The similarity in the names of the recently introduced intranasal glucocorticoid fluticasone furoate (FF; Veramyst®, GlaxoSmithKline/Avamys®, GlaxoSmithKline UK, Uxbridge, UK) and the earlier fluticasone propionate (FP; Flonase®/Flixonase®, GlaxoSmithKline) has led many to assume that the two compounds have the same active principle (fluticasone) (e.g. 1, 2). This has been compounded by FP commonly, and incorrectly, being abbreviated to fluticasone. The purpose of this letter is to highlight that FF and FP are completely different drugs with FF showing distinct and superior properties (3), and hence prevent any misprescription of these drugs in the future.
This confusion clearly stems from the unusual assigned glucocorticoid nomenclature which splits these molecules into the steroidal backbone (fluticasone) and the ester substituent (furoate/propionate). This naming convention does suggest that these derivatives could be ester prodrugs of fluticasone. In fact, a number of topical glucocorticoid esters are indeed ester prodrugs releasing the active parent glucocorticoid in the body. However, fluticasone 17α esters are remarkably stable and remain attached to the fluticasone backbone even during metabolism. Their pharmacological activity is mediated by the entire molecule (backbone + ester) and they share no common metabolites (Fig. 1) – neither FF nor FP is metabolised to fluticasone. FF and FP are therefore structurally distinct drug substances with distinct properties.
Figure 1.
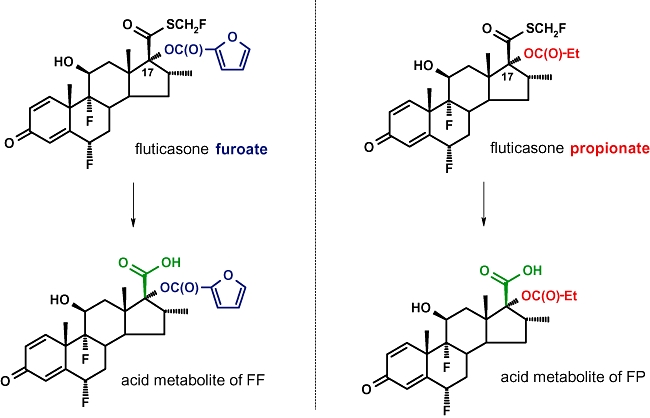
Structures of fluticasone furoate and fluticasone propionate and their major metabolites.
The furoate and propionate moieties are far from inert appendages but serve to significantly enhance the glucocorticoid activity of fluticasone, which has never itself been developed. Key interactions of FF with the glucocorticoid receptor have been elucidated by X-ray crystallography which shows the ester derived from 2-furoic acid occupying a discrete pocket on the receptor much more completely than does the smaller propionate ester of FP (4). The resulting enhanced affinity of FF for the target receptor is reflected in the lower daily dose of Veramyst (110 µg) compared with Flonase (200 µg).
The ester group also contributes to the physicochemical characteristics of the molecule which impact on solubility, dissolution rate, tissue affinity, and hence pharmacokinetic and pharmacodynamic properties. Thus, the ester derived from 2-furoic acid in FF confers higher affinity for both nasal and lung tissue compared with FP (5, 6) and recent studies with inhaled FF have shown that this translates to enhanced lung residency and once-daily efficacy in asthma (7, 8). There is already some evidence that the characteristics of FF may result in superior symptom reduction compared with FP (9, 10) or similar improvements in symptoms at less frequent dosing schedules (11), which could result in reduced health-care costs/concomitant medication use (12); however, prospective, randomised, head-to-head studies are required to provide a definitive answer. With new inhaled products containing FF in Phase III trials (Relovair®, GlaxoSmithKline) it is important for prescribers to understand that this is a novel glucocorticoid, not to be confused with FP. Moreover, the practice of abbreviating FP and FF to fluticasone should be discouraged.
References
- 1.Pearlman S. Research notes on Veramyst vs Flonase. Available at: http://organizedwisdom.com/Veramyst_vs._Flonase (accessed 1 June 2011)
- 2.The Associated Press. Glaxo, Theravance report Relovair safety data. Available at: http://www.biosciencetechnology.com/News/FeedsAP/2010/09/glaxo-theravance-report-relovair-safety-data/ (accessed 1st June 2011)
- 3.Salter M, Biggadike K, Matthews JL, West MR, Haase MV, Farrow SN, Uings IJ, Gray DW. Pharmacological properties of the enhanced-affinity glucocorticoid fluticasone furoate in vitro and in an in vivo model of respiratory inflammatory disease. Am J Physiol Lung Cell Mol Physiol. 2007;293:L660–7. doi: 10.1152/ajplung.00108.2007. [DOI] [PubMed] [Google Scholar]
- 4.Biggadike K, Bledsoe RK, Hassall AM, Kirk BE, McLay IM, Shewchuk LM, Stewart EL. X-ray crystal structure of the novel enhanced affinity glucocorticoid agonist fluticasone furoate in the glucocorticoid receptor-ligand binding domain. J Med Chem. 2008;51:3349–52. doi: 10.1021/jm800279t. [DOI] [PubMed] [Google Scholar]
- 5.Valotis A, Hogger P. Human receptor kinetics and lung tissue retention of the enhanced-affinity glucocorticoid fluticasone furoate. Respir Res. 2007;8:54. doi: 10.1186/1465-9921-8-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baumann D, Bachert C, Hogger P. Dissolution in nasal fluid, retention and anti-inflammatory activity of fluticasone furoate in human nasal tissue ex vivo. Clin Exp Allergy. 2009;39:1540–50. doi: 10.1111/j.1365-2222.2009.03306.x. [DOI] [PubMed] [Google Scholar]
- 7.Allen A, Pierre LN, Rousell VM. Fluticasone furoate (FF) a novel inhaled corticosteroid demonstrates prolonged lung absorption kinetics in man. Am J Respir Crit Care Med. 2010;181:A5408. doi: 10.1007/s40262-012-0021-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van den Berge M, Luijk B, Bareille P, Dallow N, Postma DS, Lammers J-WJ. Prolonged protection of the new inhaled corticosteroid fluticasone furoate against AMP hyperresponsiveness in patients with asthma. Allergy. 2010;65:1531–5. doi: 10.1111/j.1398-9995.2010.02414.x. [DOI] [PubMed] [Google Scholar]
- 9.Keith PK, Scadding GK. Are intranasal corticosteroids all equally consistent in managing ocular symptoms of seasonal allergic rhinitis? Curr Med Res Opin. 2009;25:2021–41. doi: 10.1185/03007990903094106. [DOI] [PubMed] [Google Scholar]
- 10.Gueron B, Demoly P, Kay S, Small M. Do patients on intranasal fluticasone furoate, mometasone furoate and fluticasone propionate experience similar numbers of symptom-free days and quality of life? A cross-sectional study in three European countries. Poster 380, XXIX Congress of the European Academy of Allergy and Clinical Immunology, London, June 5–9, 2010.
- 11.Okobu K, Nakashima M, Miyake N, Komatsubara M, Okuda M. Comparison of fluticasone furoate and fluticasone propionate for the treatment of Japanese cedar pollinosis. Allergy Asthma Proc. 2009;30:84–94. doi: 10.2500/aap.2009.30.3182. [DOI] [PubMed] [Google Scholar]
- 12.Garris C, Shah M, D'Souza A, Stanford R. Comparison of corticosteroid nasal sprays in relation to concomitant use and cost of other prescription medications to treat allergic rhinitis symptoms. Clin Drug Investig. 2009;29:515–26. doi: 10.2165/00044011-200929080-00002. [DOI] [PubMed] [Google Scholar]


