Abstract
Purpose:
Behavioral and psychiatric symptoms of dementia (BPSD) are common in Alzheimer’s disease (AD) and disrupt the effective management of AD patients. The present study explores the use of radio electric asymmetric brain stimulation (REAC) in patients who have had a poor response to pharmacological treatment.
Patients and methods:
Eight patients (five females and three males; mean [±standard deviation] age at study baseline: 69.9 ± 3.0 years) diagnosed with AD according to the DSM-IV-TR criteria (mean onset age of AD: 65.4 ± 3.5 years) were cognitively and psychometrically assessed with the Mini-Mental State Examination (MMSE), the Activity of Daily Living (ADL), the Instrumental Activity of Daily Living (IADL), and the Neuropsychiatric Inventory (NPI), prior to and after each of 2 REAC treatment cycles.
Results:
Scores on the MMSE and all subscales of the NPI (frequency, severity, and distress), the ADL, and the IADL were significantly improved following the initial REAC treatment. There was further significant improvement in all measurements (with a tendency for improvement in the IADL) after the second REAC treatment cycle.
Conclusion:
The improvement of cognitive and behavioral/psychiatric functioning following REAC treatment suggests that this innovative approach may be an effective, safe, and tolerable alternative to pharmacological treatment of AD patients, especially in the area of BPSD. Elderly patients suffering from other types of dementia may also benefit from REAC treatment.
Keywords: anxiety, depression, insomnia, behavioral and psychiatric symptoms of dementia (BPSD)
Introduction
The progressively increasing number of the elderly in the general population and, consequently, the growing prevalence of Alzheimer disease1,2 (AD) highlight the need for new specifically targeted therapeutic options.3–6 The currently available psychological7–10 and psychopharmacological11–15 treatments16 for AD provide relatively poor results. The inadequate and/or insufficient safety and tolerability profile of ‘typical’ AD therapies produce familiar social and economic difficulties.17,18 According to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR), AD includes a disruption in attention,19,20 concentration, memory,21,22 and cognition.23 While the DSM-IV-TR instructs diagnosing clinicians to specify whether cognitive symptoms of AD are accompanied by behavioral abnormalities (eg, delusions, depression, anxiety, agitation, wandering, sexual behavior alterations, sleep dysfunctions, disorientation), behavioral symptoms of AD are not included in the working definition.
The relationship between AD and the behavioral and psychiatric symptoms of dementia24 (BPSD) is becoming more prevalent. Symptoms of dementia accompany AD in about 90% of cases,25 typically arising early in the course of the disease and persisting. Unlike the steady loss of “global” cognition, throughout the course of AD, behavioral symptoms are more variable, with different types of BPSD seen among patients.26 Despite the observed inter-patient variability in number and type of behavioral symptoms encountered in AD, patients with advanced illness tend to have more behavioral symptoms than those in earlier phases of the disorder. Several long-term studies indicate that once a specific symptom occurs in a given patient, it is likely to persist or recur thereafter.
Radio electric asymmetric brain stimulation27,28 (REAC) treatment has proven efficacy in ameliorating several stress-related disorders, depression, and anxiety,29–35 and our unpublished data have shown promising results for some forms of dementias. REAC treatments are painless, noninvasive, and have a high safety and tolerability profile. On the basis these we can hypothesize that the treatments with REAC technology may be helpful in AD and, in general, in several forms of cognitive-impairment disorders.
Aim of the present study
REAC has demonstrated efficacy in improving certain psychiatric disorders such as anxiety, depression and bipolar disorder. The main goal of the present study was to investigate the efficacy of REAC in the treatment of behavioral symptoms in AD patients. In addition, effects on cognitive functioning were also investigated.
Materials and methods
The data for the current study were collected during routine therapy sessions at the Rinaldi Fontani Institute, Behavioral Disorder Department in Florence, Italy. Most patients referred to the clinic were nonresponders to typical pharmacological strategies. Eight patients (five females and three males; mean [±SD] age at study baseline: 69.9 ± 3.0 years) diagnosed with AD according to the DSM-IV-TR criteria (mean onset age of AD: 65.4 ± 3.5 years), presenting with behavioral and/or psychiatric disturbances were psychometrically assessed using the Mini-Mental State Examination36 (MMSE), the Activity of Daily Living37 (ADL), the Instrumental Activity of Daily Living38 (IADL), and the Neuropsychiatric Inventory39 (NPI), before and after 2 treatment cycles of REAC.
REAC is applied by a medical device that is based on an innovative biostimulation technology.27,28 REAC works within a typical frequency range of 2.4, 5.8, or 10.5 GHz, selected by the operator for each specific protocol. For the current study, a frequency of 10.5 GHz, with a specific absorption rate (SAR) of 7 μW/kg, was used. The neuro postural optimization (NPO) protocol consisting of a single radiofrequency burst of 500 milliseconds (ms) applied by touching the metallic tip of the REAC probe (Convogliatore di Radianza Modulante – CRM; ASMED, Italy) to the ear pavilion was performed as an initial treatment. NPO was followed by another treatment protocol, named neuro psycho physical optimization (NPPO). This protocol consisting of seven REAC radiofrequency bursts of 500 ms applied by touching the metallic tip of the REAC probe at precise points of the ear. Each therapy session lasts about 5 seconds. An NPPO treatment cycle consists of 18 therapy sessions, administered on alternate days. The mean interval between the first and the second NPPO treatment cycle is about 6 months.
ANOVA for repeated measures was performed for statistical analysis of differences in MMSE, ADL, IADL, and NPI scores prior to and following REAC treatments. P values <0.05 were considered significant.
Results
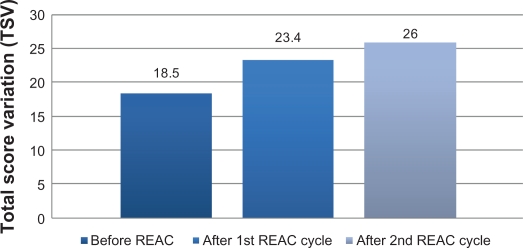
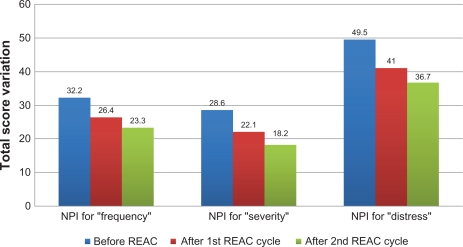
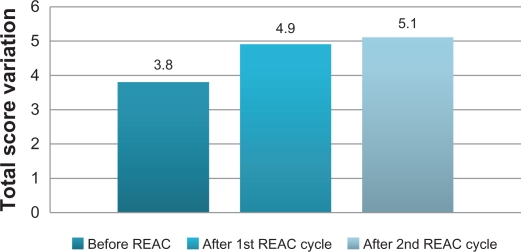
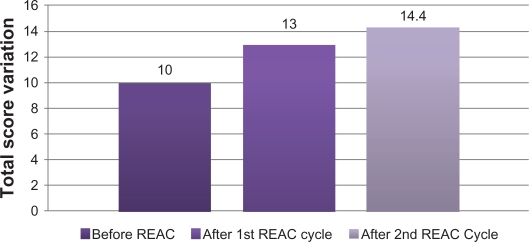
After the first REAC treatment cycle, patients showed great improvement in cognitive performance as demonstrated by a significant increase in average MMSE total score from 18.5 (±2.4) to 23.4 (±2.3) (ANOVA P < 0.01) (Table 1 and Figure 1). In addition, BPSD was improved as demonstrated by a decrease in average NPI frequency score (Table 1 and Figure 2) from 32.2 (±2.5) to 26.4 (±2.4) (ANOVA P = 0.000), NPI severity score (Table 1 and Figure 2) from 28.6 (±3.4) to 22.1 (±3.4) (ANOVA P = 0.000), and NPI distress score from (Table 1 and Figure 2) 49.5 (±3.6) to 41.0 (±2.1) (ANOVA P = 0.000). In addition, average ADL score (Table 1 and Figure 3) increased from 3.8 (±1.0) to 4.9 (±0.5) (ANOVA P = NS) and average IADL score (Table 1 and Figure 4) increased from 10.0 (±2.4) to 13.0 (±1.1) (ANOVA P = 0.000).
Table 1.
Total score variation before and after first REAC treatment cycle
| Test | TSV before REAC | TSV after 1 REAC cycle | ANOVA F | df | P |
|---|---|---|---|---|---|
| MMSE | 18.5 ± 2.4 | 23.4 ± 2.3 | 64.0588 | 14 | <0.01 |
| NPI for “frequency” | 32.2 ± 2.5 | 26.4 ± 2.4 | 309.8421 | 14 | 0.000 |
| NPI for “severity” | 28.6 ± 3.4 | 22.1 ± 3.4 | 529.000 | 14 | 0.000 |
| NPI for “distress” | 49.5 ± 3.6 | 41.0 ± 2.1 | 583.1538 | 14 | 0.000 |
| ADL | 3.8 ± 1.0 | 4.9 ± 0.5 | 33.8507 | 14 | NS |
| IADL | 10.0 ± 2.4 | 13.0 ± 1.1 | 134.8667 | 14 | 0.000 |
Abbreviations: REAC, radio electric asymmetric brain stimulation; TSV, total score variation; MMSE, Mini-Mental State Examination; NPI, Neuropsychiatric inventory; ADL, Activity of Daily Living; IADL, Instrumental Activity of Daily Living; NS, not statistically significant.
Figure 1.
Mini-Mental State Examination (MMSE) before, after the first and second REAC treatment cycle.
Abbreviations: REAC, radio electric asymmetric brain stimulation; TSV, total score variation.
Figure 2.
Neuro Psychiatric Inventory (NPI) before after first and second REAC treatment cycle.
Abbreviation: REAC, radio electric asymmetric brain stimulation.
Figure 3.
Activity of Daily Living (ADL), before, after first and second REAC treatment cycle.
Abbreviation: REAC, radio electric asymmetric brain stimulation.
Figure 4.
Instrumental Activity of Daily Living (IADL), before, after first and second REAC treatment cycle.
Abbreviation: REAC, radio electric asymmetric brain stimulation.
After the second REAC treatment cycle, there were further significant ameliorations in both cognitive and behavioral performance (Table 2). There was a significant increase in average MMSE total score (Table 2 and Figure 1) to 26.0 (±3.4) (ANOVA P < 0.001), and a significant decrease in NPI frequency score (Table 2 and Figure 2) to 23.3 (±2.3) (ANOVA P < 0.001), NPI severity score (Table 2 and Figure 2) to 18.2 (± 3.7) (ANOVA P = 0.000), and NPI distress score (Table 2 and Figure 2) to 36.7 (±4.3) (ANOVA P = 0.000). IADL total score was significantly increased after the second REAC treatment to 14.4 (±0.7) (ANOVA P = NS) (Table 2 and Figure 4). The increase in ADL total score to 5.1 ± 0.4 (Table 2 and Figure 3) was not statistically significant (ANOVA P = NS).
Table 2.
Total score value of first versus second REAC treatment cycle
| Test | TSV after 1 REAC cycle | TSV after 2 REAC cycle | ANOVA F | df | P |
|---|---|---|---|---|---|
| MMSE | 23.4 ± 2.3 | 26.0 ± 3.4 | 44.800 | 14 | <0.001 |
| NPI for “frequency” | 26.4 ± 2.4 | 23.3 ± 2.3 | 79.5455 | 14 | <0.001 |
| NPI for “severity” | 22.1 ± 3.4 | 18.2 ± 3.7 | 203.8936 | 14 | 0.000 |
| NPI for “distress” | 41.0 ± 2.1 | 36.7 ± 4.3 | 191.6526 | 14 | <0.05 |
| ADL | 4.9 ± 0.5 | 5.1 ± 0.4 | 0.4430 | 14 | NS |
| IADL | 13.0 ± 1.1 | 14.4 ± 0.7 | 0.7238 | 14 | NS |
Abbreviations: REAC, radio electric asymmetric brain stimulation; TSV, total score variation; MMSE, Mini-Mental state examination; NPI, Neuropsychiatric inventory; ADL, Activity of Daily Living; IADL, Instrumental Activity of Daily Living; NS, not statistically significant.
Discussion
REAC treatment enhanced cognitive and behavioral functioning in patients with AD. All measures of cognitive functioning were significantly improved after the initial REAC treatment and continued to improve after the second REAC cycle. Similarly, all behavioral measurements were positively affected after the first REAC treatment and most continued to improve after the second cycle.
It is likely that the effects on cognition and behavior are due to a process of synchronization of brain function caused by the microelectric stimulation of the REAC.29
While cognitive functioning benefitted from REAC treatment, the positive effects on BPSD may be of more importance in the management of AD patients. The improvement in these clinical parameters is reflected in functional terms by an increase in the quality of life of patients as reported by relatives, physicians, and care-givers and is psychometrically demonstrated by the ADL and IADL total scores. The use of REAC treatment may decrease the need to continually alter pharmacological treatments to achieve optimal results, leading to significant savings in economic resources of rehabilitation programs for AD patients.
REAC treatments alone are not likely to manage all deficits associated with neuropsychiatric disorders in the elderly. However, the results of the present study suggest that REAC treatment has a high safety, tolerability, and efficacy profile and may be useful not only in patients who have experienced poor and/or unstable responses to psychotropic drugs, but also as a first-line therapeutic option. REAC treatment may be useful not only for AD patients, but also for other forms of dementia (ie, vascular dementia or mixed conditions).
Acknowledgments
The authors thank Dr Eng Matteo Lotti Margotti for data analysis. Lucia Aravagli MD and Stefania Bini MD of Rinaldi Fontani Institute, Department of Neuro Psycho Physio Pathology, Florence, Italy, for their helpful discussions.
Footnotes
Disclosure
Salvatore Rinaldi and Vania Fontani are the inventors of the Radio Electric Asymmetric Conveyer.
Reference
- 1.Chouliaras L, Rutten BP, Kenis G, et al. Epigenetic regulation in the pathophysiology of Alzheimer’s disease. Prog Neurobiol. 2010;90(4):498–510. doi: 10.1016/j.pneurobio.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Salloway S. Introduction: the prevalence of Alzheimer’s disease – a growing crisis. CNS Spectr. 2008;13(3 Suppl 3):1. [PubMed] [Google Scholar]
- 3.Lleo A. Current therapeutic options for Alzheimer’s disease. Curr Genomics. 2007;8(8):550–558. doi: 10.2174/138920207783769549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moreira PI, Zhu X, Nunomura A, Smith MA, Perry G. Therapeutic options in Alzheimer’s disease. Expert Rev Neurother. 2006;6(6):897–910. doi: 10.1586/14737175.6.6.897. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Ruiz Espiga PJ, Echeverria A, Garcia-Torres A, Contreras A. New therapeutic options in Alzheimer’s disease. Rev Neurol. 2006;42(8):478–481. [PubMed] [Google Scholar]
- 6.Kurz A, Perneczky R. Novel insights for the treatment of Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(2):373–379. doi: 10.1016/j.pnpbp.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 7.Ballard C, Corbett A, Chitramohan R, Aarsland D. Management of agitation and aggression associated with Alzheimer’s disease: controversies and possible solutions. Curr Opin Psychiatry. 2009;22(6):532–540. doi: 10.1097/YCO.0b013e32833111f9. [DOI] [PubMed] [Google Scholar]
- 8.Mathias JL, Burke J. Cognitive functioning in Alzheimer’s and vascular dementia: a meta-analysis. Neuropsychology. 2009;23(4):411–423. doi: 10.1037/a0015384. [DOI] [PubMed] [Google Scholar]
- 9.Logsdon RG, McCurry SM, Teri L. Evidence-based psychological treatments for disruptive behaviors in individuals with dementia. Psychol Aging. 2007;22(1):28–36. doi: 10.1037/0882-7974.22.1.28. [DOI] [PubMed] [Google Scholar]
- 10.O’Connor DW, Ames D, Gardner B, King M. Psychosocial treatments of psychological symptoms in dementia: a systematic review of reports meeting quality standards. Int Psychogeriatr. 2009;21(2):241–251. doi: 10.1017/S1041610208008223. [DOI] [PubMed] [Google Scholar]
- 11.Rocca P, Marino F, Montemagni C, Perrone D, Bogetto F. Risperidone, olanzapine and quetiapine in the treatment of behavioral and psychological symptoms in patients with Alzheimer’s disease: preliminary findings from a naturalistic, retrospective study. Psychiatry Clin Neurosci. 2007;61(6):622–629. doi: 10.1111/j.1440-1819.2007.01729.x. [DOI] [PubMed] [Google Scholar]
- 12.Lauterbach EC, Victoroff J, Coburn KL, Shillcutt SD, Doonan SM, Mendez MF. Psychopharmacological neuroprotection in neurodegenerative disease: assessing the preclinical data. J Neuropsychiatry Clin Neurosci. 2010;22(1):8–18. doi: 10.1176/jnp.2010.22.1.8. [DOI] [PubMed] [Google Scholar]
- 13.Marksteiner J, Schmidt R. Treatment strategies in Alzheimer’s disease with a focus on early pharmacological interventions. Drugs Aging. 2004;21(7):415–426. doi: 10.2165/00002512-200421070-00001. [DOI] [PubMed] [Google Scholar]
- 14.Massoud F, Gauthier S. Update on the pharmacological treatment of Alzheimer’s disease. Curr Neuropharmacol. 2010;8(1):69–80. doi: 10.2174/157015910790909520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hogan DB. Progress update: pharmacological treatment of Alzheimer’s disease. Neuropsychiatr Dis Treat. 2007;3(5):569–578. [PMC free article] [PubMed] [Google Scholar]
- 16.Zec RF, Burkett NR. Non-pharmacological and pharmacological treatment of the cognitive and behavioral symptoms of Alzheimer disease. Neuro Rehabilitation. 2008;23(5):425–438. [PubMed] [Google Scholar]
- 17.Wimo A, Norlund A. Commentary on “health economics and the value of therapy in Alzheimer’s disease.” Cost-effectiveness studies. Alzheimers Dement. 2007;3(3):157–161. doi: 10.1016/j.jalz.2007.04.390. [DOI] [PubMed] [Google Scholar]
- 18.Zhu CW, Sano M. Economic considerations in the management of Alzheimer’s disease. Clin Interv Aging. 2006;1(2):143–154. doi: 10.2147/ciia.2006.1.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peretti CS, Ferreri F, Blanchard F, et al. Normal and pathological aging of attention in presymptomatic Huntington’s, Huntington’s and Alzheimer’s disease, and nondemented elderly subjects. Psychother Psychosom. 2008;77(3):139–146. doi: 10.1159/000116607. [DOI] [PubMed] [Google Scholar]
- 20.Solfrizzi V, Panza F, Torres F, et al. Selective attention skills in differentiating between Alzheimer’s disease and normal aging. J Geriatr Psychiatry Neurol. 2002;15(2):99–109. doi: 10.1177/089198870201500209. [DOI] [PubMed] [Google Scholar]
- 21.Daulatzai MA. Early stages of pathogenesis in memory impairment during normal senescence and Alzheimer’s disease. J Alzheimers Dis. 2010;20(2):355–367. doi: 10.3233/JAD-2010-1374. [DOI] [PubMed] [Google Scholar]
- 22.Scheff SW, Price DA, Schmitt FA, Mufson EJ. Hippocampal synaptic loss in early Alzheimer’s disease and mild cognitive impairment. Neurobiol Aging. 2006;27(10):1372–1384. doi: 10.1016/j.neurobiolaging.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 23.Dumont C, Voisin T, Nourhashemi F, Andrieu S, Koning M, Vellas B. Predictive factors for rapid loss on the mini-mental state examination in Alzheimer’s disease. J Nutr Health Aging. 2005;9(3):163–167. [PubMed] [Google Scholar]
- 24.Chan DC, Kasper JD, Black BS, Rabins PV. Prevalence and correlates of behavioral and psychiatric symptoms in community-dwelling elders with dementia or mild cognitive impairment: the memory and medical care study. Int J Geriatr Psychiatry. 2003;18(2):174–182. doi: 10.1002/gps.781. [DOI] [PubMed] [Google Scholar]
- 25.Garcia-Alberca JM, Lara JP, Berthier ML, et al. Can impairment in memory, language and executive functions predict neuropsychiatric symptoms in Alzheimer’s disease (AD)? Findings from a cross-sectional study. Arch Gerontol Geriatr. 2011;52(3):264–269. doi: 10.1016/j.archger.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 26.Vilalta-Franch J, Lopez-Pousa S, Turon-Estrada A, et al. Syndromic association of behavioral and psychological symptoms of dementia in Alzheimer disease and patient classification. Am J Geriatr Psychiatry. 2010;18(5):421–432. doi: 10.1097/JGP.0b013e3181c6532f. [DOI] [PubMed] [Google Scholar]
- 27.Rinaldi S, Fontani V, Inventor, Rinaldi S, Fontani V., assignee Radioelectric Asymmetric Conveyer for therapeutic use. US patent 7,333,8592001.
- 28.Rinaldi S, Fontani V. 2000. Radioelectric Asymmetric Conveyer for therapeutic use. European Patent Office – World Intellectual Property Organization. October 11, 2006. (EP20010960475 20010706)
- 29.Mannu P, Rinaldi S, Fontani V, Castagna A. Long-term treatment of bipolar disorder with a radio-electric asymmetric conveyor. Neuropsychiatr Dis Treat. 2011;7(1):373–379. doi: 10.2147/NDT.S22007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Castagna A, Rinaldi S, Fontani V, Aravagli L, Mannu P, Margotti ML. Does osteoarthritis of the knee also have a psychogenic component? Psycho-emotional treatment with a radio-electric device vs intra-articular injection of sodium hyaluronate: an open-label, naturalistic study. Acupunct Electrother Res. 2010;35(1–2):1–16. doi: 10.3727/036012910803860968. [DOI] [PubMed] [Google Scholar]
- 31.Collodel G, Moretti E, Fontani V, et al. Effect of emotional stress on sperm quality. Indian Journal of Medical Research. 2008;128(3):254–261. [PubMed] [Google Scholar]
- 32.Mannu P, Rinaldi S, Fontani V, Castagna A, Lotti Margotti M. Radio electric treatment vs es-citalopram in the treatment of panic disorders associated with major depression: an open-label, naturalistic study. Acupunct Electrother Res. 2009;34:135–149. doi: 10.3727/036012909803861040. [DOI] [PubMed] [Google Scholar]
- 33.Rinaldi S, Fontani V, Aravagli L, Margotti ML. Psychological and symptomatic stress-related disorders with radio-electric treatment: psychometric evaluation. Stress Health. 2010;26(5):350–358. [Google Scholar]
- 34.Rinaldi S, Fontani V, Aravagli L, Mannu P. Psychometric evaluation of a radio electric auricular treatment for stress related disorders: a double-blinded, placebo-controlled controlled pilot study. Health Qual Life Outcomes. 2010;8(1):31. doi: 10.1186/1477-7525-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rinaldi S, Fontani V, Moretti E, et al. A new approach on stress-related depression and anxiety: neuro-psycho-physical-optimization with radio electric asymmetric-conveyer. Indian Journal of Medical Research. 2010;132:189–194. [PubMed] [Google Scholar]
- 36.Soubelet A, Salthouse TA. Correlates of level and change in the mini-mental state examination. Psychol Assess. 2011 Apr 11; doi: 10.1037/a0023401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoshino H, Sakurai T, Hasegawa K, Yokono K. Causes of decreased activity of daily life in elderly patients who need daily living care. Geriatr Gerontol Int. 2011;11(3):297–303. doi: 10.1111/j.1447-0594.2010.00683.x. [DOI] [PubMed] [Google Scholar]
- 38.Fujiwara Y, Chaves PH, Yoshida H, et al. Intellectual activity and likelihood of subsequently improving or maintaining instrumental activities of daily living functioning in community-dwelling older Japanese: a longitudinal study. Int J Geriatr Psychiatry. 2009;24(6):547–555. doi: 10.1002/gps.2148. [DOI] [PubMed] [Google Scholar]
- 39.Zuidema SU, Buursema AL, Gerritsen MG, et al. Assessing neuropsychiatric symptoms in nursing home patients with dementia: reliability and reliable change index of the neuropsychiatric inventory and the cohen-mansfield agitation inventory. Int J Geriatr Psychiatry. 2011;26(2):127–134. doi: 10.1002/gps.2499. [DOI] [PubMed] [Google Scholar]