Abstract
An optically-controlled Ca2+-chelator 1 was developed to mimic natural calcium oscillations. Compound 1, a spiroamido-rhodamine derivative of 1,2-bis(o-aminophenoxy) ethane-N,N,N′,N′-tetraacetic acid (BAPTA), underwent cycles of reversible transitions between a colorless closed state and a fluorescent open form. The closed-state exhibited a high affinity for Ca2+ (Kd: 509 nM) with excellent selectivity over Mg2+ (Kd: 19 mM). The open isomer had a 350-fold lower Ca2+ affinity (Kd: 181 μM) while the Mg2+ affinity was not significantly affected (Kd: 14 mM).
Oscillations in the intracellular calcium (Ca2+) concentration exist in many types of cells and are believed to regulate a variety of physiological functions.1 The ability to artificially generate similar calcium oscillations in cells would be a valuable tool to understand the physiological role of the oscillatory calcium signals and to study the Ca2+ dependent biological processes.2 Photochromic probes that reversibly bind and release Ca2+ in response to light are being used as part of this approach. To be biologically useful, such probes should also fulfill the following requirements:3 (1) Since the free Ca2+ concentration in most resting cells is in a sub-micromolar range, the resting state of the probe should bind Ca2+ with a similar or lower dissociation constant, in order to store a significant amount of Ca2+ at equilibrium; (2) The two states of the probe should have at least a 10-fold difference in binding affinity for Ca2+; (3) Because the typical intracellular free Mg2+ concentration is around mM, then in order to avoid saturating by Mg2+ in cells, the probe should exhibit excellent selectivity for Ca2+ over Mg2+; (4) The required wavelength for photochemistry should not damage living cells, therefore the wavelengths should be at least above 300 nm; (5) The probe should be switchable in an aqueous environment; (6) One of the two states of the probe should incorporate a fluorescence readout allowing the user to determine the location of the switch and to monitor the release of Ca2+ in the sample.
Several attempts have been made to develop photoreversible Ca2+ chelators.4 However, the reported probes are still far away from satisfying the criteria detailed above. Two favored photochemical reactions, such as geometric isomerization of azobenzene or spiropyran to sterically disrupt the binding pocket and ring-opening of spiropyran to generate additional binding site, have been employed to provide a means to optically control the Ca2+ binding affinity. Such mechanisms suffer from the problem that Ca2+ will stablize the isomer that binds it, thus the photochemistry will be hindered by the Ca2+ binding. Moreover, none of the probes has good Ca2+/Mg2+ selectivity.
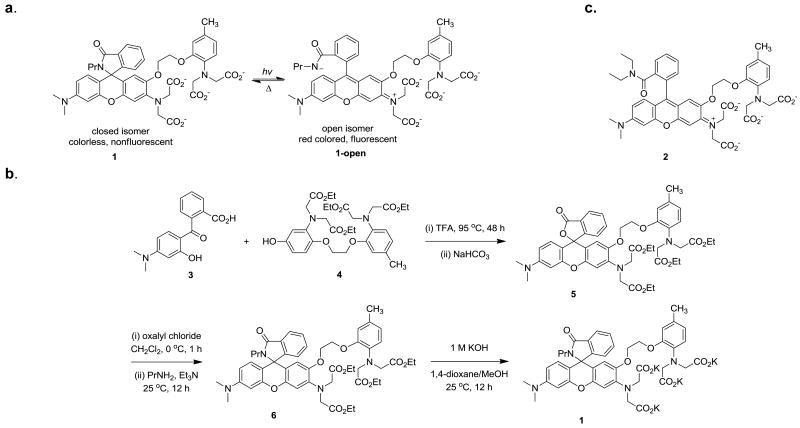
Herein, we report the design, synthesis and properties of the first photoreversible Ca2+ chelator 1 (Figure 1a) that can satisfy all the aforementioned requirements. Our approach to the design of 1 is displayed in Figure 1a. The novel photoreversible calcium chelator 1 is based on a photochromic rhodamine scaffold5 and a Ca2+-chelating moiety 1,2-bis(o-aminophenoxy) ethane-N,N,N′,N′-tetraacetic acid (BAPTA).6 The well known Ca2+-chelator BAPTA is chosen as the starting point due to its high Ca2+ affinity and excellent Ca2+/Mg2+ selectivity. Moreover, its Ca2+ affinity can be easily adjusted by modulating the substitutions on its benzene rings. Electron-withdrawing or donating substituents will decrease or increase the affinity for Ca2+ respectively. These properties have been used to develop caged calcium complexes.6b However, cage compounds are less suitable for the study of oscillatory calcium signals due to the irreversible photochemical reactions. Reversible modulation of the electron density of the BAPTA moiety would lead to release or bind Ca2+ without steric disruption of the binding site. A suitable photochromic reaction is envisioned to be the well established transformation between the closed and open form of rhodamine amide derivatives5 as shown in Figure 1a. The photoinduced ring-opening reaction generates the rhodamine chromophore, which significantly descreases the electron density in the BAPTA part thus produces the desired drop in Ca2+ affinity. The open isomer reverts thermally to the closed form with a characteristic lifetime of a few milliseconds in polar solvents,5 restoring the affinity for Ca2+. The design strategy proves to work well, though further optimization is still needed to improve the photochromic properties of the scaffold.
Figure 1.
(a) Design and (b) synthesis of rhodamine-based Ca2+ chelator 1; (c) a model compound 2 for the open form of 1.
The synthesis of the reversible Ca2+ chelator 1 is summarized in Figure 1b. The key starting fragments 3 and 4 were prepared according to reported procedures7,8 with slight modifications. Condensation of 3 with 4 was conducted in neat TFA at elevated temperature to afford rhodamine 5, which was isolated as the colorless ring-closed isomer. The spirolacton form was further confirmed by the characteristic carbon signal near 84 ppm in 13C NMR spectrum.5 Target compound 1 was obtained by amidation of rhodamine 5 with propylamine followed by hydrolysis of the four ethyl esters in the BAPTA part. To estimate the Ca2+ binding affinity of the short-lived open form of chelator 1, a model compound 29 (Figure 1c), which is structurally and electronically similar to the open form of 1 and exists only in the open state, was also designed. Rhodamine 2 was synthesized from intermediate 5 using similar conditions as described for compound 1 (See Supporting Information for details).
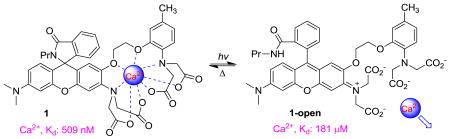
Rhodamine-based chelator 1 dissolved freely in aqueous solution at physiological pH. UV-Vis absorption spectra of 1 at various levels of free Ca2+ in MOPS buffer at pH 7.2 were displayed in Figure 2a. In the absence of Ca2+, the spectrum showed a maximum at 240 nm (ε: 34200 M-1-cm1) with a shoulder at 320 nm (ε: 5600 M-1·cm-1). There was no absorbance in the visible range, indicating that compound 1 only existed as the closed form under such conditions. The closed form was practically non-fluorescent. Addition of Ca2+ to the solution did not result in any detectable formation of the open isomer. Small but reproducible absorption changes in the UV region were observed upon Ca2+ binding: the peak at 240 nm was slightly blue-shifted and the absorbance at 320 nm decreased with addition of Ca2+. The titration were repeated three times and the data were analyzed by a Hill plot6a (Figure 2b), i.e. a plot of log [(A – Amin)/(Amax – A)] versus log [Ca2+]free, where Amin is the absorbance of the free chelator, Amax is the absorbance of the Ca2+ complex, and A is the absorbance at an intermediate Ca2+ level, all measured at the same wavelength. The Hill plot for absorbance at 320 nm gave a straight line with slope = 1 consistent with a 1 : 1 binding for compound 1 with Ca2+. The x intercept indicated the dissociation constant (Kd) of the complex to be 509 nM. The binding characteristics of the closed form of 1 with Mg2+ were analyzed analoguously (Supporting Information, Figure S3). The effect of Mg2+ binding on the absorption spectrum was much smaller compared with the effect of Ca2+, presumabally because Mg2+ binds mainly to just one half of the chelator and does not perturb the spectrum of the other parts.6,8 The Mg2+ dissociation constant for 1 was found to be 19 mM with a 1 : 1 binding stoichiometry. Thus the closed form of 1 has high affinity for Ca2+ and exhibits excellent selectivity for Ca2+ over Mg2+.
Figure 2.
(a) Absorption spectra of 1 (20 μM) as a function of [Ca2+]free (10 mM MOPS, 100 mM KCl, pH 7.2). Further increase in [Ca2+]free after 39 μM had little effect on the spectrum indicating complete saturation of 1 by Ca2+ was achieved; (b) Hill plot of the absorbance measured at 320 nm.
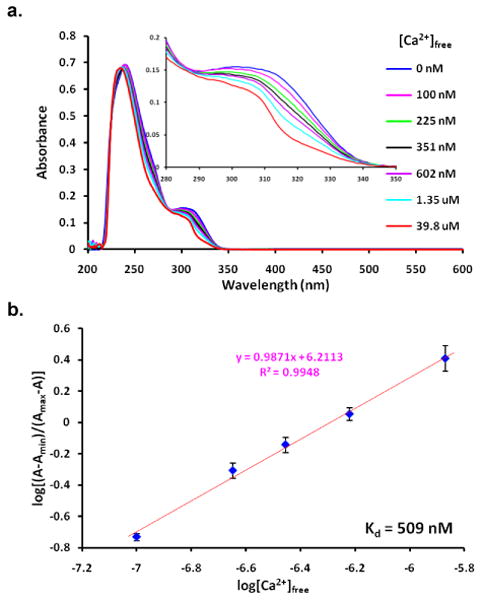
The open form of 1 is short-lived,5 preventing the direct measurement of the binding affinities. Thus a model compound 2 was used to estimate the dissociation constants of the metal complex of 1-open. Compound 2 absorbs maximally at 575 nm (ε: 98000 M-1·cm-1) and emits at around 600 nm, which are pretty much as expected for rhodamine derivatives. Figure 3a shows the fluorescence spectra of 2 at different Ca2+ concentrations. The fluorescence intensities were enhanced by addition of Ca2+. However, Ca2+ binding caused little change in the wavelength of emission. The titration data were fitted to Hill plot to give an apparent Kd of 181 μM with a 1 : 1 binding for Ca2+. The affinity for Ca2+ was 350-fold lower than that of the closed form of 1, indicating the success of our design strategy. The binding of 2 with Ca2+ reduced the absorbance peak at 575 nm with little change in peak position (Supporting Information, Figure S2). The Ca2+ dissociation constant was found to be 164 μM from the absorption studies, which agreed well within experimental errors with that from emission titration. Complexation of 2 with Mg2+ was also examined spectroscopically (Supporting Information, Figure S4). The Mg2+ dissociation constant was found to be 14 mM from the fluorescence studies, which is very similar as that for the closed form of compound 1. Unlike Ca2+ chelation, the Mg2+ binding caused little change in absorption spectra of 2. These results reflect that Mg2+ binds primarily to only half of the chelator.
Figure 3.
(a) Emission spectra of 2 (2 μM, λex = 575 nm) as a function of [Ca2+]free (10 mM MOPS, 100 mM KCl, pH 7.2). Further increase in [Ca2+]free after 1.68 mM had little effect on the spectrum indicating complete saturation of 2 by Ca2+ was achieved; (b) Hill plot of the emission measured at 600 nm.
The optical switching properties of chelator 1 were investigated in a buffered solution (10 mM MOPS, 100 mM KCl, pH 7.2). The formation of the open isomer 1-open was not detected when a solution of 1 was irradiated with UV light, probably because the lifetime of the open state was too short to be detected.5 Bovine serum albumin (BSA) was found to stabilize the open isomer probably by interactions within the anion binding site of BSA. Thus BSA (1 mg/mL) was added to the solution in the following studies. An example of photoswitching is shown in Figure 4. When 1 was irradiated with 312 nm UV light, a visible absorption band (575 nm) corresponding to the open form of 1 appeared. The photo-induced open form of 1 emitted at around 600 nm. The absorption and emission spectra of 1-open were very similar to these of compound 2, further confirming that 2 is a good model compound for the open form of 1. The open isomer converted back to the close form thermally with a half life-time (t1/2) about 58 s at room temperature in the dark. The above process can be repeated several cycles without significant changes in the absorption spectrum. Addition of excess Ca2+ or Mg2+ to the solution had little effect on the photoswitching, except for the decrease of the absorbance at the photostationary state probably due to the increased rate of thermal ring-closure (Supporting Information, Figure S6-7). In the presence of Ca2+, the half life-time of the open form of 1 was decreased to 34 s. This value was found to be 28 s in the presence of Mg2+.
Figure 4.
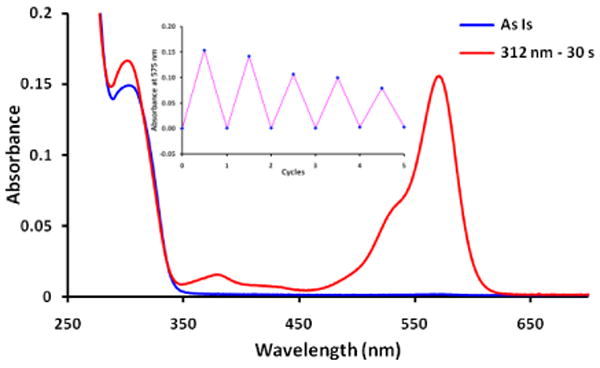
Optical switching of 1 (20 μM, 10 mM MOPS, 100 mM KCl, 1 mg/mL BSA, pH 7.2) with 312 nm UV light irradiation for 30 s followed by thermal ring-closure in the dark at 20 °C. Inset shows absorbance at 575 nm for five cycles of the switching process.
In summary, a novel chelator 1 that reversibly binds and releases Ca2+ in response to light was rationally designed and synthesized to mimic calcium oscillations. A new design concept was introduced for the development of reversible Ca2+ chelators. Unlike previously reported probes4, in which the Ca2+ affinities were manipulated through geometry changes or creation of additional binding sites, the Ca2+ affinity of 1 was modulated by harnessing the changes in electronic properties of the BAPTA moiety during photoswitching. Compound 1 exhibits high affinity for Ca2+ (Kd: 509 nM) with excellent Ca2+/Mg2+ selectivity (Kd for Mg2+: 19 mM) in the resting state. Upon irradiation of UV light, compound 1 was converted to the open isomer 1-open, which has a much lower affinity for Ca2+ (Kd: 181 μM) while the affinity for Mg2+ is not significantly affected (Kd: 14 mM). The open form reverts back thermally and the process can be repeated for several cycles. To the best of our knowledge, this is the first demonstration of a reasonably satisfactory reversible Ca2+ chelator that might be used to generate defined waveforms of calcium ions in a sample perhaps to mimic natural oscillations of calcium ions in cells and tissue, although increase in the Ca2+ affinity of the resting state and further tuning of the photochemical properties, i.e. the required wavelength for photochemistry, thermal stability of the open isomer, quantum yield of the photochemistry and optical control of the ring-closure reaction, are still desired. Additional future work in this direction is in progress.
Supplementary Material
Acknowledgments
This work was financially supported by NIH (PN2EY018241).
Footnotes
Supporting Information Available Experimental procedures, characterization data for the new compounds and details of spectroscopic studies. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Fewtrell C. Annu Rev Physiol. 1993;55:427–454. doi: 10.1146/annurev.ph.55.030193.002235. [DOI] [PubMed] [Google Scholar]; (b) Tsien RW, Tsien RY. Annu Rev Cell Biol. 1990;6:715–760. doi: 10.1146/annurev.cb.06.110190.003435. [DOI] [PubMed] [Google Scholar]; (c) Thul R, Bellamy TC, Roderick HL, Bootman MD, Coombes S. Adv Exp Med Biol. 2008;641:1–27. doi: 10.1007/978-0-387-09794-7_1. [DOI] [PubMed] [Google Scholar]
- 2.Dolmetsch RE, Xu K, Lewis RS. Nature. 1998;392:933–936. doi: 10.1038/31960. [DOI] [PubMed] [Google Scholar]
- 3.Ellis-Davies GCR. Methods Enzymol. 2003;360:226–238. doi: 10.1016/s0076-6879(03)60112-6. [DOI] [PubMed] [Google Scholar]
- 4.(a) Kumar S, Hernandez D, Hoa B, Lee Y, Yang JS, McCurdy A. Org Lett. 2008;10:3761–3764. doi: 10.1021/ol801406b. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kumar S, Chau C, Chau G, McCurdy A. Tetrahedron. 2008;64:7097–7105. doi: 10.1016/j.tet.2008.05.083. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Momotake A, Arai T. Tetrahedron Lett. 2003;44:7277–7280. [Google Scholar]; (d) Sakata T, Jackson DK, Mao S, Marriott G. J Org Chem. 2008;73:227–233. doi: 10.1021/jo7019898. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Filley J, Ibrahim MA, Nimlos MR, Watt AS, Blake DM. J Photochem Photobiol, A. 1998;117:193–198. [Google Scholar]; (f) Roxburgh CJ, Sammes PG. Eur J Org Chem. 2006:1050–1056. [Google Scholar]
- 5.(a) Foelling J, Belov V, Kunetsky R, Medda R, Schoenle A, Egner A, Eggeling C, Bossi M, Hell SW. Angew Chem, Int Ed. 2007;46:6266–6270. doi: 10.1002/anie.200702167. [DOI] [PubMed] [Google Scholar]; (b) Knauer KH, Gleiter R. Angew Chem. 1977;89:116–117. [Google Scholar]
- 6.(a) Tsien RY. Biochemistry. 1980;19:2396–2404. doi: 10.1021/bi00552a018. [DOI] [PubMed] [Google Scholar]; (b) Adams SR, Kao JPY, Grynkiewicz G, Minta A, Tsien RY. J Am Chem Soc. 1988;110:3212–3220. [Google Scholar]
- 7.Liu QH, Yan XL, Guo JC, Wang DH, Li L, Yan FY, Chen LG. Spectrochim Acta, Part A. 2009;73A:789–793. doi: 10.1016/j.saa.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 8.Grynkiewicz G, Poenie M, Tsien RY. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 9.Similar compounds based on rhodol and BAPTA were developed by Clarke and co-workers for ratiometric imaging of calcium ion. For details, see: Simth GA, Metcalfe JC, Clarke SD. J Chem Soc, Perkin Trans 2. 1993:1195–1204.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.