Abstract
Objective
Assessed the convergent and discriminant validity of a water load symptom provocation test (WL-SPT) in creating visceral sensations similar to the naturally occurring sensations experienced by children with functional abdominal pain.
Methods
Participants were pediatric patients with functional abdominal pain (N = 110) and healthy school children (N = 120) between the ages of 8 and 16 years. Pain patients completed questionnaires describing gastrointestinal (GI) and nongastrointestinal (non-GI) symptoms associated with their typical abdominal pain episodes. Weeks later, the WL-SPT was administered to pain patients and well children. Before and immediately following the WL-SPT, children rated their symptoms.
Results
The WL-SPT produced (a) significant increases in children’s GI symptoms that were reliably predicted by the children’s naturally occurring GI symptoms, and (b) significantly greater increases in GI symptoms in pain patients than in well children.
Conclusions
The WL-SPT produces clinically relevant symptoms for laboratory studies of children with functional abdominal pain.
Keywords: children, functional abdominal pain, pain perception, recurrent abdominal pain, visceral hypersensitivity
Abdominal pain is the most common recurrent pain condition in children and adolescents (McGrath, 1990). Although recurrent abdominal pain is sometimes mistakenly referred to as a diagnosis, it is in fact only a description of symptoms (von Baeyer & Walker, 1999). The pain is typically functional, that is, medical evaluations rarely yield evidence of organic disease (Dorn et al., 2003; Stickler & Murphy, 1979; Walker, Garber, Van Slyke, & Greene, 1995). Most pediatric patients evaluated for recurrent abdominal pain meet the recently developed diagnostic criteria for a functional gastrointestinal (GI) disorder associated with abdominal pain (Rasquin-Weber et al., 1999; Walker et al., 2004).
Several investigators have used a cold pressor procedure (immersion of the forearm into cold water) as a laboratory pain stimulus to test the hypothesis that autonomic nervous system dysfunction is a biological substrate for pediatric recurrent abdominal pain (e.g., Apley, Tulloh, & Haslam, 1971; Battistella, Carra, Zaninotto, Ruffilli, & Da Dalt, 1992; Feuerstein, Barr, Francoeur, Houle, & Rafman, 1982; Rubin, Barbero, & Sibinga, 1967). Although results have been mixed, the most comprehensive of these studies (Feuerstein et al., 1982) found no differences between children with and without abdominal pain on measures of digital blood volume, heart rate, muscle tension, subjective pain reports, or behavioral responses to cold pressor pain.
A critical shortcoming of using the cold pressor procedure in studies of abdominal pain patients is that it induces somatic rather than visceral pain. Visceral sensations, including abdominal pain and discomfort, are associated with spinal and vagal-sacral sensory nerves and involve different pain transmission systems than somatic sensations (Zeltzer, Bush, Chen, & Riveral, 1997). Thus, observations of pain perception and pain behavior based on the cold pressor may not generalize to how patients respond to their abdominal pain. In vivo studies involving visceral sensation rarely have been conducted with pediatric patients, in part because an acceptable experimental procedure for inducing these sensations in children has not been identified.
Laboratory studies of visceral abdominal sensation in adults use balloon distention via a tube inserted into the upper or lower GI tract and inflated to induce visceral discomfort (e.g., Mertz, Naliboff, Munakata, Niazi, & Mayer, 1995). Results of many such studies with adults (e.g., Bouin et al., 2002; Mertz et al., 1995; Trimble, Farouk, Pryde, Douglas, & Heading, 1995) and two studies with children (DiLorenzo et al., 2001; Van Ginkel, Voskuijl, Benninga, Taminiau, & Boeckxstaens, 2001) suggest that visceral hyperalgesia may play an important role in functional abdominal pain. Although this laboratory paradigm promises to yield considerable information on visceral pain in adults, its use for pediatric studies is limited because the procedure is invasive and may produce unacceptably high levels of discomfort. In addition, the most widely used balloon placement, in the rectum, produces primarily local sensations rather than more general abdominal discomfort.
In contrast to balloon distention in the GI tract, the water load (WL) test is a noninvasive procedure that induces visceral sensations associated with gastric distention. Previously used for clinical studies of gastric myoelectrical activity (Koch, Bingaman, Muth, & Ouyang, 1997; Koch, Hong, & Xu, 2000), the WL test requires the patient to drink water until “full” while electrogastrograms (EGGs) are recorded by means of electrodes on the surface of the patient’s abdomen. Symptoms of abdominal distress are assessed before water ingestion and at subsequent intervals during EGG recording. Although the purpose of these studies is to evaluate gastric dysrhythmias, they also have shown that adult patients with functional GI disorders reported being “full” after ingesting smaller quantities of water and reported more GI symptoms (e.g., nausea, bloating) following water ingestion compared to well controls (Koch et al., 1997, 2000).
The incidental finding that the WL test induced GI symptoms suggested to us that it might serve as a safe, noninvasive method for inducing visceral discomfort in children for experimental purposes. Laboratory studies of spouses’ responses to chronic pain patients when they are experiencing pain have yielded important information regarding social reinforcement of symptoms and disability in adult pain patients (e.g., Romano et al., 1995). We reasoned that the WL test might be used to provoke abdominal discomfort for laboratory studies of the role of contextual factors in children’s experience of abdominal discomfort. Before adopting the WL as a symptom provocation test (WL-SPT) for laboratory studies, however, it was important to demonstrate that it in fact produced sensations similar to, but less intense than, those associated with naturally occurring episodes of abdominal pain.
Thus, the first aim of this study was to test the validity of the WL as a WL-SPT to produce abdominal discomfort similar to that experienced by pediatric patients with functional abdominal pain. We predicted that, for children with functional abdominal pain, the WL test would produce abdominal pain, discomfort, and other GI symptoms similar to those experienced during their naturally occurring episodes of abdominal pain. That is, we predicted both that abdominal pain, discomfort, and other GI symptoms would reliably increase in response to the WL test, and that these increases would be associated with patients’ reports of GI symptoms typically experienced during their bouts of abdominal pain. Thus, patients reporting high levels of GI symptoms during naturally occurring episodes of abdominal pain would report higher levels of GI symptoms following the WL than patients reporting low levels of GI symptoms during abdominal pain episodes. Because children with functional abdominal pain also report high levels of more general somatization symptoms (Routh, Ernst, & Harper, 1988; Walker, Garber, & Greene, 1991), it was important to examine the possibility that symptoms produced by the WL test merely reflected general symptom-reporting tendencies rather than symptoms specific to WL-related visceral sensation. Therefore, we also assessed non-GI symptoms and predicted that any increases in these symptoms following the WL test would be small relative to the increases observed for GI symptoms. Furthermore, we expected that increases in non-GI symptoms would not be associated with the non-GI symptoms typically reported by children with functional abdominal pain. In this way, we tested the convergent and discriminant validity of the WL as a GI WL-SPT in children with functional abdominal pain.
Our second aim was to test the utility of the WL test in discriminating patients with functional abdominal pain from well children. There are two ways in which the WL might discriminate pain patients from well children. First, it is possible that pain patients would reach the sensation of fullness at a lower volume of water intake than well children. This would be consistent with prior research showing that adult patients with functional dyspepsia consume less water in the WL test than healthy volunteers (Boeckxstaens, Hirsch, van den Elzen, Heisterkamp, & Tytgat, 2001; Koch et al., 2000). However, participants in previous studies had visual cues regarding the amount of water they consumed and it is possible that these cues, rather than internal sensations of fullness, influenced the amount of water they required to reach perceived fullness. In addition, prior studies did not control for body mass index (BMI) in assessing group differences in the volume of water consumed. Therefore, we modified Koch’s WL procedure to eliminate visual cues and controlled for BMI in testing the hypothesis that pediatric patients with functional abdominal pain would report fullness at a lower volume of water ingestion than well children.
The WL test also might discriminate functional abdominal pain patients from well children in terms of the impact of water ingestion on symptoms. Prior research has shown that, compared to healthy volunteers, adult patients with functional dyspepsia reported significantly higher levels of GI symptoms in response to water ingestion (Boeckxstaens et al., 2001; Koch et al., 2000). However, these studies did not control for baseline symptoms in assessing the impact of water ingestion on symptoms, even though baseline symptoms were higher in the functional pain group than in healthy volunteers in one of the studies (Boeckxstaens et al., 2001). Therefore, in this study, we controlled for baseline symptoms in testing the hypothesis that patients with functional abdominal pain would report significantly greater increases in GI symptoms than well children following the WL test. In another innovation, we also assessed changes in symptoms of positive and negative affect (NA) and predicted that the WL test would produce greater increases in negative affect and greater decreases in positive affect (PA) for pain patients than for well children.
Method
Participants
Abdominal Pain Patients
The patient sample (N = 110) consisted of children ages 8 through 16 years (M = 11.23; SD = 1.94) referred to a pediatric gastroenterology clinic for evaluation of abdominal pain. Eligibility criteria included (a) at least three episodes of abdominal pain that interfered with activities during the previous 3 months (Apley & Naish, 1958; Apley, 1975), (b) no chronic illness or disability, (c) living with a parent, and (d) no organic disease revealed in the medical evaluation by the referring provider or the pediatric gastroenterology service. The sample was 60% female. Participants were 93% Caucasian, 4% African-American, 2% Asian, and 1% Hispanic. Duration of abdominal pain ranged from 3 months to 11 years (M = 71.95 months; SD = 194.78).
Well Children
Participants (N = 120) in a public school survey of children’s health were eligible for the study if they (a) ranged in age from 8 to 15 years, (b) were living with a parent, (c) reported on the school survey that they had experienced no more than two episodes of abdominal pain in the past 2 weeks, (d) obtained a score on the Children’s Somatization Inventory (Walker & Greene, 1989) below the sample median for their gender, and (e) by parent report, had no chronic illness or disability. The sample ranged in age from 8 to 15 years (M = 11.46; SD = 2.11) and was 50% female. Participants were 95% Caucasian, 2% African-American, 1% Asian, and 2% from other or unknown ethnic backgrounds. T test and chi-square analyses revealed no demographic differences between the samples of abdominal pain patients and well children.
Recruitment
Patients
Following the research protocol approved by the Institutional Review Board, parents of children scheduled for evaluation of abdominal pain at a pediatric gastroenterology clinic were identified by clinic staff and contacted by telephone several days before their initial clinic visit. Those who expressed interest in participating in the study were screened for eligibility and were asked to arrive early for their child’s appointment. Members of the research team obtained informed consent at the clinic. A research protocol was administered before each child’s medical evaluation. A trained interviewer read the questionnaire items to children in a private room, and children selected answers from a printed response sheet. Children whose medical evaluation revealed no evidence of organic disease were invited to return to the medical center several weeks after their clinic visit to participate in the present laboratory study. Of the 240 patients who participated in the initial assessment, 198 met eligibility criteria for the laboratory study and were contacted by telephone. Of these, 110 (56%) completed the laboratory study. The remaining families declined participation or were unable to keep their appointment at the laboratory; 15 families agreed to participate but did not keep their scheduled appointments, and 73 families declined, primarily due to distance from the medical center (more than 80% of families lived outside the county). Patients who participated did not differ from those who declined with respect to duration of abdominal pain, age or gender. Written consent to participate in the laboratory study was obtained from the child and his or her parent before initiating the study protocol.
Well Children
The sample of well children was recruited from participants in a health survey conducted in the elementary and middle schools of a neighboring county. The survey was approved by the Institutional Review Board. Consent for participation in the survey included consent to be contacted by telephone regarding the laboratory study. Families of 189 children who completed the survey and met eligibility criteria for the laboratory study were contacted by telephone. Of these, 120 (63%) participated in the laboratory study (12 families agreed to participate but did not keep their scheduled appointments, and 57 families declined, primarily due to time constraints and distance from the medical center).
Instruments and Measures
WL-SPT
We modified the WL test used in Koch’s motility studies (Koch et al., 1997) so that children would not have visual or physical cues about how much water they were ingesting. A backpack water bag, such as those worn by long distance bicyclists, was filled with room temperature bottled water. The bag was connected to a 30-in. long, 5/16-in. diameter plastic tube with a drinking valve attached at the end of the tube. The bag was hung inside a large canvas backpack on the wall next to the child, with only the tube and valve exposed, so that children could neither see nor feel the change in water level as they drank. This arrangement helped to insure that children would focus on their own internal sensations, rather than external cues, to assess their perceived fullness. Children were shown how to use the drinking valve and were told to drink until they felt “completely full”, as illustrated on the Fullness Rating Scale (described below). The equipment was cleaned and a new drinking valve was provided for each child.
In the original WL studies, Koch instructed research participants to drink water for 5 min, “until completely full” (Koch et al., 1997). We pilot tested the WL-SPT and discovered that after 5 min many children still did not report feeling “completely full” on our Fullness Rating Scale. Because our intention was to induce perceived fullness, rather than to standardize drinking time or volume, we allowed the children to drink water for up to 15 min Children who did not reach fullness within 5 min were asked to rate their level of fullness on the Fullness Rating Scale at 5-min intervals. This procedure was implemented to insure that children did not push themselves to drink water beyond the point of perceived fullness. Thirteen children (6%) requested and were allowed to discontinue drinking water when they felt almost but not completely full. Questionnaires were administered before and immediately following water ingestion.
Measures
Abdominal Pain and Discomfort
The Faces Pain Scale-Revised (FPS-R) was used to rate children’s levels of abdominal pain and discomfort. The FPS-R was developed by Bieri and colleagues (1990) and recently revised by Hicks and colleagues (2001). It is a self-report visual analog pain scale appropriate for children as young as 6 years of age. The revised scale consists of a series of six drawings of facial displays that range from neutral (coded “0”) to high levels of pain (coded “5”). In this study, children were asked to rate both abdominal pain (“how much does your stomach hurt”) and abdominal discomfort (“how much does your stomach feel bad or uncomfortable?”). Due to their high correlation (r > .72 across administrations), ratings of abdominal pain and discomfort were summed and averaged to create an Abdominal Pain/Discomfort Index.
The FPS-R was administered to abdominal pain patients at the time of their medical evaluation and to both patients and well children during the laboratory study. At their clinic visit, patients were asked, “When you have a stomach ache, how much does your stomach usually hurt?” and “When you have a stomach ache, how much does your stomach usually feel bad or uncomfortable?” In the laboratory, the FPS-R was administered before and immediately following water ingestion. The rating scale was presented and children were asked, “How much does your stomach hurt right now?” and “How much does your stomach feel bad or uncomfortable right now?” Internal consistency was adequate for the Abdominal Pain/Discomfort Index at both the clinic assessment (α = .85) and in the laboratory (α = .86).
Indices of GI and non-GI Symptoms
A symptom checklist was developed for this study to assess symptoms experienced by patients in association with abdominal pain episodes. Symptoms on the checklist were derived from a longer measure of children’s physical symptoms, the Children’s Somatization Inventory (Walker & Greene, 1989), an instrument designed to assess somatic symptoms in studies of children with recurrent abdominal pain. Children were asked to rate how much they felt four GI symptoms (stomach ache, nausea/upset stomach, feel like throwing up, and sick) and eight Non-GI symptoms (e.g., dizzy, tired, headache, and back ache) on a five-point numerical rating scale with responses ranging from “none” (coded “0”) to “a whole lot” (coded “4”). Ratings of GI and Non-GI symptom items were summed and averaged to create a GI Symptom Index and a Non-GI Symptom Index, respectively. Scores on the both indices could range from 0 to 4.
The symptom checklist was administered to abdominal pain patients at the time of their medical evaluation. For each symptom, they were asked, “How much do you also feel this when you have a stomach ache?” At the laboratory, both pain patients and well children completed the symptom checklist before and immediately following water ingestion. For each symptom, children were asked, “How much are you feeling this right now?”
Internal consistency was adequate for the GI Symptom Index in pain patients at the clinic assessment (three-item index; α = .78) and for both pain patients and well children following water ingestion in the laboratory (four-item index; α = .87). For the non-GI Symptom Index, internal consistency also was adequate for pain patients at the clinic assessment (α = .77) and for both patients and well children following water ingestion in the laboratory (α = .74). The correlation between the GI Symptom Index and the non-GI Symptom Index was r = .38 (p < .001), indicating that these symptom constellations are relatively independent.
PA and NA
PA and NA were assessed in pain patients and well children before and following water ingestion in the laboratory. Children were asked to rate how much they felt each of three positive emotions (excited, happy, and full of energy) and four negative emotions (scared, annoyed, nervous/worried, and upset) on a five-point numerical rating scale with responses ranging from “none” (coded “0”) to “a whole lot” (coded “4”). Ratings of items assessing PA and NA were summed and averaged to create indices of PA and NA, respectively. Scores on the indices could range from 0 to 4. Reliability was adequate for indices of PA (α = .75) and NA (α = .64).
Fullness Rating Scale
The Fullness Rating Scale was developed to assess children’s perception of fullness at baseline and following water ingestion in the laboratory. The scale consisted of a series of five simple line drawings of the human body, with an outline of the stomach inside each body drawing. The stomachs were shaded to represent different levels of fullness ranging from completely empty (coded “0”) to completely full (coded “4”). Children were asked to indicate how “full” they felt after water ingestion by selecting one of the drawings.
Adverse Event
One child vomited following water ingestion. During debriefing, the child reported that he had pushed himself to drink water beyond the point of feeling full. In subsequent administrations of the WL, children were cautioned that vomiting was a possibility if they continued to drink water beyond the point of feeling completely full. Data from the child who vomited were excluded from data analysis.
Results
Aim 1: To test whether the WL-SPT produces abdominal discomfort similar to that experienced by pediatric patients with functional abdominal pain. Analyses for this aim were based on the patient sample and used data from both the clinic and laboratory assessments.
Preliminary Analyses
Five participants in the patient sample had incomplete data at the clinic assessment and were omitted from analyses for Aim 1, yielding a total sample of N = 105. Prior to hypothesis testing, scores on symptom measures were inspected for univariate outliers, defined as scores greater than three standard deviations above the mean. Eleven patients with outlying scores were identified; nine patients had one extreme score and two patients had two extreme scores. Outlying scores were windsorized (recoded to three standard deviations above the mean) (Tabachnick & Fidell, 2001).
Next, the data were examined to assess whether extending the period for water ingestion beyond the 5 min used in Koch’s study (Koch et al., 1997) influenced the volume ingested or any of the symptom outcomes. Approximately half of the participants (53%) required more than 5 min to reach a state of perceived fullness. A bivariate Pearson correlation revealed no relation between duration of drinking time and amount of water consumed (r = .13, ns). Multiple regression analyses controlling for baseline symptom scores revealed that drinking time was not a significant predictor of changes in Abdominal Pain/Discomfort, GI, or non-GI symptoms from pre-to postwater ingestion. Therefore, duration of drinking time was not used as a covariate in data analysis.
Analyses also were conducted to assess whether the volume of water ingested predicted symptom increases following water ingestion. Results of multiple regression analyses controlling for BMI indicated that the amount of water ingested was not a significant predictor of changes in abdominal pain/discomfort, GI, or non-GI symptoms following the WL-SPT. Therefore, volume of water consumed was not used as a covariate in data analysis for Aim 1.
Hypothesis 1a: In children with functional abdominal pain, the WL-SPT will produce symptoms similar to those they experience during naturally occurring episodes of abdominal pain.
Table I summarizes descriptive statistics for the measures administered to patients at the clinic as well as pre-and postwater ingestion in the laboratory. Multiple regression analyses examined whether symptom scores representing naturally occurring pain episodes assessed at the clinic predicted symptom scores following water ingestion in the laboratory. In all analyses, the postwater ingestion score was first regressed on the corresponding prewater ingestion score to control for baseline symptom levels.
Table I.
Means, Standard Deviations, and Ranges on Symptom Indices for Patients with Functional Abdominal Pain at the Clinic Assessment and Pre- and Postwater Ingestion in the Laboratory
| Pain/Discomfort Index (range 0–5) | Gastrointestinal Index (range 0–4) | Nongastrointestinal Index (range 0–4) | |
|---|---|---|---|
| Clinic assessment | |||
| M | 3.16 | 2.12 | 1.19 |
| SD | 1.05 | 0.90 | 0.67 |
| Observed range | 1–5 | 0–4 | 0–3 |
| Laboratory assessment—prewater ingestion | |||
| M | 0.53 | 0.20 | 0.33 |
| SD | 0.75 | 0.36 | 0.28 |
| Observed range | 0–3 | 0–2 | 0–2 |
| Laboratory assessment—postwater ingestion | |||
| M | 1.76 | 0.84 | 0.43 |
| SD | 1.2 | 0.86 | 0.43 |
| Observed range | 0–5 | 0–4 | 0–2 |
Due to missing data, N = 105 for the clinic assessment and N = 110 for the laboratory assessment.
As predicted, scores on the Abdominal Pain/Discomfort Index obtained at the clinic significantly predicted scores on the Abdominal Pain/Discomfort Index following water ingestion in the laboratory (β = .23, p < .01). Similarly, scores on the GI Symptom Index obtained at the clinic significantly predicted scores on the GI Symptom Index scores following water ingestion in the laboratory (β = .26, p < .01).
In contrast and as expected, scores on the non-GI Symptom Index obtained at the clinic did not significantly predict scores on the Abdominal Pain/Discomfort Index or the non-GI Symptom Index following the WL-SPT in the laboratory (β = .12, ns and β = .02, ns, respectively), although they predicted scores on the GI Symptom Index following water ingestion, (β = .18, p < .05). Scores on the Abdominal Pain/Discomfort and GI Symptom Indices obtained at the clinic did not predict non-GI Symptom Index scores following water ingestion in the laboratory (β = .07, ns and β = −.003, ns, respectively).
Hypothesis 1b: In children with functional abdominal pain, the WL-SPT will produce significantly greater increases in GI symptoms than non-GI symptoms.
Results of paired t tests indicated a significant increases from pre- to postwater ingestion on all symptom measures: for Abdominal Pain/Discomfort, t(104) = 11.58, p < .001; for GI Symptoms, t(104) = 8.59, p < .001; for non-GI Symptoms, t(104) = 3.67, p < .001. However, as predicted, GI Symptom Index scores increased significantly more than non-GI Symptom Index scores, t(104) =7.53, p < .001.
Aim 2: To test the utility of the WL-SPT in discriminating patients with functional abdominal pain from well children.
Analyses for Aim 2 used both the pain patient and well groups and were based only on data obtained in the laboratory. Before hypothesis testing, the data from the well group were examined for univariate outliers, as had been done for the patient group, reported above. In the well group, 12 children had outlying scores: nine children had one extreme score, two children had two extreme scores, and one child had four extreme scores. As with the patient group, outlying scores were windsorized (recoded as three standard deviations above the group mean) (Tabachnick & Fidell, 2001). The number of participants with outlying scores did not differ significantly by group.
Hypothesis 2a: Pediatric patients with functional abdominal pain will reach perceived fullness at a lower volume of water ingestion than well children.
Controlling for BMI, a group (2) by gender (2) analysis of variance yielded a group effect that approached significance, F(225) = 3.50, p < .06. Pain patients ingested less water (M = 608.15 mL, SD = 272.98) than well children (M = 674.88, SD = 313.91).
Hypothesis 2b: The WL-SPT will produce significantly greater increases in GI symptoms and NA and significantly greater decreases in PA for patients with functional abdominal pain than for well children.
Before testing Hypothesis 2b, the data were examined to assess whether drinking time and volume of water consumed influenced symptom outcomes. Multiple regression analyses indicated that neither drinking time nor volume of water consumed was a significant predictor of changes in Abdominal Pain/Discomfort, GI Symptoms, non-GI symptoms, PA, or NA for pain patients or for well children. Therefore, drinking time and volume were not used as covariates in the following data analyses.
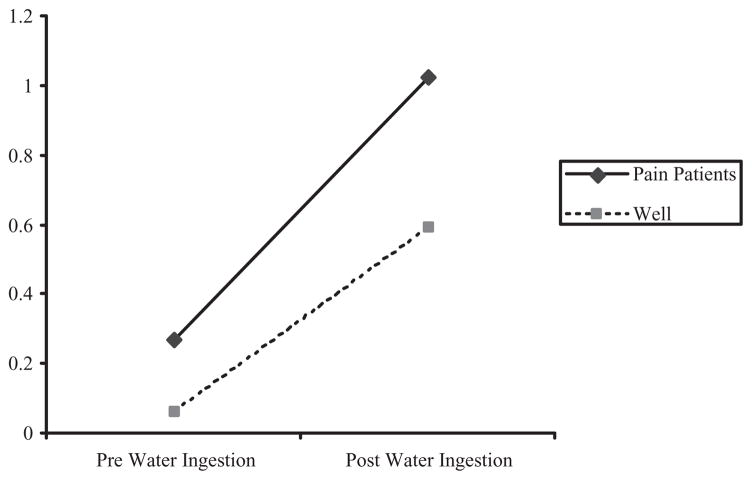
A series of 2 (pre- vs. postwater ingestion) × 2 (group) × 2 (gender) repeated measures analyses of variance (ANOVAs) examined changes in symptom levels from baseline to postwater ingestion. The five symptom measures were Abdominal Pain/Discomfort, GI Symptom Index, non-GI Symptom Index, PA, and NA (see Table II). As predicted, data analysis revealed a significant Group by Time interaction effect on the GI Symptom Index, F(226) = 6.02, p < .05. Compared to well children, pain patients had significantly greater increases in GI symptoms from pre- to postwater ingestion (Fig. 1). The Group by Time interaction was not significant on the Abdominal Pain/Discomfort Index; instead, a significant Time effect, F(226) = 264.14, p < .01, indicated that both pain patients and well children reported significant increases in abdominal pain and discomfort from pre- to postwater ingestion.
Table II.
Means, Standard Deviations, and Observed Ranges on Symptom Indices Pre- and Postwater Ingestion in the Laboratory by Group
| Abdominal Pain/Discomfort (range 0–5) | Gastrointestinal Index (range 0–4) | Nongastrointestinal Index (range 0–4) | Positive affect (PA) Index (range 0–4) | Negative affect (NA) Index (range 0–4) | |
|---|---|---|---|---|---|
| Pain patients (N = 110)—prewater ingestion | |||||
| M | 0.52 | 0.27 | 0.32 | 1.46 | 0.21 |
| SD | 0.74 | 0.39 | 0.28 | 0.95 | 0.31 |
| Observed range | 0–3 | 0–2 | 0–2 | 0–4 | 0–2 |
| Pain patients (N = 110)—postwater ingestion | |||||
| M | 1.81 | 1.02 | 0.45 | 1.12 | 0.16 |
| SD | 1.22 | 0.88 | 0.46 | 0.91 | 0.30 |
| Observed range | 0–5 | 0–4 | 0–2 | 0–4 | 0–2 |
| Well children (N = 120)—prewater ingestion | |||||
| M | 0.23 | 0.06 | 0.19 | 1.83 | 0.18 |
| SD | 0.42 | 0.14 | 0.17 | 0.96 | 0.26 |
| Observed range | 0–2 | 0–1 | 0–1 | 0–4 | 0–1 |
| Well children (N = 120)—postwater ingestion | |||||
| M | 1.43 | 0.59 | 0.29 | 1.55 | 0.06 |
| SD | 1.14 | 0.69 | 0.27 | 1.00 | 0.14 |
| Observed range | 0–5 | 0–3 | 0–2 | 0–4 | 0–1 |
Figure 1.
Increases in gastrointestinal (GI) symptoms from pre- to postwater ingestion in pain patients and well children.
As expected, there was no significant Time by Group interaction on the non-GI Symptom Index. A significant Group effect indicated that, summing scores for pre- and postwater ingestion, the pain group had higher non-GI Symptom scores than the well group, F(226) = 17.00, p < .01. A significant two-way interaction of Time by Gender, F(226) = 4.35, p < .05, indicated that males reported a greater increase in non-GI symptoms than females from pre- to postwater ingestion. A significant main effect of Time, F(226) = 40.30, p < .01, reflected an overall increase in non-GI symptoms from pre- to post-water ingestion for both pain patients and well children. There were no other significant main or interaction effects.
A significant Group effect was observed on the PA Index, indicating that the well group reported higher PA scores overall than the pain group, F(226) = 11.05, p < .01. A significant effect for Time, F(226) = 48.66, p < .01, indicated that PA decreased significantly from pre- to postwater ingestion for both pain patients and well children. There were no other significant main or interaction effects involving PA.
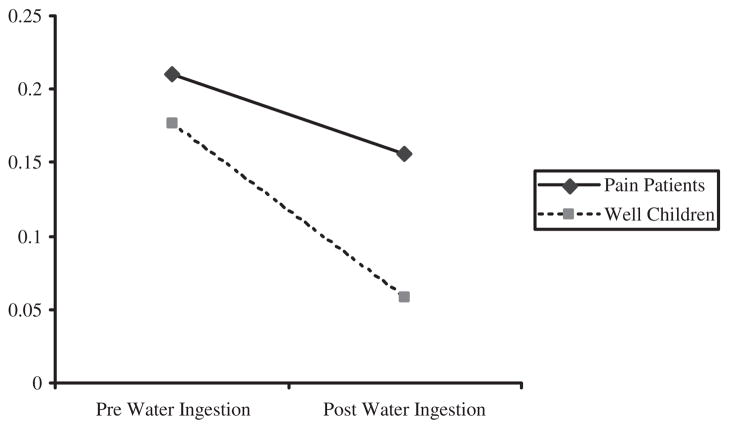
On the NA Index, a significant Time by Group interaction effect, F(226) = 5.89, p < .05, indicated that NA decreased significantly more for well children than for pain patients following water ingestion (Fig. 2). In addition, a significant interaction of Time by Gender, F(226) = 22.16, p < .01, indicated that females reported greater decreases in NA from pre- to postwater ingestion than males.
Figure 2.
Decreases in negative affect (NA) following water ingestion for pain patients and well children.
Effect Sizes for the WL-SPT
Calculation of effect sizes between pre- and postwater ingestion scores was conducted using pooled standard deviation estimates across group. For the GI Symptom Index, the WL-SPT had a very large effect on pain patients (d = 1.28) and a smaller but still sizeable effect on well children (d = .89). The WL-SPT had a very large effect on the Abdominal Pain/Discomfort Index for both groups (d = 1.38 and 1.29 for pain patients and well children, respectively). As expected, the WL-SPT had only a minor effect on the non-GI Symptom Index (d = .41 and .31 for patients and well children, respectively). Although the WL-SPT had only a minor effect on NA in pain patients (d = −.21), a medium effect was observed for well children (d = −.46). The level of NA remained relatively stable in pain patients but decreased from pre-to postwater ingestion in well children. A small effect size was observed for PA, with both groups decreasing slightly in PA following water ingestion (d = −.35 and −.30 for patients and well children, respectively).
Discussion
Results of this study support the convergent and discriminant validity of a WL-SPT that creates visceral sensations similar to the naturally occurring sensations experienced by children with functional abdominal pain. Patients’ ratings of abdominal pain and other GI symptoms experienced during typical pain episodes significantly predicted similar symptoms following water ingestion, controlling for baseline symptoms before water ingestion. Thus, the WL-SPT and naturally occurring episodes of abdominal pain converged in producing abdominal symptoms. Moreover, the test produced significantly greater increases in GI symptoms than non-GI symptoms. This pattern of results suggests that the symptoms produced by the WL-SPT did not simply reflect elevated symptom reporting by pain patients.
Laboratory studies of pain and discomfort are useful for assessing the impact of contextual factors on pain perception and behavior in children and adults (e.g., Romano et al., 1995). To date, however, this methodology has not been available for studies of children with abdominal pain because we have not had an appropriate method to induce visceral sensations in the laboratory. The WL-SPT developed for this study shows considerable promise in this regard. The abdominal discomfort and GI symptoms induced by the WL-SPT were similar in nature but not as intense as those associated with naturally occurring bouts of abdominal pain. Specifically, the WL-SPT produced levels of abdominal discomfort and GI symptoms three times greater than those observed at baseline, but these levels were still only half of those associated with a typical pain episode. This level of discomfort was acceptable to children and their parents—an important consideration for clinical research.
In developing the WL as a WL-SPT, we introduced modifications to the WL procedure used for studies of gastric motility (e.g., Koch et al., 1997).1 First, based on our discovery that 5 min was insufficient time for children to drink water to the point of feeling “completely full”, we asked children to rate their level of fullness at intervals during the procedure and allowed them to drink water for up to 15 min. This modification enabled nearly all children to reach a perceived state of complete fullness. Subsequent data analysis indicated that duration of drinking time did not influence the volume of water consumed or symptom outcomes. Thus, future studies in which the perception of complete fullness is the desired stimulus should consider adopting a fullness rating scale and allowing children sufficient time to reach a state of perceived fullness. Of course, extending the time for drinking also extends the time for gastric emptying and may not be appropriate in studies of gastric motility or fundic accommodation where standardization of water volume, rather than perceived fullness, is the goal.
We also introduced a modification to the WL test to prevent children from using external cues to assess their state of fullness. In our pilot work, children drank water from glasses, as in prior studies. However, we observed that children typically reported feeling completely full after emptying a glass, and rarely left a glass partially full. This behavior suggested that children’s perceptions of fullness were not based exclusively on internal sensations but were influenced by external cues related to the glasses of water (empty vs. full). Thus, we devised a system in which children drank from a tube attached to a water reservoir that was hidden from view.
In addition to validating the WL-SPT as a procedure that induces clinically relevant symptoms in children with functional abdominal pain, we assessed its utility in discriminating abdominal pain patients from well children. The WL-SPT produced significantly greater increases in GI symptoms in pain patients than well children. This finding is consistent with the adult literature on functional abdominal pain (Boeckxstaens et al., 2001; Koch et al., 2000) and is even more notable because, unlike prior reports, our analyses controlled for baseline symptoms. Assessment of baseline symptom levels also allowed us to calculate effect sizes for the WL-SPT. The effect of the WL-SPT on GI Symptoms was substantial for pain patients (Cohen’s d = 1.28), lending further support to its utility as a WL-SPT for these patients. The fact that it also had a moderate effect on GI Symptoms for well children (Cohen’s d = .89) suggests that it may have utility in studies assessing the behavior of well children and their parents during experimentally induced abdominal discomfort.
The WL-SPT also discriminated between pain patients and well children in their affective reaction to abdominal discomfort. The level of NA before water ingestion was similar in the two groups. However, NA decreased significantly and nearly reached zero in well children following water ingestion, but remained relatively stable in pain patients. Thus, whereas both groups may have experienced mild distress in anticipation of the procedure, only well children appeared to have recover following the actual experience. The role of affect in the experience of visceral sensations could be explored further in studies that manipulate NA before administering the WL-SPT.
This study revealed a trend for pain patients to have a lower threshold for the perception of fullness than well children, that is, pain patients reported fullness at a lower volume of water consumption, controlling for BMI, than well children. Related studies have consistently found that patients with functional abdominal symptoms consumed significantly less water than healthy volunteers (e.g., Boeckxstaens et al., 2001; Koch et al., 2000), but these studies have not eliminated visual cues from the experimental procedure and have not controlled for BMI in assessing group differences in water consumption. Additional research controlling for these potential confounds is needed in both children and adults.
This study does not allow us to evaluate whether differences between pain patients and well children reflect differences in sensory function or psychological influences on symptom perception. Even studies that use fMRI to monitor brain activity during balloon distention in the gut cannot establish conclusively whether GI hypersensitivity or psychological hypervigilance to gut sensations account for differences between patients with and without functional abdominal pain (cf. Mertz, et al., 1995). We would argue, however, that the question of hypersensitivity versus hypervigilance is based on a dualistic perspective that has limited utility for understanding functional pain. From a biopsychosocial perspective (e.g., Drossman, 1996), the more important question is how physiological and psychological events interact in the experience of pain. The WL-SPT provides a means for us to manipulate these events in the laboratory.
The study has several limitations that should be considered in interpreting the results and planning future research. It is possible that our sample of pain patients included subgroups that may have responded differently to the WL-STP. For example, DiLorenzo and colleagues have demonstrated, using an electronic barostat, that different symptom phenotypes are associated with different sites of visceral hyperalgesia in patients with functional abdominal pain (DiLorenzo et al., 2001). Future research should assess whether the impact of the WL-SPT differs for patients with upper, periumbilical, and lower abdominal pain associated with functional GI disorders defined by the Rome symptom criteria (Rasquin-Weber et al., 1999). Another limitation of the study was that the WL produced symptoms similar in nature but not as intense as naturally occurring discomfort. Although ethical considerations prohibit induction of severe discomfort, we would expect that our findings with a low level of discomfort would only be stronger at a higher level of discomfort.
In summary, we have demonstrated the convergent and discriminant validity of a WL-SPT that produces GI symptoms similar to those experienced naturally by children with functional abdominal pain and discriminates those patients from well children. This procedure may be useful in experimental laboratory studies that manipulate variables such as affect and social context to increase our understanding of mechanisms of functional abdominal pain and to identify promising targets for intervention to alleviate suffering.
Acknowledgments
This research was supported by a grant to the investigators from the National Institute on Child Health and Development (HD23264), by a core grant (HD15052) to the John F. Kennedy Center, Vanderbilt University, and by grant M01 RR-00095 to Vanderbilt University Medical Center from the National Center for Research Resources, National Institutes of Health. The authors thank the staff of the Pediatric Gastroenterology, Hepatology and Nutrition Clinic at Vanderbilt University for assistance with patient recruitment and medical evaluations. We also thank Renee Cox, Kari Freeman, Sarah Leek, Angela Profeta, Kristen Reeslund, and Kelly Voss for their contributions to data collection.
Footnotes
Instructions for the WL test vary somewhat across laboratories. In contrast to Koch, Boeckxstaens instructed participants to drink 100 mL of water from a beaker every minute until reaching “very severe” discomfort (Boeckxstaens et al., 2001). In a study of healthy school children, Sood and colleagues asked children to drink as much water as possible “in three minutes or until they felt too full to continue” (Sood et al., 2002). The shorter drinking time was intended to minimize the effect of gastric emptying. However, the authors appear not to have assessed whether the children in fact achieved fullness within 3 min.
References
- Apley J. The child with abdominal pains. London: Blackwell; 1975. [Google Scholar]
- Apley J, Naish N. Recurrent abdominal pains: A field survey of 1000 school children. Archives of Diseases of Childhood. 1958;33:165–170. doi: 10.1136/adc.33.168.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apley J, Tulloh CG, Haslam DR. Pupillary reaction in children with recurrent abdominal pain. Archives of Diseases in Childhood. 1971;46:337–340. doi: 10.1136/adc.46.247.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battistella PA, Carra S, Zaninotto M, Ruffilli R, Da Dalt L. Pupillary reactivity in children with recurrent abdominal pain. Headache. 1992;32:105–107. doi: 10.1111/j.1526-4610.1992.hed3202105.x. [DOI] [PubMed] [Google Scholar]
- Bieri D, Reeve RA, Champion GD, Addicoat L, Ziegler JB. The Faces Pain Scale for the self-assessment of the severity of pain experience by children: Development, initial validation, and preliminary investigation for ratio scale properties. Pain. 1990;41:139–150. doi: 10.1016/0304-3959(90)90018-9. [DOI] [PubMed] [Google Scholar]
- Boeckxstaens GE, Hirsch DP, van den Elzen BD, Heisterkamp SH, Tytgat GN. Impaired drinking capacity in patients with functional dyspepsia: Relationship with proximal stomach function. Gastroenterology. 2001;121:1054–1063. doi: 10.1053/gast.2001.28656. [DOI] [PubMed] [Google Scholar]
- Bouin M, Plourde V, Boivin M, Riberdy M, Lupien F, Laganiere M, et al. Rectal distention testing in patients with irritable bowel syndrome: Sensitivity, specificity, and predictive values of pain sensory thresholds. Gastroenterology. 2002;122:1771–1777. doi: 10.1053/gast.2002.33601. [DOI] [PubMed] [Google Scholar]
- DiLorenzo C, Youssef NN, Sigurdsson L, Scharff L, Griffiths J, Wald A. Visceral hyperalgesia in children with functional abdominal pain. Journal of Pediatrics. 2001;139:838–843. doi: 10.1067/mpd.2001.118883. [DOI] [PubMed] [Google Scholar]
- Dorn LD, Campo JC, Thato S, Dahl RE, Lewin D, Chandra R, et al. Psychological comorbidity and stress reactivity in children and adolescents with recurrent abdominal pain and anxiety disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2003;42:66–75. doi: 10.1097/00004583-200301000-00012. [DOI] [PubMed] [Google Scholar]
- Drossman DA. Gastrointestinal illness and the biopsychosocial model. Journal of Clinical Gastroenterology. 1996;22:252–254. doi: 10.1097/00004836-199606000-00002. [DOI] [PubMed] [Google Scholar]
- Feuerstein M, Barr R, Francoeur E, Houle M, Rafman S. Potential biobehavioral mechanisms of recurrent abdominal pain in children. Pain. 1982;13:287–298. doi: 10.1016/0304-3959(82)90018-5. [DOI] [PubMed] [Google Scholar]
- Hicks CL, von Baeyer CL, Spafford PA, van Korlaar I, Goodenough B. The Faces Pain Scale-Revised: Toward a common metric in pediatric pain measurement. Pain. 2001;93:173–183. doi: 10.1016/S0304-3959(01)00314-1. [DOI] [PubMed] [Google Scholar]
- Koch KL, Bingaman S, Muth E, Ouyang A. Effects of physiological gastric distention on nausea, stomach fullness, satiety and gastric myoelectrical activity in patients with irritable bowel syndrome. Gastroenterology. 1997;112:A763. [Google Scholar]
- Koch KL, Hong SP, Xu L. Reproducibility of gastric myoelectrical activity and the water load test in patients with dysmotility-like dyspepsia symptoms and in control subjects. Journal of Clinical Gastroenterology. 2000;31:125–129. doi: 10.1097/00004836-200009000-00007. [DOI] [PubMed] [Google Scholar]
- McGrath PA. Pain in children: Nature, assessment, and treatment. New York: Guilford; 1990. [Google Scholar]
- Mertz H, Naliboff BD, Munakata J, Niazi N, Mayer EA. Altered rectal perception is a biological marker of patients with irritable bowel syndrome. Gastroenterology. 1995;109:40–52. doi: 10.1016/0016-5085(95)90267-8. [DOI] [PubMed] [Google Scholar]
- Rasquin-Weber A, Hyman PE, Cucchiara S, Fleisher DR, Hyams JS, Milla PJ, et al. Childhood functional gastrointestinal disorders. Gut. 1999;45:60–68. doi: 10.1136/gut.45.2008.ii60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano JM, Turner JA, Jensen MP, Friedman LS, Bulcroft RA, Hops H, et al. Chronic pain patient – spouse behavioral interactions predict patient disability. Pain. 1995;63:353–360. doi: 10.1016/0304-3959(95)00062-3. [DOI] [PubMed] [Google Scholar]
- Routh DK, Ernst AR, Harper DC. Recurrent abdominal pain in children and somatization disorder. In: Routh DK, editor. Handbook of pediatric psychology. New York: Guilford Press; 1988. pp. 492–504. [Google Scholar]
- Rubin LS, Barbero GJ, Sibinga MS. Pupillary reactivity in children with recurrent abdominal pain. Psychosomatic Medicine. 1967;29:111–120. doi: 10.1097/00006842-196703000-00002. [DOI] [PubMed] [Google Scholar]
- Sood MR, Schwankovsky LM, Rowhani A, Zangen T, Ziring D, Furtado T, et al. Water load test in children. Journal of Pediatric Gastroenterology and Nutrition. 2002;35:199–201. doi: 10.1097/00005176-200208000-00017. [DOI] [PubMed] [Google Scholar]
- Stickler GB, Murphy DB. Recurrent abdominal pain. American Journal of Diseases of Children. 1979;133:486–489. doi: 10.1001/archpedi.1979.02130050030006. [DOI] [PubMed] [Google Scholar]
- Tabachnick BS, Fidell LS. Using multivariate statistics. 4. Needham Heights, MA: Allyn & Bacon; 2001. [Google Scholar]
- Trimble KC, Farouk R, Pryde A, Douglas S, Heading RC. Heightened visceral sensation in functional gastrointestinal disease is not site-specific. Evidence for a generalized disorder of gut sensitivity. Digestive Diseases and Sciences. 1995;40:1607–1613. doi: 10.1007/BF02212678. [DOI] [PubMed] [Google Scholar]
- Van Ginkel R, Voskuijl WP, Benninga MA, Taminiau JAJM, Boeckxstaens GE. Alterations in rectal sensitivity and motility in childhood irritable bowel syndrome. Gastroenterology. 2001;120:838–843. doi: 10.1053/gast.2001.20898. [DOI] [PubMed] [Google Scholar]
- von Baeyer CL, Walker LS. Children with recurrent abdominal pain: Issues in the selection and description of research participants. Journal of Developmental and Behavioral Pediatrics. 1999;20:307–313. doi: 10.1097/00004703-199910000-00001. [DOI] [PubMed] [Google Scholar]
- Walker LS, Garber J, Greene JW. Somatization symptoms in pediatric abdominal pain patients: Relation to chronicity of abdominal pain and parent somatization. Journal of Abnormal Child Psychology. 1991;19:379–394. doi: 10.1007/BF00919084. [DOI] [PubMed] [Google Scholar]
- Walker LS, Garber J, Van Slyke DA, Greene JW. Long-term health outcomes in patients with recurrent abdominal pain. Journal of Pediatric Psychology. 1995;20:233–245. doi: 10.1093/jpepsy/20.2.233. [DOI] [PubMed] [Google Scholar]
- Walker LS, Greene JW. Children with recurrent abdominal pain and their parents: More somatic complaints, anxiety, and depression than other patient families? Journal of Pediatric Psychology. 1989;14:231–243. doi: 10.1093/jpepsy/14.2.231. [DOI] [PubMed] [Google Scholar]
- Walker LS, Lipani T, Greene JW, Caines K, Stutts J, Polk DB, et al. Recurrent abdominal pain: Symptom subtypes based on the Rome II criteria for pediatric functional gastrointestinal disorders. Journal of Pediatric Gastroenterology and Nutrition. 2004;38:187–191. doi: 10.1097/00005176-200402000-00016. [DOI] [PubMed] [Google Scholar]
- Zeltzer LK, Bush JP, Chen E, Riveral A. A psychobiologic approach to pediatric pain: Part 1. History, physiology, and assessment strategies. Current Problems in Pediatrics. 1997;27:225–258. doi: 10.1016/s0045-9380(97)80025-4. [DOI] [PubMed] [Google Scholar]