Abstract
Kaposi’s sarcoma-associated herpesvirus (KSHV) is necessary for KS, a highly vascularized tumor predominated by endothelial-derived spindle cells that express markers of lymphatic endothelium. Following KSHV infection of TIME cells, an immortalized human dermal microvascular endothelial cell (DMVEC) line, expression of many genes specific to lymphatic endothelium, including VEGFR3, podoplanin, LYVE-1, and Prox-1, is significantly increased. Increases in VEGFR3 and podoplanin protein are also demonstrated following latent infection. Examination of cytokine secretion showed that KSHV infection significantly induces hIL-6 while strongly inhibiting secretion of IL-8, a gene product that is decreased by differentiation of blood to lymphatic endothelial cells. These studies support the hypotheses that latent KSHV infection of blood endothelial cells drives their differentiation to lymphatic endothelial cells.
Keywords: KSHV, HHV-8, Endothelial, Lymphatic, Human herpesvirus, Angiogenesis, VEGF receptor3, IL-6, Podoplanin, Kaposi’s sarcoma
Introduction
Kaposi’s sarcoma (KS), the most frequent malignancy in AIDS patients, is currently the most commonly reported tumor in parts of Africa (Wabinga et al., 1993). KS tumors are highly vascularized and exhibit extensive neoangiogenesis. KS tumors also contain increased levels of several pro-inflammatory cytokines, including hIL-6 (Oxholm et al., 1989). The most prominent cell in the KS tumor is the spindle cell, a cell of endothelial origins, though there are other cell types present in the tumor including infiltrating lymphocytes. KS spindle cells express elevated levels of vascular endothelial growth factor receptor 3 (VEGFR3) and podoplanin, two specific markers of the lymphatic endothelium (Jussila et al., 1998; Skobe et al., 1999), which led to the hypothesis that KS spindle cells are lymphatic in origin (Jussila et al., 1998; Skobe et al., 1999; Weninger et al., 1999). The lymphatic endothelium controls the fluid and lymphocyte uptake into lymph nodes and is architecturally distinct from the blood vascular endothelium. Lymphangiogenesis is a separate process from blood or hemangiogenesis, utilizing different VEGF family members, primarily VEGF-C and D and their receptor VEGFR3 (Makinen et al., 2001).
Kaposi’s sarcoma-associated herpesvirus (KSHV also known as human herpesvirus 8 or HHV-8) is present in spindle cells of all KS tumors and is necessary for KS progression (Aluigi et al., 1996). KSHV is also associated with two B-cell lymphoproliferative diseases, primary effusion lymphoma (PEL) (Cesarman et al., 1995) and AIDS-associated plasmablastic multicentric Castleman’s disease (MCD) (Soulier et al., 1995). KSHV is a gamma herpesvirus, further subclassified as a rhadinovirus. At least 80 genes are encoded on its 165-kilobase genome (Neipel et al., 1997; Russo et al., 1996). Like all herpesviruses, KSHV has both a lytic and a latent phase. During latency, a small subset of the coding capacity of KSHV is expressed. In KS tumors and primary effusion lymphomas, KSHV is found predominantly in the latent state, although there is always a low background of lytic replication (Staskus et al., 1997).
TIME cells are human dermal microvascular endothelial cells (DMVECs) immortalized with h-tert, the active subunit of telomerase, and are efficiently infected with KSHV. By 24 h postinfection, nearly all of the TIME cells support latent infection while around 1–3% undergo lytic replication, similar to the percentages in KS tumors and PELs (Lagunoff et al., 2002). The studies presented here demonstrate that KSHV infection of TIME cells induces many changes that are consistent with differentiation from blood to lymphatic endothelial cells (BEC to LEC). This supports the hypothesis that KSHV infection of BECs in vivo causes them to differentiate into LECs as they transform into spindle cells.
Results
Markers of lymphatic endothelial cells are induced by KSHV infection
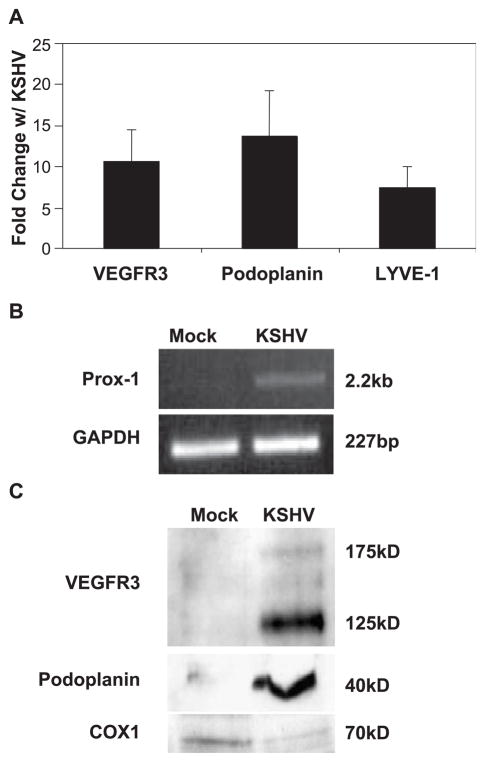
To examine major markers of lymphatic endothelial cells, we used quantitative real-time RT-PCR to examine expression of VEGFR3, podoplanin, and LYVE-1 in TIME cells infected with KSHV or with KSHV that was UV irradiated. UV-irradiated virus can bind and enter cells but there is no viral gene expression. Infection rates were determined by seeding a subset of infected cells onto slides at the time of harvest and staining with antibodies to a latent marker (LANA) and a lytic marker (ORF 59). Only experiments where greater than 90% of the cells expressed latent markers and less than 5% of the cells expressed lytic markers were analyzed. No staining was seen in uninfected cells or cells infected with UV-irradiated virus. Expression of VEGFR3, podoplanin, and LYVE-1 is increased approximately 10-, 13-, and 7-fold, respectively, in KSHV-infected cells (Fig. 1A). Prox-1 is required for the developmental expression of VEGFR3 and podoplanin as BECs differentiate into LECs in vivo (Wigle and Oliver, 1999), thus making the Prox-1 pathway a likely cellular target of KSHV. We could not amplify an RT-PCR product using primers specific for Prox-1 when using mRNA from uninfected or UV-irradiated KSHV-infected TIME cells, but we could amplify product from mRNA isolated from KSHV-infected TIME cells (Fig. 1B). The 2.2-kb product was subcloned, sequenced, and found to be full-length Prox-1 mRNA (data not shown). This indicates that uninfected TIME cells express little, if any, Prox-1, and the gene is upregulated by KSHV infection. Interestingly, we could easily amplify Prox-1 using mRNA from uninfected primary DMVECs (data not shown). It was previously shown that commercially available DMVECs are a mixture of blood and lymphatic endothelial cells (Makinen et al., 2001). Thus, it is likely that the contaminating LECs express the Prox-1 detected. The complete lack of detectable Prox-1 in TIME cells provides evidence that TIME cells are purely BEC in origin.
Fig. 1.
KSHV infection of TIME cells induces specific markers of lymphatic endothelial cells. (A) Depicted is the fold change of LEC-specific transcripts in KSHV-infected TIME cells compared to UV-irradiated virus-treated cells at 48 h postinfection as determined by real-time RT-PCR (N = 3–5). All values were normalized to GAPDH expression and error bars represent the standard error of the mean. (B) Shown is a representational gel of Prox-1 and GAPDH mRNA amplification by semi-quantitative RT-PCR from mock- and KSHV-infected TIME cells. (C) Western blot analysis of VEGFR3 podoplanin in mock- and KSHV-infected TIME cells. Cox1 is included as a loading control.
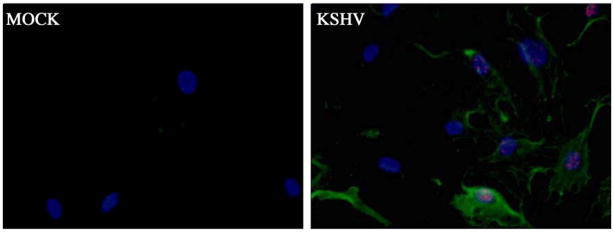
We also analyzed the protein levels of lymphatic endothelial-specific markers. Cell lysates from uninfected and KSHV-infected TIME cells were analyzed by Western blot using antibodies to VEGFR3 and podoplanin. Both were initially below the threshold of detection by Western blot, but easily detected following infection (Fig. 1C). We also examined expression of podoplanin in cells using immunofluorescence. There was only light background staining with an anti-podoplanin antibody in uninfected cells while there is strong cytoplasmic membrane staining in KSHV-infected TIME cells (Fig. 2). Importantly, around 90% of these cells express LANA and less than 1% express markers of lytic replication, indicating expression of lymphatic markers is due to latent gene expression. Also of note, the high level of podoplanin staining is only seen in LANA-positive cells in the infected cell panel (Fig. 2). Taken together, these data indicate that latent KSHV infection of BECs leads to differentiation to LECs.
Fig. 2.
Podoplanin expression is increased in latently infected TIME cells. TIME cells that are >90% latently infected and <1% lytically infected with KSHV and matched mock-infected cells were fixed 72 h after infection and co-stained with anti-podoplanin antibody (green), anti-LANA (red), and DAPI (blue) to stain the nuclei. Only those infected cells that express LANA exhibit cytoplasmic membrane staining for podoplanin.
Global changes in mRNA expression after KSHV infection
To identify global changes in gene expression caused by KSHV infection of endothelial cells, we used cDNA microarrays to examine transcripts from uninfected versus KSHV-infected TIME cells during a 4-day time course of infection and also UV-irradiated virus versus KSHV-infected TIME cells to control for contaminants in the inoculum that might effect host cell gene expression. Analysis was done on glass slides spotted with approximately 15000 or 13000 features in duplicate. As above, at the time of harvest, subsets of cells were seeded onto slides and stained with antibodies to LANA and ORF59. The cells infected with UV-irradiated virus did not express LANA or ORF59, while in the wild-type-infected cells, greater than 90% of the cells stained for LANA and less than 5% of the cells expressed ORF59. A total of eight true biological replicates were done in duplicate at three different time points, 24, 48, and 96 h after infection with another set of duplicates performed at 48 h with UV-irradiated virus-treated TIME cells as the control. The effects of changes in gene expression in cells undergoing lytic replication are minimal because changes in expression in less than 5% of the cells will not significantly impact the overall gene expression patterns. In addition, it was recently demonstrated that there is host gene shut off in lytically infected cells (Glaunsinger and Ganem, 2004).
To analyze genes altered by KSHV infection, we chose to only examine genes that were altered in all eight experiments, indicating that the alterations were sustained over the course of 4 days and, because we used UV-irradiated virus in two experiments, alterations also required viral gene expression. Cellular genes (147), about 1% of the total genes on the array, are altered at all time points more than 1.8-fold in intensity with a P value of less than 0.001. Expression of 86 genes is significantly increased at all time points (Table 1) while 61 genes are significantly repressed by infection with KSHV (Table 2). More than 10% of the genes that were identified as altered by KSHV infection are similarly differentially expressed in BECs versus LECs or in BECs differentiated into LECs by forced expression of Prox-1 (Hirakawa et al., 2003; Hong et al., 2002; Petrova et al., 2002). These changes are indicated in bold in Tables 1 and 2. We have confirmed many of these results by real-time RT-PCR looking at transcripts altered by KSHV infection as well as some that were unchanged on the arrays. Over 30 transcripts have been analyzed by real-time RT-PCR and to date all the genes tested are consistent with the results from the microarrays (see Figs. 1A, 2, 3A and data not shown). Expression of podoplanin is significantly increased on the array (listed as T1A-2), confirming results from Fig. 1. VEGFR3 is not spotted on the array.
Table 1.
Genes upregulated by KSHV infection of TIME cells
| Sequence name | Sequence description | Accession no. | Fold change |
|---|---|---|---|
| SOCS3 | suppressor of cytokine signaling 3 | T72915 | 18.01 |
| FLJ13220 | hypothetical protein FLJ13220 | AA083577 | 7.37 |
| CEACAM1 | carcinoembryonic antigen-related cell adhesion molecule 1 (biliary glycoprotein) | AA406571 | 6.02 |
| IL6 | interleukin 6 (interferon, beta 2) | W31016 | 5.82 |
| LIPG | lipase, endothelial | T49529 | 5.22 |
| GBP5 | guanylate binding protein 5 | AA487252 | 4.49 |
| SLC21A2 | solute carrier family 21 (prostaglandin transporter), member 2 | AA037014 | 4.33 |
| CALCRL | calcitonin receptor-like | N45301 | 3.96 |
| T1A-2 | lung type-I cell membrane-associated glycoprotein (podoplanin) | AA046430 | 3.79 |
| PMP22 | peripheral myelin protein 22 | R26732 | 3.47 |
| DEPP | decidual protein induced by progesterone | N55269 | 3.34 |
| SLC2A14 | solute carrier family 2 (facilitated glucose transporter), member 14 | T97782 | 3.27 |
| TMEM2 | transmembrane protein 2 | H75703 | 3.15 |
| T1A-2 | lung type-I cell membrane-associated glycoprotein (podoplanin) | AA149827 | 3.06 |
| SSB | Sjogren syndrome antigen B (autoantigen La) | H29484 | 2.96 |
| STOM | stomatin | R62817 | 2.85 |
| FHIT | Homo sapiens cDNA FLJ46040 fis, clone SPLEN2036475 | AA256123 | 2.79 |
| CA2 | carbonic anhydrase II | H23187 | 2.76 |
| DEPP | decidual protein induced by progesterone | R33362 | 2.74 |
| LTBP4 | latent transforming growth factor beta binding protein 4 | R87406 | 2.69 |
| PRSS2 | protease, serine, 2 (trypsin 2) | AA284528 | 2.68 |
| EST_293336 | 2.58 | ||
| ADAMTS1 | a disintegrin-like and metalloprotease (reprolysin type) with thrombospondin type 1 motif, 1 | R76276 | 2.56 |
| DAF | decay accelerating factor for complement (CD55, Cromer blood group system) | R09561 | 2.56 |
| ROM1 | retinal outer segment membrane protein 1 | H84113 | 2.45 |
| FLJ11795 | hypothetical protein FLJ11795 | AA459693 | 2.43 |
| HIF1A | hypoxia-inducible factor 1, alpha subunit (basic helix-loop-helix transcription factor) | AA598526 | 2.38 |
| IFIT2 | interferon-induced protein with tetratricopeptide repeats 2 | N63988 | 2.35 |
| ATF3 | activating transcription factor 3 | H21041 | 2.33 |
| KIAA1450 | KIAA1450 protein | W31540 | 2.31 |
| PBEF | pre-B-cell colony-enhancing factor | 2.3 | |
| KIAA0963 | KIAA0963 protein | AA476273 | 2.26 |
| CD47 | CD47 antigen (Rh-related antigen, integrin-associated signal transducer) | R92801 | 2.25 |
| PALM2 | paralemmin 2 | AA488418 | 2.23 |
| IL6ST | interleukin 6 signal transducer (gp130, oncostatin M receptor) | T60500 | 2.2 |
| SOD2 | superoxide dismutase 2, mitochondrial | AA487750 | 2.2 |
| FLT1 | fms-related tyrosine kinase 1 (vascular endothelial growth factor/vascular permeability factor receptor) | AA149508 | 2.18 |
| ARRB1 | Homo sapiens mRNA; cDNA DKFZp762M127 (from clone DKFZp762M127) | H20911 | 2.17 |
| GARP | glycoprotein A repetitions predominant | AA122287 | 2.13 |
| E2-EPF | ubiquitin carrier protein | AA464019 | 2.12 |
| NRCAM | neuronal cell adhesion molecule | R25521 | 2.11 |
| LGALS9 | lectin, galactoside-binding, soluble, 9 (galectin 9) | AA434102 | 2.08 |
| C6orf4 | chromosome 6 open reading frame 4 | R78558 | 2.08 |
| FLJ31265 | hypothetical protein FLJ31265 | H80258 | 2.07 |
| SERPINB1 | serine (or cysteine) proteinase inhibitor, clade B (ovalbumin), member 1 | R54664 | 2.07 |
| UBE2L6 | ubiquitin-conjugating enzyme E2L 6 | AA292074 | 2.06 |
| CTSL | cathepsin L | W73874 | 2.06 |
| RPL7A | ribosomal protein L7a | H23421 | 2.04 |
| NP | nucleoside phosphorylase | AA430382 | 2.03 |
| KIAA0960 | KIAA0960 protein | N92895 | 2.02 |
| KIAA1554 | KIAA1554 protein | H17860 | 2.02 |
| KIAA1450 | KIAA1450 protein | N52875 | 2 |
| IL1RL1 | interleukin 1 receptor-like 1 | AA128153 | 2 |
| EST_139250 | 1.99 | ||
| P2RY5 | purinergic receptor P2Y, G-protein coupled, 5 | N90783 | 1.98 |
| PROS1 | protein S (alpha) | T74191 | 1.97 |
| PPAP2B | phosphatidic acid phosphatase type 2B | T72119 | 1.96 |
| FLJ22269 | hypothetical protein FLJ22269 | AA463986 | 1.95 |
| MMRN | multimerin | AA423867 | 1.95 |
| SLC38A2 | solute carrier family 38, member 2 | R27255 | 1.94 |
| ARHJ | ras homolog gene family, member J | W92399 | 1.93 |
| LAPTM4B | lysosomal associated protein transmembrane 4 beta | AA600214 | 1.93 |
| PRO1073 | PRO1073 protein | AA065090 | 1.92 |
| NPC1 | Niemann-Pick disease, type C1 | AA634267 | 1.92 |
| SP110 | SP110 nuclear body protein | R54613 | 1.91 |
| EST_357298 | Homo sapiens mRNA; cDNA DKFZp762M127 (from clone DKFZp762M127) | W93688 | 1.89 |
| CD53 | CD53 antigen | AA132090 | 1.88 |
| MGP | matrix Gla protein | AA155913 | 1.87 |
| MTSG1 | mitochondrial tumor suppressor gene 1 | AA621202 | 1.87 |
| M160 | scavenger receptor cysteine-rich type 1 protein M160 | N69291 | 1.87 |
| MG61 | Homo sapiens cDNA clone IMAGE:5092935, partial cds | AA486843 | 1.85 |
| EPAS1 | endothelial PAS domain protein 1 | AA680300 | 1.85 |
| RALA | v-ral simian leukemia viral oncogene homolog A (ras related) | H97948 | 1.85 |
| WARS | tryptophanyl-tRNA synthetase | AA664040 | 1.84 |
| NNMT | nicotinamide N-methyltransferase | T72089 | 1.84 |
| ETS2 | v-ets erythroblastosis virus E26 oncogene homolog 2 (avian) | T46844 | 1.84 |
| HS3ST1 | heparan sulfate (glucosamine) 3-O-sulfotransferase 1 | T55714 | 1.84 |
| KIAA0564 | KIAA0564 protein | R24860 | 1.84 |
| PTGS2 | prostaglandin-endoperoxide synthase 2 (prostaglandin G/H synthase and cyclooxygenase) | AA644211 | 1.84 |
| KIAA0963 | KIAA0963 protein | AA485433 | 1.84 |
| FLJ25084 | hypothetical protein FLJ25084 | R62633 | 1.83 |
| GDAP1 | ganglioside-induced differentiation-associated protein 1 | H15302 | 1.83 |
| RANBP1 | RAN binding protein 1 | 1.83 | |
| SPRY4 | Homo sapiens cDNA FLJ26120 fis, clone SYN00419 | R70506 | 1.82 |
| CXCL16 | chemokine (C-X-C motif) ligand 16 | AA416552 | 1.82 |
| POPDC3 | popeye domain containing 3 | N20482 | 1.82 |
Bold denotes similar regulation in lymphatic endothelial cells.
Table 2.
Genes downregulated by KSHV infection of TIME cells
| Sequence name | Sequence description | Accession no. | Fold change |
|---|---|---|---|
| EST_261253 | Homo sapiens cDNA FLJ43100 fis, clone CTONG2003100 | H98248 | −1.82 |
| EST_23588 | Homo sapiens, Similar to hypothetical protein LOC216438, clone IMAGE:4444559, mRNA, partial cds | R38369 | −1.83 |
| EST_322537 | Homo sapiens MSTP157 (MST157) mRNA, complete cds | W15263 | −1.83 |
| TPM1 | tropomyosin 1 (alpha) | W58009 | −1.83 |
| VASP | Homo sapiens transcribed sequence with strong similarity to protein pir:S51797 (H. sapiens) S51797 vasodilator-stimulated phosphoprotein—human | AA410429 | −1.84 |
| CDC6 | CDC6 cell division cycle 6 homolog (S. cerevisiae) | H59203 | −1.85 |
| BIRC5 | baculoviral IAP repeat-containing 5 (survivin) | AA460685 | −1.86 |
| ITGA10 | integrin, alpha 10 | H43656 | −1.87 |
| MN1 | meningioma (disrupted in balanced translocation) 1 | R59212 | −1.87 |
| FOSL1 | Homo sapiens mRNA; cDNA DKFZp666J045 (from clone DKFZp666J045) | T82817 | −1.88 |
| CTCF | CCCTC-binding factor (zinc finger protein) | H89996 | −1.88 |
| UBE2C | ubiquitin-conjugating enzyme E2C | AA430504 | −1.89 |
| AKR1C1 | aldo-keto reductase family 1, member C1 (dihydrodiol dehydrogenase 1; 20-alpha (3-alpha)-hydroxysteroid dehydrogenase) | R93124 | −1.89 |
| MYBL2 | v-myb myeloblastosis viral oncogene homolog (avian)-like 2 | AA456878 | −1.9 |
| DOC1 | downregulated in ovarian cancer 1 | W69790 | −1.9 |
| EST_782847 | Homo sapiens transcribed sequences | AA448283 | −1.9 |
| EST_415584 | Homo sapiens transcribed sequence with moderate similarity to protein ref:NP_060265.1 (H. sapiens) hypothetical protein FLJ20378 [Homo sapiens] | W78816 | −1.91 |
| CARP | cardiac ankyrin repeat protein | AA486364 | −1.92 |
| EST_501430 | Homo sapiens cDNA FLJ31353 fis, clone MESAN2000264. | AA115248 | −1.92 |
| PVRL3 | poliovirus receptor-related 3 | R62780 | −1.92 |
| SEC15L1 | SEC15-like 1 (S. cerevisiae) | AA187002 | −1.93 |
| UAP1 | UDP-N-acteylglucosamine pyrophosphorylase 1 | N68465 | −1.93 |
| CXCL1 | chemokine (C-X-C motif) ligand 1 (melanoma growth stimulating activity, alpha) | W42723 | −1.95 |
| COL1A2 | collagen, type I, alpha 2 | W93067 | −1.96 |
| IL8 | interleukin 8 | AA102526 | −1.97 |
| PTTG1 | pituitary tumor-transforming 1 | AA430032 | −1.97 |
| ALCAM | activated leukocyte cell adhesion molecule | R39862 | −1.98 |
| EGR3 | early growth response 3 | R39111 | −1.98 |
| FJX1 | four jointed box 1 (Drosophila) | H72460 | −2 |
| HMGA1 | high mobility group AT-hook 1 | AA448261 | −2.02 |
| KRT7 | keratin 7 | AA489569 | −2.03 |
| HRB2 | HIV-1 rev binding protein 2 | W52272 | −2.05 |
| EST_201562 | Homo sapiens transcribed sequences | R97050 | −2.05 |
| EST_171753 | Homo sapiens surfactant associated protein F mRNA, partial sequence, mRNA sequence | H18335 | −2.06 |
| RGS4 | regulator of G-protein signaling 4 | AA007419 | −2.07 |
| EPLIN | epithelial protein lost in neoplasm beta | AA491501 | −2.09 |
| MICAL2 | flavoprotein oxidoreductase MICAL2 | AA412582 | −2.1 |
| EST_130826 | Homo sapiens transcribed sequences | R22189 | −2.11 |
| FN14 | IMAGE EST: 135791; Unigene: Hs.10086; type I transmembrane protein Fn14 | −2.12 | |
| CDCA1 | cell division cycle associated 1 | AA421171 | −2.19 |
| EST_140301 | Homo sapiens cDNA FLJ11041 fis, clone PLACE1004405. | R66924 | −2.19 |
| ESDN | endothelial and smooth muscle cell-derived neuropilin-like protein | AA431438 | −2.24 |
| IRS1 | insulin receptor substrate 1 | AA460841 | −2.27 |
| PDCD1L1 | programmed cell death 1 ligand 1 | R78465 | −2.29 |
| RRM2 | ribonucleotide reductase M2 polypeptide | AA187351 | −2.29 |
| EST_140966 | Homo sapiens LOC158525 (LOC158525), mRNA | R66502 | −2.31 |
| HRB2 | HIV-1 rev binding protein 2 | AA251800 | −2.32 |
| TRB@ | Homo sapiens TCR BV3 mRNA for T cell receptor beta chain (CDR3 region), partial cds, isolate:HTLV-1 myopathy case3, clone:Tax tetramer-5. | N91921 | −2.34 |
| DJ667H12.2 | hypothetical protein DJ667H12.2 | AA608531 | −2.37 |
| CCNA2 | cyclin A2 | AA608568 | −2.37 |
| ESDN | endothelial and smooth muscle cell-derived RG_TX_tbl2neuropilin-like protein | N21309 | −2.39 |
| ESDN | endothelial and smooth muscle cell-derived neuropilin-like protein | H99543 | −2.44 |
| COL1A2 | collagen, type I, alpha 2 | AA490172 | −2.56 |
| EST_80186 | Homo sapiens T cell receptor beta chain BV20S1 BJ1-5 BC1 mRNA, complete cds | T64380 | −2.84 |
| SEMA3C | sema domain, immunoglobulin domain (Ig), short basic domain, secreted, (semaphorin) 3C | AA042990 | −3.12 |
| LRRC17 | leucine rich repeat containing 17 | AA423870 | −3.19 |
| XRCC1 | X-ray repair complementing defective | AA425139 | −3.25 |
| CDKN2B | repair in Chinese hamster cells 1 cyclin-dependent kinase inhibitor 2B (p15, inhibits CDK4) | N34320 | −3.45 |
Bold denotes similar regulation in lymphatic endothelial cells.
Fig. 3.
KSHV infection of TIME cells alters transcripts involved in angiogenesis. Depicted is the fold change of angiogenesis-related transcripts in KSHV-infected TIME cells compared to UV-irradiated virus treated cells at 48 h postinfection as determined by real-time RT-PCR (N = 3–5). All values were normalized to GAPDH expression and error bars represent the standard error of the mean.
Angiogenic genes
KSHV infection alters the expression of several genes involved in angiogenesis. Included in the significantly altered genes is FLT1 (VEGFR1), which is increased about 2-fold on average (Table 1). Interestingly, FLT1 was previously shown to be decreased in LECs versus BECs (Hirakawa et al., 2003), indicating that KSHV is also altering gene regulation unrelated to lymphatic differentiation. A set of experiments was done to further quantify the alteration in expression of genes involved in angiogenesis and to probe transcripts that were not present on the array. Quantitative real-time RT-PCR was done using commercially available TaqMan probes for each transcript. As before, replicates (N = 3–5) were done on different days with different viral stocks. As the microarray experiments had shown, expression of VEGFR1 (FLT1) in TIME cells is significantly increased, approximately 5-fold, following KSHV infection, while VEGFR2 (KDR) is relatively unchanged (Fig. 3). None of the VEGF family members were greatly increased in expression. In fact, most (VEGF-A, VEGF-B, and placental growth factor (PGF)) appeared to be slightly decreased, while VEGF-C was slightly and consistently increased (Fig. 3). Interestingly, expression of VEGF-B and PGF is decreased in LECs versus BECs (Hirakawa et al., 2003; Petrova et al., 2002), again consistent with KSHV-induced differentiation. Cultured LECs, but not BECs, respond to VEGF-C, both as a mitogen and chemoattractant (Makinen et al., 2001), and VEGF-C is present in KS lesions (Skobe et al., 1999). VEGF-C was previously shown to be decreased significantly in LECs versus BECs. However, it was not repressed by forced Prox-1 expression in BECs (Petrova et al., 2002), only in mixed culture primary DMVECs (Hong et al., 2002). Thus, KSHV infection of BECs appears to drive the differentiation to LECs, while the increase in VEGFR3 expression and the concomitant induction of VEGF-C raise the possibility of an autocrine loop for cell activation.
We also found that carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1) was increased about 18-fold (Fig. 3), which was consistently one of the most induced genes upon BEC to LEC differentiation (Hirakawa et al., 2003; Petrova et al., 2002).
Cytokines and KSHV infection
Because many cytokines are expressed in KS tumors and cytokine dysregulation has been proposed to play an important role in KS genesis, the alteration of cytokines was further analyzed. One of the most highly induced genes is hIL-6. hIL-6 is a cytokine involved in B-cell activation but may also play many other roles including a role in tumor formation. Importantly, hIL-6 is present at high levels in the KS tumor (Oxholm et al., 1989). The expression of hIL-6 after infection was further analyzed by real-time RT-PCR, where its transcript was increased approximately 44-fold on average after infection (Fig. 4A). Inhibition of cytokines may play a role in KS formation by immune evasion or more directly through changes in the activation of different cell types. Expression of IL-8, a chemokine that is capable of attracting neutrophils and T-cells (Hoffmann et al., 2002), is significantly inhibited at all three time points after KSHV infection. As described above, the inhibition of IL-8 is also consistent with the differentiation of TIME cells from blood to lymphatic endothelium. The expression of IL-8 was further analyzed by real-time RT-PCR, where a decrease of approximately 8-fold was seen at 48 h postinfection with KSHV (Fig. 4A).
Fig. 4.
KSHV infection of endothelial cells alters cytokine production and secretion. (A) Depicted is the fold change of IL6 and IL8 transcripts in KSHV-infected TIME cells compared to UV-irradiated virus treated cells at 48 h postinfection as determined by real-time RT-PCR (N = 3). All values were normalized to GAPDH expression and error bars represent the standard error of the mean. (B) A cytokine antibody array was used to monitor secreted cytokines from KSHV-infected and mock-infected primary human DMVECs by probing the antibody-spotted membrane with diluted supernatants from 48 to 96 h postinfection.
To analyze the protein levels of IL-6, IL-8, and a large number of other cytokines, a cytokine antibody array was used to monitor secreted proteins, as described in Materials and methods. Media from uninfected and infected cells were used to probe a membrane spotted with antibodies to 79 different cytokines and the relative levels of cytokines were determined. A representative experiment is shown from uninfected and KSHV-infected primary DMVECs at both 48 and 96 h postinfection (Fig. 4B). Similar results were obtained with TIME cells at 48 and 96 h postinfection (data not shown). Matching the mRNA expression data, hIL-6 protein levels in the media was significantly increased while IL-8 protein levels in the media were strongly decreased by KSHV infection. The only other cytokine that was altered was monocyte chemoattractant protein-1 (MCP-1), which was decreased at both 48 and 96 h postinfection. MCP-1 expression is also decreased in LECs versus BECs (Hirakawa et al., 2003; Petrova et al., 2002), again consistent with KSHV-infection reprogramming BECs to behave like LECs. These data also demonstrate that the cytokine changes are not unique to TIME cells but are also seen after infection of primary endothelial cells.
Discussion
In vivo, KS spindle cells express markers of the lymphatic endothelium and it was proposed that KS was lymphatic endothelium in origin (Weninger et al., 1999). The data presented here indicate that, rather than infecting lymphatic endothelial cells, KSHV could infect blood or circulating endothelial precursor cells and drive them to differentiate into lymphatic endothelial cells as they become spindle cells. Developmental work has shown that the lymphatic vasculature buds from the existing blood vasculature as a result of the initiation of a distinct differentiation pathway in the endothelial cells (Oliver and Harvey, 2002). Expression of Prox-1 is specific to lymphatic endothelial cells and mice that were deficient in Prox-1 had a normal blood vasculature but had no lymphatic system. While we could detect Prox-1 in primary microvascular endothelial cells (Carroll and Lagunoff, data not shown), which are known to contain significant percentages of LECs, we could not detect expression in TIME cells, indicating that TIME cells do not contain LECs and are BEC derived. Furthermore, only after infection with KSHV could we detect Prox-1 mRNA in TIME cells (Fig. 1B). We obtained quantitative data for three other major markers of lymphatic endothelium, VEGFR3, podoplanin, and LYVE-1 (Fig. 1A), and data demonstrating an increase in protein levels for two of the markers, VEGFR3 and podoplanin (Fig. 1C). Global analysis of TIME cell changes after infection yielded many more changes consistent with differentiation into the lymphatic endothelium. Ten percent of the most significant alterations after KSHV latent infection was differentially expressed between BECs and LECs and many of these changes were also altered when Prox-1 was used to drive differentiation of BECs to LECs (bold in Tables 1 and 2). Taken together, these data demonstrate that KSHV recapitulates many of the aspects of differentiation from BEC to LEC via Prox-1, but the fact that some of the changes are similar to other array data comparing BECs to LECs (Hirakawa et al., 2003; Petrova et al., 2002), but not with forced expression of Prox-1 alone in BECs (Hong et al., 2002; Petrova et al., 2002), suggests that KSHV may induce differentiation effects distinct from Prox-1 in addition to the changes programmed by Prox-1.
There is a large amount of neo-angiogenesis in KS tumors. Whether KSHV infection directly induces angiogenesis or if the effects are indirect is unknown. If KSHV directly induces angiogenesis, this study indicates that factors involved in lymphangiogenesis not hemangiogenesis should be analyzed. Lymphangiogenesis does not revolve around VEGF-A, but rather VEGF-C or VEGF-D is the main growth factor. The receptor for VEGF-C or D is VEGFR3, which is highly upregulated in infected cells. Interestingly, VEGF-C was shown to induce growth of KS spindle cells in culture (Marchio et al., 1999). While these cells are abnormal in chromosome number and no longer maintain KSHV, they were most likely infected with KSHV in vivo and subsequently lost the virus upon growth in culture. Thus, it is possible that the differentiation to lymphatic endothelium is irreversible in these cells and they now respond to VEGF-C because they are lymphatic endothelial cells. There are other changes in genes involved in angiogenesis that are not completely consistent with lymphatic differentiation. The two most prominent are VEGFR1, which is increased slightly on the array and by real-time RT-PCR, and the slight increase in VEGF-C. An increase in VEGF-C is very interesting because that could induce an autocrine loop in infected cells. Further work is underway to determine the role of VEGF-C in infected cells. VEGFR1 has been described as both a negative and a positive regulator of hemangiogenesis. If it is acting positively, it would be interesting to have an infected cell that can respond gratuitously to both lymphangiogenic and hemeangiogenic stimuli simultaneously.
Changes in the cytokine milieu have been proposed to play a major role in KS pathogenesis. The studies presented here identified three cytokines that are altered following KSHV infection. hIL-6 is one of the most highly upregulated genes following KSHV infection while IL-8 is one of the most significantly downregulated genes. MCP-1 secretion is also decreased upon infection with KSHV. IL-8 and MCP-1 decreases are consistent with differentiation from blood to lymphatic endothelial cells. In KS tumors, hIL-6 is found in high levels. As with VEGFR3 and podoplanin, changes in infected TIME cells in culture mimic gene expression seen in KS tumors in vivo. The role of hIL-6 in KS tumors is unknown but may be part of the interplay of infiltrating lymphocytes and spindle cells.
Three other studies have analyzed host gene expression following KSHV infection of endothelial cells. Two studies used commercially available primary DMVECs (Naranatt et al., 2004; Poole et al., 2002). The third study used a DMVEC line that was immortalized with E6 and E7 from human papilloma virus (Moses et al., 2002), two potent oncogenes. None of these studies noted the large number of lymphatic endothelial cell changes that we found here. Both studies done at later times postinfection contained a few changes consistent with lymphatic differentiation, but neither noted the major markers of lymphatic endothelium. It is possible that the arrays used did not have many of the lymphatic-specific genes. For example, the cDNA array we used did not contain VEGFR3, the changes were determined solely by real-time RT-PCR and at the protein level. However, it is important to note again that commercially available primary cells contain a significant percentage of lymphatic endothelial cells and thus many of the changes due to differentiation could be dampened. We have analyzed many of the genes described in primary DMVECs as well, and all of the genes are altered in a similar, yet in some cases diminished, fashion (Carroll and Lagunoff, unpublished). The most recent study examined cells at 2 and 4 h (Naranatt et al., 2004), before latency has been established and there is much more complex viral gene expression not directly relevant to latent gene expression (Krishnan et al., 2004). In the E6/E7 immortalized DMVECs, the authors found that VEGFR2 is upregulated (Moses et al., 2002). This hallmark of blood endothelial cells is not seen after latent infection of primary cells in the other study or our experiments (Poole et al., 2002). Of note, E7 can destabilize chromosomes leading to aneuploidy (Duensing and Munger, 2002), and E6 targets p53 for degradation (Scheffner et al., 1990), which can have multiple effects on angiogenesis and tumorigenesis (Ravi et al., 2000).
Overall, this study demonstrates the potential for KSHV to alter the differentiation state of endothelial cells. Because KS spindle cells express markers of LECs and KSHV infection of BECs in culture leads to upregulation of lymphatic markers, we believe that KSHV may infect blood endothelial cells or other endothelial cell types in vivo and drive their differentiation to lymphatic endothelial cells. However, the question remains, what is the role of lymphatic differentiation in KSHV biology? It is possible that differentiation induces the cells to a state where latency is better established or where the infected cells are better suited to expand and spread though the body via the lymphatic system and VEGFR3-mediated mitogenic stimulation. Further studies are ongoing to determine the functional effects of the alterations of these genes on endothelial cells and how they might be advantageous for KSHV.
Materials and methods
Cells
BCBL-1 cells are a KSHV-positive, EBV-negative B-cell line derived from a primary effusion lymphoma maintained as described elsewhere (Renne et al., 1996). TIME cells are a tert-immortalized dermal microvascular endothelial cell line (DMVEC) maintained as described elsewhere (Lagunoff et al., 2002). Primary human DMVECs (Clonetics) were cultured under the same conditions as the TIME cells.
Virus isolation and infections
Virus was isolated as before (Lagunoff et al., 2002) and was titered on TIME cells to determine the optimal amount of virus to achieve greater than 90% latent infection and less than 5% lytic infection, as determined by immunofluorescence assay (Lagunoff et al., 2002) using anti-LANA (a kind gift from A. Polson and D. Ganem) and anti-ORF59 (a kind gift from B. Forghani or purchased from ABI). Alexafluor 488 or 594 nm labeled secondary antibodies were used (Molecular Probes, Oregon). Slides were mounted in DAPI containing mounting media, Vectashield (Vector Laboratories, Inc.), then scored on a fluorescent microscope. Immunofluorescence staining with anti-podplanin antibody, purchased from AngioBio Co. (Del Mar, CA), was done similarly as above except methanol was used to fix the cells and fetal bovine serum was used as a block. The pictures in Fig. 2 were taken for each fluor under identical conditions between mock- and KSHV-infected cells.
RNA isolation and cDNA generation
Total RNA was isolated from TIME cells using RNAbee as recommended by the manufacturer (Tel-Test, Texas). Enrichment of polyadenylated mRNA was done using the Oligotex Kit as recommended by the manufacturer (Qiagen). For the first set of experiments, 8 μg of polyA-enriched RNA was labeled for microarray as described elsewhere (Geiss et al., 2001). For the second round of experiments, mRNA was amplified using the RiboAmp RNA Amplification Kit, as recommended by the manufacturer (Arcturus). Labeled cDNA was then made as described above. cDNA for real-time RT-PCR was generated from 100 ng of isolated mRNA using the SuperScript First-Strand Synthesis System according to the manufacturers directions (Invitrogen).
Microarray analysis
Slides for analysis were spotted at the University of Washington’s Center for Expression Array as described elsewhere (Geiss et al., 2001). Labeled cDNAs from the first set of experiments were hybridized to approximately 15000 gene array spotted in duplicate on two slides. Each experiment was done with the control and experimental cDNA labeled with cy3 and cy5 and then repeated with the labels reversed. The second round of experiments was done on approximately 13000 gene array identical to the original array except for the absence of approximately 2000 spots. RNA for each time point was isolated from different infections, performed on different days, using different viral stocks. Microarray data were analyzed using the University of Washington’s Center for Expression Array and the Resolver Biosoftware Package (Rosetta Inpharmatics).
Real-time and semi-quantitative RT-PCR
cDNA was generated as stated above, and commercially available TaqMan probes (Assays-on-Demand) were used, following the manufacturers directions (Applied Biosystems). iTaq DNA Polymerase (Biorad) was used for the amplification of 2 μl of cDNA with the TaqMan probes, and the reactions were monitored on an Icycler thermocycler (Biorad). Changes in relative abundance of transcript were normalized to GAPDH expression (Threshold cycle, CT) compared to control expression in UV-irradiated KSHV-treated TIME cells using the following equation (CT of experimental gene in control cells minus CT of GAPDH) minus (CT of experimental gene in KSHV-infected cells minus CT of GAPDH). For semi-quantitative RT-PCR, 5–160 ng of mRNA was amplified using the OneStep RT-PCR kit (Qiagen) as recommended using the following 5′ to 3′ primers; Prox-1 F: CCCGGATCCGTGATGCCTGACCATGAC, Prox-1 R: AAACCCGTC-GACTTTCTACTCATGAAGCAGCTC and GAPDH F: AAGGTGAAGGTCGGAGTCAACG, GAPDH R: TGGAAGATGGTGATGGGATTTC. The Prox-1 primers are tailed with restriction sites. The product resulting from RT-PCR with these primers was sequenced and found to perfectly match the human Prox-1 sequence.
Western blot analysis
Forty-eight hours postinfection, KSHV-infected and mock-infected TIME cells were lysed in RIPA lysis buffer (50 mM Tris–HCL, pH 7.6, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 1% sodium deoxycholate, and 0.1% sodium dodecyl sulfate supplemented with 0.5 mM dithiothreitol, 0.4 mM phenylmethylsulfonyl fluoride, 2 μg/ml each of aprotinin, pepstatin, and leupeptin, and 1 mM sodium ortho-vanadate). Protein in the lysis supernatant was quantified by BCA assay (Pierce), and 20 or 40 μg of total protein was subjected to SDS-PAGE. After transfer to PVDF (Immobilon-P, Millipore), the blot was probed with the following primary antibodies: rabbit anti-podoplanin (Research Diagnostic Inc, RDI-102PA40), rabbit anti-VEGFR3 (Santa Cruz, SC-321), and mouse anti-COX1 (Cayman Chemical, 160110), then washed and probed with either goat anti-mouse or goat anti-rabbit (Jackson ImmunoResearch Laboratories). Chemiluminescence was then performed using ECL+ Plus (Amersham) and exposing to Blue X-Ray Film (Phenix Research Products).
Cytokine antibody array
DMVECs were infected with KSHV or mock infected for 42 and 90 h. Media were replaced with serum-free media and left on the cells at 37 °C for 6 h. The serum-free media were then harvested, cellular debris was centrifuged out, and the supernatant was diluted 1:5 and used as the probe on a Human Cytokine Array V spotted with antibodies to 79 different cytokines (RayBiotech, Inc.). The membrane was treated as recommended by the manufacturer. After the final wash the membrane was developed using ECL+ Plus Western Blotting Detection System (Amersham Biosciences).
Acknowledgments
The authors would like to thank Angelique Van’t Wout and Gary Geiss for help with array analysis software. ML is supported by the PEW scholars program in the biological sciences sponsored by the PEW charitable trust and by a grant (1RO1CA97934-01A1) from the National Cancer Institute. PAC is partially supported by training grant T32-CA09229 from the National Cancer Institute.
Footnotes
Note added in proof
Two manuscripts demonstrating similar KSHV induced lymphatic differentiation were published while this manuscript was in press, Y.-K. Hong et al. Nature Genetics, 2004, 36: 683–85 and H.-W. Wang et al. Nature Genetics, 2004, 36: 687–93.
References
- Aluigi MG, Albini A, Carlone S, Repetto L, De Marchi R, Icardi A, Moro M, Noonan D, Benelli R. KSHV sequences in biopsies and cultured spindle cells of epidemic, iatrogenic and Mediterranean forms of Kaposi’s sarcoma. Res Virol. 1996;147 (5):267–275. doi: 10.1016/0923-2516(96)82285-0. [DOI] [PubMed] [Google Scholar]
- Cesarman E, Chang Y, Moore PS, Said JW, Knowles DM. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med. 1995;332 (18):1186–1191. doi: 10.1056/NEJM199505043321802. [DOI] [PubMed] [Google Scholar]
- Duensing S, Munger K. Human papillomaviruses and centrosome duplication errors: modeling the origins of genomic instability. Oncogene. 2002;21 (40):6241–6248. doi: 10.1038/sj.onc.1205709. [DOI] [PubMed] [Google Scholar]
- Geiss GK, An MC, Bumgarner RE, Hammersmark E, Cunningham D, Katze MG. Global impact of influenza virus on cellular pathways is mediated by both replication-dependent and-independent events. J Virol. 2001;75 (9):4321–4331. doi: 10.1128/JVI.75.9.4321-4331.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaunsinger B, Ganem D. Lytic KSHV infection inhibits host gene expression by accelerating global mRNA turnover. Mol Cell. 2004;13 (5):713–723. doi: 10.1016/s1097-2765(04)00091-7. [DOI] [PubMed] [Google Scholar]
- Hirakawa S, Hong YK, Harvey N, Schacht V, Matsuda K, Libermann T, Detmar M. Identification of vascular lineage-specific genes by transcriptional profiling of isolated blood vascular and lymphatic endothelial cells. Am J Pathol. 2003;162 (2):575–586. doi: 10.1016/S0002-9440(10)63851-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann E, Dittrich-Breiholz O, Holtmann H, Kracht M. Multiple control of interleukin-8 gene expression. J Leukocyte Biol. 2002;72 (5):847–855. [PubMed] [Google Scholar]
- Hong YK, Harvey N, Noh YH, Schacht V, Hirakawa S, Detmar M, Oliver G. Prox1 is a master control gene in the program specifying lymphatic endothelial cell fate. Dev Dyn. 2002;225 (3):351–357. doi: 10.1002/dvdy.10163. [DOI] [PubMed] [Google Scholar]
- Jussila L, Valtola R, Partanen TA, Salven P, Heikkila P, Matikainen MT, Renkonen R, Kaipainen A, Detmar M, Tschachler E, Alitalo R, Alitalo K. Lymphatic endothelium and Kaposi’s sarcoma spindle cells detected by antibodies against the vascular endothelial growth factor receptor-3. Cancer Res. 1998;58 (8):1599–1604. [PubMed] [Google Scholar]
- Krishnan HH, Naranatt PP, Smith MS, Zeng L, Bloomer C, Chandran B. Concurrent expression of latent and a limited number of lytic genes with immune modulation and antiapoptotic function by Kaposi’s sarcoma-associated herpesvirus early during infection of primary endothelial and fibroblast cells and subsequent decline of lytic gene expression. J Virol. 2004;78 (7):3601–3620. doi: 10.1128/JVI.78.7.3601-3620.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagunoff M, Bechtel J, Venetsanakos E, Roy AM, Abbey N, Herndier B, McMahon M, Ganem D. De novo infection and serial transmission of Kaposi’s sarcoma-associated herpesvirus in cultured endothelial cells. J Virol. 2002;76 (5):2440–2448. doi: 10.1128/jvi.76.5.2440-2448.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makinen T, Veikkola T, Mustjoki S, Karpanen T, Catimel B, Nice EC, Wise L, Mercer A, Kowalski H, Kerjaschki D, Stacker SA, Achen MG, Alitalo K. Isolated lymphatic endothelial cells transduce growth, survival and migratory signals via the VEGF-C/D receptor VEGFR-3. EMBO J. 2001;20 (17):4762–4773. doi: 10.1093/emboj/20.17.4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchio S, Primo L, Pagano M, Palestro G, Albini A, Veikkola T, Cascone I, Alitalo K, Bussolino F. Vascular endothelial growth factor-C stimulates the migration and proliferation of Kaposi’s sarcoma cells. J Biol Chem. 1999;274 (39):27617–27622. doi: 10.1074/jbc.274.39.27617. [DOI] [PubMed] [Google Scholar]
- Moses AV, Jarvis MA, Raggo C, Bell YC, Ruhl R, Luukkonen BG, Griffith DJ, Wait CL, Druker BJ, Heinrich MC, Nelson JA, Fruh K. Kaposi’s sarcoma-associated herpesvirus-induced upregulation of the c-kit proto-oncogene, as identified by gene expression profiling, is essential for the transformation of endothelial cells. J Virol. 2002;76 (16):8383–8399. doi: 10.1128/JVI.76.16.8383-8399.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naranatt PP, Krishnan HH, Svojanovsky SR, Bloomer C, Mathur S, Chandran B. Host gene induction and transcriptional reprogramming in Kaposi’s sarcoma-associated herpesvirus (KSHV/HHV-8)-infected endothelial, fibroblast, and B cells: insights into modulation events early during infection. Cancer Res. 2004;64 (1):72–84. doi: 10.1158/0008-5472.can-03-2767. [DOI] [PubMed] [Google Scholar]
- Neipel F, Albrecht JC, Fleckenstein B. Cell-homologous genes in the Kaposi’s sarcoma-associated rhadinovirus human herpesvirus 8: determinants of its pathogenicity? J Virol. 1997;71 (6):4187–4192. doi: 10.1128/jvi.71.6.4187-4192.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver G, Harvey N. A stepwise model of the development of lymphatic vasculature. Ann N Y Acad Sci. 2002;979:159–165. doi: 10.1111/j.1749-6632.2002.tb04876.x. (discussion 188–96) [DOI] [PubMed] [Google Scholar]
- Oxholm A, Oxholm P, Permin H, Bendtzen K. Epidermal tumour necrosis factor alpha and interleukin 6-like activities in AIDS-related Kaposi’s sarcoma. An immunohistological study. APMIS. 1989;97 (6):533–538. doi: 10.1111/j.1699-0463.1989.tb00827.x. [DOI] [PubMed] [Google Scholar]
- Petrova TV, Makinen T, Makela TP, Saarela J, Virtanen I, Ferrell RE, Finegold DN, Kerjaschki D, Yla-Herttuala S, Alitalo K. Lymphatic endothelial reprogramming of vascular endothelial cells by the Prox-1 homeobox transcription factor. EMBO J. 2002;21 (17):4593–4599. doi: 10.1093/emboj/cdf470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole LJ, Yu Y, Kim PS, Zheng QZ, Pevsner J, Hayward GS. Altered patterns of cellular gene expression in dermal microvascular endothelial cells infected with Kaposi’s sarcoma-associated herpesvirus. J Virol. 2002;76 (7):3395–3420. doi: 10.1128/JVI.76.7.3395-3420.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravi R, Mookerjee B, Bhujwalla ZM, Sutter CH, Artemov D, Zeng Q, Dillehay LE, Madan A, Semenza GL, Bedi A. Regulation of tumor angiogenesis by p53-induced degradation of hypoxia-inducible factor 1alpha. Genes Dev. 2000;14 (1):34–44. [PMC free article] [PubMed] [Google Scholar]
- Renne R, Zhong W, Herndier B, McGrath M, Abbey N, Kedes D, Ganem D. Lytic growth of Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) in culture. Nat Med. 1996;2 (3):342–346. doi: 10.1038/nm0396-342. [DOI] [PubMed] [Google Scholar]
- Russo JJ, Bohenzky RA, Chien MC, Chen J, Yan M, Maddalena D, Parry JP, Peruzzi D, Edelman IS, Chang Y, Moore PS. Nucleotide sequence of the Kaposi sarcoma-associated herpesvirus (HHV8) Proc Natl Acad Sci USA. 1996;93 (25):14862–14867. doi: 10.1073/pnas.93.25.14862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffner M, Werness BA, Huibregtse JM, Levine AJ, Howley PM. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63 (6):1129–1136. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- Skobe M, Brown LF, Tognazzi K, Ganju RK, Dezube BJ, Alitalo K, Detmar M. Vascular endothelial growth factor-C (VEGF-C) and its receptors KDR and flt-4 are expressed in AIDS-associated Kaposi’s sarcoma. J Invest Dermatol. 1999;113 (6):1047–1053. doi: 10.1046/j.1523-1747.1999.00798.x. [DOI] [PubMed] [Google Scholar]
- Soulier J, Grollet L, Oksenhendler E, Cacoub P, Cazals-Hatem D, Babinet P, d’Agay MF, Clauvel JP, Raphael M, Degos L, et al. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman’s disease. Blood. 1995;86 (4):1276–1280. [PubMed] [Google Scholar]
- Staskus KA, Zhong W, Gebhard K, Herndier B, Wang H, Renne R, Beneke J, Pudney J, Anderson DJ, Ganem D, Haase AT. Kaposi’s sarcoma-associated herpesvirus gene expression in endothelial (spindle) tumor cells. J Virol. 1997;71 (1):715–719. doi: 10.1128/jvi.71.1.715-719.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wabinga HR, Parkin DM, Wabwire-Mangen F, Mugerwa JW. Cancer in Kampala, Uganda, in 1989–91: changes in incidence in the era of AIDS. Int J Cancer. 1993;54 (1):26–36. doi: 10.1002/ijc.2910540106. [DOI] [PubMed] [Google Scholar]
- Weninger W, Partanen TA, Breiteneder-Geleff S, Mayer C, Kowalski H, Mildner M, Pammer J, Sturzl M, Kerjaschki D, Alitalo K, Tschachler E. Expression of vascular endothelial growth factor receptor-3 podoplanin suggests a lymphatic endothelial cell origin of Kaposi’s sarcoma tumor cells. Lab Invest. 1999;79 (2):243–251. [PubMed] [Google Scholar]
- Wigle JT, Oliver G. Prox1 function is required for the development of the murine lymphatic system. Cell. 1999;98 (6):769–778. doi: 10.1016/s0092-8674(00)81511-1. [DOI] [PubMed] [Google Scholar]