of the radioactivity concentrations in the voxels within the VOI divided by the mean radioactivity concentration in the voxels which is a sensitive measure of metabolic heterogeneity [20].
Results
Table I gives the individual mean arterial blood concentrations of galactose during each PET-recording. Good approximation to steady-state of the arterial concentrations of galactose was obtained in all 10 studies; on average, the concentrations did not deviate more than 2% within each experimental time period. For calculation of approximate Vmax values, the individual mean of the four concentrations from the same PET recording was accordingly used. The individual mean arterial blood concentrations of galactose did not differ significantly between the two days (p = 0.34).
Table I.
Approximate maximum hepatic removal rate, Vmax, of galactose
| Experiment | Arterial concentration of galactose (mM)
|
Hepatic Vmax for galactose (mmol/l tissue/min)
|
Hepatic Vmax–total for galactose (mmol/liver/min)
|
|||
|---|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 1 | Day 2 | Day 1 | Day 2 | |
| 1 | 3.13 | 4.30 | 0.27 | 0.45 | 0.28 | 0.41 |
| 2 | 6.09 | 7.06 | 0.59 | 0.90 | 0.44 | 0.60 |
| 3 | 5.05 | 5.93 | 0.76 | 0.82 | 0.57 | 0.60 |
| 4 | 6.07 | 4.99 | 0.61 | 0.44 | 0.47 | 0.41 |
| 5 | 5.53 | 5.80 | 0.75 | 0.69 | 0.52 | 0.60 |
| Mean ± SEM | 5.17 ± 0.54 | 5.62 ± 0.46★ | 0.60 ± 0.09 | 0.66 ± 0.09† | 0.46 ± 0.05 | 0.52 ± 0.05‡ |
The results were calculated using the whole liver volume of interest.
Not significantly different from Day 1 (p = 0.34; paired t-test).
Not significantly different from Day 1 (p = 0.49; paired t-test); COVestimate = 19%.
Not significantly different from Day 1 (p = 0.16; paired t-test); COVestimate = 14%.
The approximate Vmax values are also given in Table I, both expressed as mmol galactose/l tissue/min and as mmol/liver/min. No statistically significant differences between the two days were observed for either of the two estimates (p = 0.49 and p = 0.16, respectively). The COVestimate improved from 19% to 14% when correcting for liver volume.
For the five small VOIs, the estimated Vmax–local in each region was significantly different between the two days (p < 0.01). However, when looking at the regional fraction of the total Vmax (Vmax–local/Vmax–total), the difference was statistically insignificant (p = 0.94), with an average fraction of 1.11 (range 0.97–1.19) and a mean COVestimate of 7% (range 0.01–21%).
The mean COVtissue of radioactivity concentration within the whole liver VOIs was 15.6% (range 14.0–16.7%) on Day 1. No difference between Day 1 and Day 2 was observed (p = 0.7). For the small VOIs, the mean COVtissue was 9.8% (range 8.8–10.4%) on Day 1; no difference between Day 1 and Day 2 was observed (p = 0.5). The COVtissue values for the small VOIs were significantly lower than COVtissue for the whole liver VOIs (p < 0.01).
Discussion
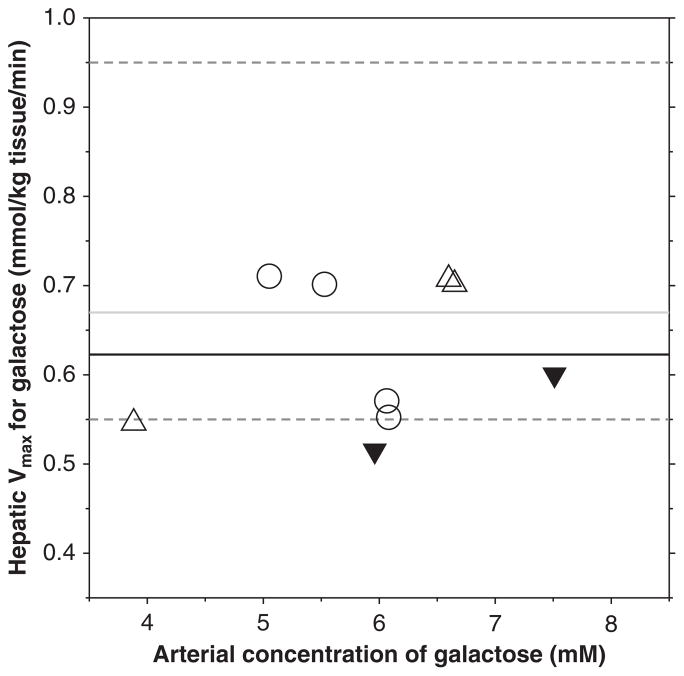
Based on seven successive determinations in one healthy person, Tygstrup found a COV of 8.1% for the classic GEC and based on duplicate measurements in 20 subjects an overall reproducibility of about 10% [4]. The mean COVestimate of 14% for the approximate hepatic Vmax–total for galactose measured in the present study using FDGal PET/CT is slightly higher, but considered comparable to the well-established classic GEC method. Figure 1 shows the individual estimates of Vmax–total from the pigs in the present study (Day 1) together with the results from Sørensen et al. [14] and two previously unpublished pig studies (Sørensen, unpublished observations). The PET-estimated mean Vmax of 0.62 mmol/kg tissue/min compares well with the mean hepatic Vmax for galactose of 0.67 mmol/kg tissue/min determined in the intact pig by means of liver vein catheterization (range 0.55–0.95 mmol/kg tissue/min) [16]. This supports the view that the FDGal PET method provides good estimates of the hepatic Vmax for galactose.
Figure 1.
Estimates of approximate Vmax for galactose in pig liver using FDGal PET (experiments no. 2–5; Day 1 in the present study (○)), three pigs from [14] (△), and two previously unpublished pigs (▼) (Sørensen, unpublished observations). The black line represents the mean Vmax from all PET studies, the grey line is the mean in vivo Vmax in pigs with the dashed lines representing upper and lower observations measured using liver vein catheterization [16]. FDGal = 2-[18F]fluoro-2-deoxy-D-galactose.
When correcting for the volume of the VOI, i.e. when calculating the Vmax–total, the COVestimate improved from 19% to 14%. This agrees with the observations that the hepatic GEC is unaffected by changes in hepatic blood flow and blood volume [21–23] which might cause the minor differences in liver volumes observed between the two days. Also, as the PET camera records radioactivity concentrations in terms of radioactivity per volume tissue, any changes in blood volume within a VOI could affect the tissue radioactivity concentration, an effect the correction for minor differences in VOI volumes also eliminates.
The Vmax–total on Day 1 in experiment 1 was only 0.28 mmol/min which is somewhat lower than expected [14,16] and lower than the results from the other four experiments. It may be ascribed to normal biological variation, but it could also be that the arterial galactose concentration of 3.13 mM reached in that particular experiment may have been too low to ensure near-saturation of the hepatic galactose metabolism. As mentioned, the calculated values of Vmax only approximate the true Vmax by the factor c/(c + Km); if, however, c≫Km the approximate Vmax becomes very close to the true Vmax [15]. The mean Km for hepatic galactose metabolism in pigs in vivo is 0.25 mM [16], but with individual measurements ranging from 0.12 to 0.58 mM [16]. Thus, Km in the pig in experiment 1 may very well be higher than the mean Km of 0.25 mM and the metabolism hence not near-saturated which is essential to get a reliable estimate of Vmax [14,15]. Moreover, if the enzymatic system is not near-saturated, the kinetics depend not only on the enzymatic Vmax and Km for galactose but also on hepatic blood flow and time-dependent distribution of the tracer and unlabeled substrate [14,15]. In the in vivo situation, with a concentration gradient of substrate from inlet to outlet of the sinusoid [24], the logarithmic mean sinusoidal concentration ĉ is introduced, which is defined as ĉ = (ci–co)/ln(ci/co), where ci and co are the sinusoidal inlet and outlet substrate concentrations, respectively [24]. Because this requires access to blood sampling from a liver vein (co) and since PET is traditionally based on compartmental analysis, ĉ is not used in PET studies. At a sufficiently high inlet concentration, however, the difference between ĉ and ci can safely be ignored, which is the case at near-saturation [14,24]. For practical purposes and based on the present observations, it is recommended that an arterial concentration above 5 mM is used in order to fully ensure near-saturation of the hepatic galactose metabolism when determining the hepatic GEC using FDGal PET.
For the small VOIs, the pair-wise difference in Vmax–local was statistically significant between the two days. This may be ascribed to minor differences in placement of the VOIs in the liver tissue, although all effort was put into avoiding this. Again, differences in relative blood volume may affect the PET data, especially in smaller regions. When normalizing the Vmax in each region to the Vmax–total, the difference between the two days was statistically insignificant (p = 0.94) suggesting that for smaller regions, such normalization would be beneficial when evaluating possible day-to-day variations.
The COVtissue of radioactivity concentration within the VOIs was on average 15.6% (range 14.0–16.7%) on Day 1 which can be used as a reference of functional heterogeneity within the normal pig liver [20]. The COVtissue was lower for the smaller regions (mean 9.8%, range 8.8–10.4%) which may be explained by a higher probability of including larger blood vessels in the large VOIs than in the smaller VOIs. This could also explain why the fractional Vmax, viz. Vmax–local/Vmax–total, tended to be slightly higher than unity with a mean value of 1.11.
In conclusion, the overall reproducibility of the FDGal PET-GEC method for determining approximated Vmax for galactose in pig livers with a COV of about 14% is comparable to the classic and clinically validated GEC method. The blood concentration of galactose needed to ensure near-saturation of the galactokinase seems, however, to be higher than the previously assumed 3 mM and concentrations above at least 4–5 mM are recommended to ensure near-saturation in pigs. We expect that the method can easily be translated to human studies, making it possible for the first time to assess regional differences in metabolic liver function in both healthy and cirrhotic livers. The method also enables studies of the effects of local treatments on the metabolic function in normal and/or cirrhotic liver tissue.
Acknowledgments
The author thanks the highly skilled staff at the PET Center, Aarhus University Hospital, especially veterinarian Aage Kristian Astrup Olsen without whom the project could not have been conducted. The author would also like to thank Susanne Keiding for fruitful discussions on the paper. The study was supported by grants from the NIH (R01 DK074419-01), the Danish Medical Research Council (271-06-0357), the Danish Cancer Society (DP06114), Aarhus University Foundation, the Novo Nordisk Foundation, and the A.P. Møller Foundation for the Advancement of Medical Science.
Footnotes
Declaration of interest: The author reports no conflicts of interest. The author alone is responsible for the content and writing of the paper.
References
- 1.Tygstrup N. Determination of the hepatic elimination capacity (Lm) of galactose by single injection. Scand J Clin Lab Invest Suppl. 1966;18:118–25. [PubMed] [Google Scholar]
- 2.Tygstrup N. Effect of sites of blood sampling in determination of the galactose elimination capacity. Scand J Clin Lab Invest. 1977;37:333–8. doi: 10.3109/00365517709092638. [DOI] [PubMed] [Google Scholar]
- 3.Tygstrup N, Winkler K. Kinetics of galactose elimination. Acta Physiol Scand. 1954;32:354–62. doi: 10.1111/j.1748-1716.1954.tb01182.x. [DOI] [PubMed] [Google Scholar]
- 4.Tygstrup N. Determination of the hepatic galactose elimination capacity after a single intravenous injection in man. The reproducibility and the influence of uneven distribution. Acta Physiol Scand. 1963;58:162–72. doi: 10.1111/j.1748-1716.1963.tb02638.x. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt LE, Ott P, Tygstrup N. Galactose elimination capacity as a prognostic marker in patients with severe acetaminophen-induced hepatotoxicity: 10 years’ experience. Clin Gastroenterol Hepatol. 2004;2:418–24. doi: 10.1016/s1542-3565(04)00128-4. [DOI] [PubMed] [Google Scholar]
- 6.Merkel C, Marchesini G, Fabbri A, Bianco S, Bianchi G, Enzo E, et al. The course of galactose elimination capacity in patients with alcoholic cirrhosis: possible use as a surrogate marker for death. Hepatology. 1996;24:820–3. doi: 10.1053/jhep.1996.v24.pm0008855183. [DOI] [PubMed] [Google Scholar]
- 7.Jepsen P, Vilstrup H, Ott P, Keiding S, Andersen PK, Tygstrup N. The galactose elimination capacity and mortality in 781 Danish patients with newly-diagnosed liver cirrhosis: a cohort study. BMC Gastroenterol. 2009;30:50. doi: 10.1186/1471-230X-9-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Redaelli CA, Dufour JF, Wagner M, Schilling M, Hüsler J, Krähenbühl L, et al. Preoperative galactose elimination capacity predicts complications and survival after hepatic resection. Ann Surg. 2002;235:77–85. doi: 10.1097/00000658-200201000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zoedler T, Ebener C, Becker H, Roeher HD. Evaluation of liver function tests to predict operative risk in liver surgery. HPB Surg. 1995;9:13–18. doi: 10.1155/1995/47538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nadalin S, Testa G, Malagó M, Beste M, Frilling A, Schroeder T, et al. Volumetric and functional recovery of the liver after right hepatectomy for living donation. Liver Transplant. 2004;10:1024–9. doi: 10.1002/lt.20182. [DOI] [PubMed] [Google Scholar]
- 11.Jochum C, Beste M, Penndorf V, Farahani MS, Testa G, Nadalin S, et al. Quantitative liver function tests in donors and recipients of living donor liver transplantation. Liver Transplant. 2006;12:544–9. doi: 10.1002/lt.20627. [DOI] [PubMed] [Google Scholar]
- 12.Keiding S. Galactose clearance measurements and liver blood flow. Gastroenterology. 1988;94:477–81. doi: 10.1016/0016-5085(88)90440-4. [DOI] [PubMed] [Google Scholar]
- 13.Winkler K, Henriksen JH, Tygstrup N. Hepatic, renal, and total body galactose elimination in the pig. Am J Physiol Gastrointest Liver Physiol. 1993;265:G9–14. doi: 10.1152/ajpgi.1993.265.1.G9. [DOI] [PubMed] [Google Scholar]
- 14.Sørensen M, Munk OL, Mortensen FV, Olsen AK, Bender D, Bass L, et al. Hepatic uptake and metabolism of galactose can be quantified in vivo by 2-18Ffluoro-2-deoxygalactose positron emission tomography. Am J Physiol Gastrointest Liver Physiol. 2008;295:G27–36. doi: 10.1152/ajpgi.00004.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keiding S, Sørensen M. Hepatic removal kinetics: importance for quantitative measurements of liver function. In: Rodés J, Benhamou JP, Blei AT, Reichen J, Rizzetto M, editors. Textbook of hepatology: from basic science to clinical practice. 3. London: Blackwell Publishing; 2007. pp. 468–78. [Google Scholar]
- 16.Keiding S, Johansen S, Winkler K. Hepatic galactose elimination kinetics in the intact pig. Scand J Clin Lab Invest. 1982;42:253–9. [PubMed] [Google Scholar]
- 17.Kurz G, Wallenfels K. D-Galactose, UV-test mit galactose-dehydrogenase. In: Bergmeyer HU, editor. Methoden der Enzymatischen Analyse. Weinheim/Bergstr: Verlag Chemie; 1970. pp. 1241–4. [Google Scholar]
- 18.Gjedde A. Calculation of cerebral glucose phosphorylation from brain uptake of glucose analogs in vivo: a reexamination. Brain Res Rev. 1982;257:237–74. doi: 10.1016/0165-0173(82)90018-2. [DOI] [PubMed] [Google Scholar]
- 19.Patlak CS, Blasberg RG, Fenstermacher JD. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J Cereb Blood Flow Metab. 1983;3:1–7. doi: 10.1038/jcbfm.1983.1. [DOI] [PubMed] [Google Scholar]
- 20.Keiding S, Munk OL, Vilstrup H, Nielsen DT, Roelsgaard K, Bass L. Hepatic microcirculation assessed by positron emission tomography of first-pass ammonia metabolism in porcine liver. Liver Int. 2005;25:171–6. doi: 10.1111/j.1478-3231.2005.01032.x. [DOI] [PubMed] [Google Scholar]
- 21.Jacobsen KR, Ranek L, Tygstrup N. Liver function and blood flow in normal man during infusion of vasopressin. Scand J Clin Lab Invest. 1969;24:279–84. doi: 10.3109/00365516909080163. [DOI] [PubMed] [Google Scholar]
- 22.Greenway CV, Innes IR, Pushka KL, Scott GD, Sitar DS. Changes in hepatic blood volume on galactose and indocyanine green uptake by cat liver. Am J Physiol Gastrointest Liver Physiol. 1989;256:G524–31. doi: 10.1152/ajpgi.1989.256.3.G524. [DOI] [PubMed] [Google Scholar]
- 23.Keiding S, Vilstrup H, Hansen L. Importance of flow and haematocrit for metabolic function of perfused rat liver. Scand J Clin Lab Invest. 1980;40:355–9. doi: 10.3109/00365518009092655. [DOI] [PubMed] [Google Scholar]
- 24.Bass L, Keiding K, Winkler K, Tygstrup N. Enzymatic elimination of substrates flowing through the intact liver. J Theor Biol. 1976;61:393–409. doi: 10.1016/0022-5193(76)90026-6. [DOI] [PubMed] [Google Scholar]