Introduction
The importance of non-shared environment lay hidden within quantitative genetic studies since they began nearly a century ago. Quantitative genetic methods, such as twin and adoption methods, were designed to tease apart nature and nurture in order to explain family resemblance. For nearly all complex phenotypes, it has emerged that the answer to the question of the origins of family resemblance is nature—things run in families primarily for genetic reasons. However, the best available evidence for the importance of environmental influence comes from this same quantitative genetic research because genetic influence never explains all of the variance for complex phenotypes, and the remaining variance must be ascribed to environmental influences.
Yet it took many decades for the full meaning of these findings to emerge. If genetics explains why siblings growing up in the same family are similar, but the environment is important, then it must be the case that the salient environmental effects do not make siblings similar. That is, they are not shared by children growing up in the same family—they must be ‘non-shared’. This implication about non-shared environmental import lay fallow in the field of quantitative genetics because the field’s attention was then firmly on the nature–nurture debate. ‘Nurture’ in the nature–nurture debate was implicitly taken to mean shared environment because from Freud onwards, theories of socialization had assumed that children’s environments are doled out on a family-by-family basis. In contrast, the point of non-shared environment is that environments are doled out on a child-by-child basis. Note that the phrase ‘non-shared environment’ is shorthand for a component of phenotypic variance—it refers to ‘effects’ rather than ‘events’, as discussed later.
The 1987 paper reprinted in this issue of the International Journal of Epidemiology1 brought together evidence for the importance of non-shared environment in the development of personality, psychopathology and cognitive abilities, expanding on a previous paper.2 The purpose of the present commentary is to reflect on non-shared environment three decades after the topic emerged. Progress and problems in studying non-shared environment were reviewed in 2001;3 rather than providing a systematic update of this burgeoning field, my current goal is to suggest some new directions for research in this area.
The 1987 paper was published with 32 commentaries and our response,4 which I recommend. These commentaries and the response to them raised many of the issues that resurfaced during the following decades, such as the following:
Non-shared environmental effects need to be distinguished from error of measurement (yes).
Non-additive genetic variance can account for non-shared environmental effects (no).
Genotype–environment interaction and correlation can account for non-shared environmental effects (no).
Prenatal factors can contribute to non-shared environmental effects (yes).
Non-shared environmental effects may be more influential in extreme situations such as abusive families (yes).
Perceptions of environment can be an important source of non-shared experience (yes).
Non-shared environment can involve chance in the sense of idiosyncratic experiences (yes).
It is noteworthy that none of the 1987 commentaries disagreed with the fundamental phenomenon that children growing up in the same family are very different. There was also general agreement that most of the environmental variance is of the non-shared variety. As I reflect on the following decades of research on non-shared environmental influence, the basic finding of the importance of non-shared environment has not been seriously challenged, which seems surprising to me given its far-reaching implications for understanding how the environment works. It should be emphasized that the message is not that all environmental variance for all traits is non-shared but rather that most environmental influence for most traits is non-shared. Some significant shared environmental variance has been found for some traits.5,6 For example, for antisocial behaviour in adolescence, shared environment accounts for ~15% of the total phenotypic variance, although even here non-shared environment accounts for much more of the variance, ~40%.7 Another example of significant shared environmental influence is academic achievement, where the effect is surprisingly modest in its magnitude given that this result is based on siblings growing up in the same family and being taught in the same school, often by the same teacher in the same classroom.8 Intelligence (IQ) is a third example, first mooted in the 1987 paper, showing significant shared environmental influence in childhood that diminishes to insignificant levels by adolescence to be subsumed by genetic and non-shared environmental influences, a suggestion subsequently confirmed in several studies and meta-analyses.9,10
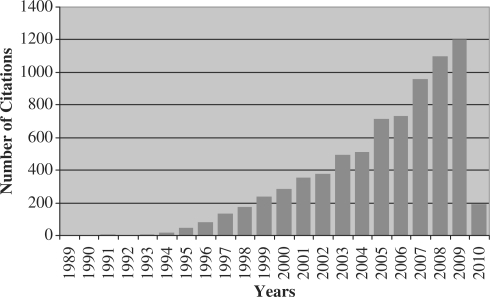
A search for ‘nonshared environment OR non-shared environment’ using ISI Web of Science (February 2010) yields 371 entries, after excluding a few inappropriate entries. These 371 entries certainly underestimate the total number of papers in the area—e.g. the search does not even identify our 1987 paper or 29 of the 32 connected commentaries. In part this is due to the fact that non-shared environment has been called by other, less searchable names such as unique, specific and individual environment. Nonetheless, a ‘citation report’ of the 371 entries (Figure 1) is interesting for three reasons. First, it was not until the mid-1990s, a decade after the first papers, that non-shared environment began to be cited. Second, since then, citations of non-shared environment have steadily increased to more than a thousand per year by 2008.
Figure 1.
Citations per year for 371 entries for ‘nonshared environment OR non-shared environment' from ISI Web of Science (March 2010)
The third point from Figure 1 is that >95% of these publications are from the behavioural sciences. The few exceptions are close to the behavioural sciences as well. For example, 10 papers are listed for the ISI subject area of ‘endocrinology and metabolism’ but these are all about body weight. Since 1991, reviews of the genetics of body mass index find that nearly all environmental influences are non-shared.11–13 This is an important finding because theories of individual differences in weight highlight environmental factors such as nutrition and lifestyle that ought to be shared by children growing up in the same family.
Beyond the behavioural sciences
The 1987 paper focused on the behavioural sciences because it was written for Behavioral and Brain Sciences and because the behavioural sciences were the battlefield for the nature–nurture wars of the past century. However, non-shared environment may be just as important for other domains in the life sciences. For example, identical twin concordances for cardiovascular disease are only ~30%.14 (Because identical twins are genetically identical for inherited DNA sequence variation, differences within pairs can be attributed to non-shared environment.) For cancers, identical twin concordances are even lower, ~10–20%.15
Non-shared environment also seems to be the major source of environmental influence for other diseases such as diabetes, ulcers, childhood eczema, asthma and allergic rhinitis, although these domains are not nearly as well studied.16 Examples of other domains in which non-shared environmental influence has been shown to be important include: longevity,17,18 psoriasis,19 stress urinary incontinence,20 menstrual symptomatology,21 abdominal aortic aneurysms,22 serum vitamin D status,23 serum lipids and apolipoproteins,24 uric acid and liver enzymes,25 and collectin surfactant protein D serum levels.26 Non-shared environmental factors may be important even for infections in the family.27–29
In summary, one suggested direction for non-shared environmental research is to go beyond the behavioural sciences. In particular, epidemiology represents a special opportunity because it is primed to go beyond its traditional focus on differences between families to consider differences within families.30
Children in the same family are very different, but why?
This question was the title of our response to the 32 commentaries on our 1987 paper. Answers to the question are the gauge of progress in understanding non-shared environment. The 1987 paper suggested a new research agenda: to study more than one child per family in order to investigate why they are so different. Three steps were recommended for this program of non-shared environmental research:
Identify differential experiences.
Identify associations between differential experiences and differential outcomes.
Identify causal associations between differential experiences and differential outcomes.
In this section, I summarize progress as I see it in these three steps.
Step 1: what are the specific sources of non-shared environmental effects?
Table 1 in the 1987 paper outlined possible sources of non-shared environmental effects such as family composition, parental treatment, sibling interactions and extra-familial influences such as peers in addition to non-systematic factors. The important point here is that any environmental factor can be viewed in terms of its contribution to non-shared environmental effects, as long as it can be assessed separately for each child (child-specific). For example, parental warmth and control have been assessed in hundreds of studies as the two ‘super-factors’ of parenting which are then correlated with children’s outcomes. These studies have traditionally assumed that parenting is a shared environmental factor in the sense that only one child per family was considered and parent–child associations were analyzed ‘between’ families. However, by studying more than one child per family and by targeting parenting that is specific to each child, it is possible to investigate the extent to which parents’ warmth and control differ for children ‘within’ families. Research of this kind has shown that parents do treat their children differently. If you ask parents about their differential parenting they report only modest differential parenting (sibling correlations of ~0.70) but if you ask children about it you might think they were raised in different families (sibling correlations of ~0.25). Observations of parent–child interactions support not the parents’ but the children’s view (sibling correlations of ~0.20).31
Sources of non-shared environmental variance can also be found in events such as divorce that are shared by children in a family. This issue illustrates well the point mentioned earlier, i.e. non-shared environment is not about events but rather is about effects on phenotypes: the shared event of parental divorce may be experienced differently by children in the same family, and thus have non-shared environmental influence.32 Similarly, siblings generally attend the same school, but members of a sibling pair perceive their school and classroom experience differently, even identical twins in the same classroom.33,34 Indeed, even prenatal life in the same womb can lead to differences.35,36 As an aside, one could argue that we should not study non-shared environmental effects in the human species until we solve the puzzle of why genetically identical inbred mice growing up prenatally in the same womb at the same time and raised by the same mother in a controlled laboratory environment are so different.37
Furthermore, although non-shared environment is often referred to as non-shared ‘family’ environment, it is not limited to families. Life outside the family such as school and peers can contribute to non-shared experiences.38 Nor is non-shared environment limited to children: as siblings grow up and leave their family, they live increasingly separate, non-shared lives. Adult life events are mostly non-shared even by identical twins.39
It is also possible that non-systematic factors, such as accidents and illnesses and other idiosyncratic experiences, initiate differences between siblings. Compounded over time, small chance differences in experience might lead to large differences in outcome, as discussed in the next section. The 1987 paper concluded that ‘one gloomy prospect is that the salient environment might be unsystematic, idiosyncratic, or serendipitous events such as accidents, illnesses and other traumas, as biographies often attest’ (p. 8). It is striking how often biographies and autobiographies point to chance as a tipping point to explain why siblings are so different.16 Illnesses and accidents also feature in interviews with parents of discordant identical twins in explaining why they thought their children differed.40 Support for chance as a source of non-shared environmental influence comes from quantitative genetic research that suggests that non-shared environmental effects are trait specific and age specific. That is, non-shared environmental effects on one trait are largely uncorrelated with such effects on other traits and non-shared environmental effects at one age are largely uncorrelated with such effects at other ages.8
Chance might not be such a gloomy prospect for non-shared environmental research.41 Some events that we call chance might be unpredictable yet orderly, just as chaos theory refers to non-linear dynamic systems highly sensitive to initial conditions.42 Chance might only be a label for our current inability to identify the environmental processes by which children growing up in the same family come to be so different.
The simple answer to the first step in the non-shared environment research agenda is that nearly all child-specific measures of the family environment show some differences between children growing up in the same family. However, much less is known about specific sources of non-shared experience outside the family.
Step 2: what specific sources of non-shared environmental effects are associated with outcomes?
Much less progress has been made in relation to the second step in the non-shared environment program of research: identifying associations between differential experiences and differential outcomes. The 1987 paper stimulated much research on this topic and half a dozen books.16,31,43-46 In 2000, a meta-analysis of 43 papers addressing this second step concluded that ‘measured non-shared environmental variables do not account for a substantial portion of the non-shared variability’.41 It seems to me that this pessimistic review served to slow down research relating non-shared environment to outcomes.
Looking at the same studies, an optimist could conclude that this research was off to a good start.3 The proportion of total variance accounted for in outcomes such as adjustment, personality and cognition was 0.01 for family constellation, 0.02 for differential parental behaviour, 0.02 for differential sibling interaction and 0.05 for differential peer or teacher interaction. Moreover, these effects are largely independent and they add up to account for 13% of the total variance. If non-shared environment accounts for ~40% of the variance in these domains, we could say the cup is already more than one quarter full.
These findings for non-shared environment look even better when compared to results emerging from genome-wide association studies in which <5% of the variance of highly heritable traits such as height and weight can be explained by replicated associations with DNA markers.47 The big question now in molecular genetics is how to identify the ‘missing’ heritability;48 the big question for non-shared environment is how to identify the ‘missing’ non-shared environment. The motivation for identifying non-shared environment should be at least as strong as the motivation for identifying DNA associations. As an aside, other interesting parallels exist between genetics and environment such as pleiotropy (each gene affects many traits) and polygenicity (each trait is affected by many genes). It seems safe to predict that each non-shared experience will affect many traits and that each trait will be influenced by many non-shared experiences. One important difference is that non-shared environmental influences can be investigated at a composite level with variables such as parenting. Although many DNA markers can be aggregated in a polygenic risk index,49 each DNA polymorphism is independent in the sense that it is uncorrelated with all other polymorphisms except its close neighbours.
An updated meta-analysis of associations between specific sources of non-shared environment and outcomes is needed but beyond the scope of this paper. However, I have three impressions from such research during the past decade. First, there has been slow but steady progress towards identifying specific sources of non-shared environment associated with outcomes. Recent research examines possible contextual moderators of the links between non-shared experiences and outcomes.50 Second, non-shared experiences outside the family are increasingly targets for these investigations.38,51 Third, a larger proportion of this research involves genetically sensitive designs, especially twin studies, which is the topic of the following section. Although studies of non-twin siblings are valuable in pointing to potential associations between non-shared environmental factors and outcomes, the next step in the non-shared environment research program attempts to untangle genetic and environmental contributions to these associations. As large twin samples with environmental measures become more abundant, it makes sense to skip Step 2 and move to Step 3. However, in epidemiology, Step 2 will continue to be valuable as a crucible for between-family research: unless a between-family variable can be shown to operate within families, in the sense that it is experienced differently by siblings and has differential effects on them, it cannot be an important environmental source of individual differences in development because these are non-shared.
Step 3: what specific sources of non-shared environmental effects are causally associated with outcomes?
When differential experiences (X) are shown to relate to differential outcomes (Y), another hurdle remains before such a correlation can be interpreted causally. That is, although the goal is to identify X that causes Y, as in any correlation Y can also cause X. Developmental psychologists have long been aware of this possibility which has come to be known as the ‘direction of effects in socialization’ issue.52 Direction of effects was discussed in relation to shared environmental effects—e.g. is parenting the cause or effect of children’s behaviour? The issue is just as relevant for non-shared environmental effects—is differential parenting the cause or effect of sibling differences? Longitudinal cross-lagged analyses of the links between parenting and children’s outcomes can help to sort out the direction of effects.53,54
In addition to X causing Y and Y causing X, a correlation can occur because a third factor causes both X and Y. One candidate ‘third factor’ of particular relevance for the current discussion is genetics. Siblings differ for genetic reasons as well as for reasons of non-shared environment because siblings are on average 50% similar genetically for additive genetic effects, which means that they are 50% different genetically. For heritable traits, siblings will of course differ at least in part for genetic reasons. But what about the environment—how can environmental measures be heritable? One possibility is that differential parenting reflects genetic differences in characteristics of their children. However, genetics can also affect parenting more directly. This raises a topic that has been called the ‘nature of nurture’,55 which in my view is the second most important finding from behavioural genetics after non-shared environment. As implausible as it might seem, most ostensible measures of the environment show significant genetic influence when investigated as dependent measures in dozens of twin and adoption studies. A review of 55 studies involving 35 environmental measures found an average heritability of ~25% for familial as well as extra-familial measures of the environment.39 A recent example is a developmental study of 1800 twin pairs interviewed retrospectively about peer group deviance which showed heritabilities of peer group deviance from 0.40 to 0.50 from childhood to young adulthood.56 In fact, very few measures of experience examined in genetically sensitive designs do not show significant genetic influence. It is important to be clear: of course, environments per se are not inherited. Genetic influence comes into the picture because these environmental measures are not independent of the person. For example, many life events and stressors are not things that happen to people passively—people contribute to their experiences to some extent. In quantitative genetics, this nature-of-nurture theme is known as genotype–environment correlation, which refers to genetic influence on exposure to environments thus creating correlations between genetic propensities and experiences.57
If genetic factors are important not only for outcomes but also for measures of the environment, then it follows that it is important to control for possible genetic contributions to correlations between non-shared environmental measures and outcomes. The first of two major methods for accomplishing this is the identical twin differences design.
Identical twin differences design
Studying differences within pairs of identical twins provides a simple and direct test of non-shared experience that by definition controls for the role of genetics. Because identical twins reared in the same family are identical genetically, their differences in experience and in outcome can only be due to non-shared environment and error of measurement. Thus, non-shared environmental effects are implicated if identical twin differences in experience correlate with their differences in outcome.58
During the past decade, more than a dozen such studies have reported evidence for non-shared environmental effects controlling for genetic confounding, primarily between differential parenting and adjustment.59–67 A few studies have looked beyond the family, e.g. between classroom environments and adjustment68 and academic achievement.34
An exciting new direction for non-shared environmental research is to use discordant identical twins in an attempt to identify biomarkers and biomechanisms of non-shared environment at the most basic ‘–omic’ levels of the transcriptome, epigenome and proteome. Even the genome can be a source of non-shared environmental differences within pairs of identical twins, such as de novo copy number variants.69 The epigenome—more specifically the methylome (DNA methylation across the genome)—is especially promising for non-shared environmental studies because the methylome is both responsive to the environment and governs gene expression, thus potentially creating a pathway from environmental effects through gene expression to behaviour.70 Moreover, because changes in DNA methylation are transmitted from mother to daughter chromatids during mitosis, epigenetic changes in response to environmental factors may be long-lasting and mediate phenotypic outcomes later in life. The long-lasting but reversible nature of DNA methylation makes it an especially exciting target for creating a biological foundation upon which to build an understanding of non-shared environmental influence as well as to provide biomarkers for non-shared environmental change.
Non-shared environmental effects on DNA methylation could be the result of random stochastic events71 accumulating over the millions of mitotic divisions occurring during the lifetime of two monozygotic (MZ) twins.72 However, DNA methylation has been shown to vary systematically as a function of numerous environmental factors such as nutritional, chemical, physical and psychosocial factors,73–75 and may be especially influenced by the prenatal environment.70 Environmental influences on the epigenome have been studied most in relation to cancer,76,77 although external influences on DNA methylation are likely to be widespread even for loci not involved in the disease. Animal studies also provide strong support for the role of environmental epigenetics in disease susceptibility78 and behaviour.79
MZ twins have been shown to differ substantially in their degree of methylation, which suggests that epigenetic processes are likely to be influenced by non-shared environmental influences.70,80 Moreover, preliminary research indicates that systematic epigenetic differences can be found within behaviourally discordant MZ pairs.81,82 Several studies that have focused on genes implicated in psychiatric morbidity have found MZ twin differences in DNA methylation.83,84 The most highly cited paper in this area showed epigenetic differences in lymphocyte DNA within 40 MZ pairs of a wide age range, with a suggestion that the greatest differences occurred within pairs of identical twins with divergent lifestyles as well as in older twins.85
Full multivariate genetic design
The identical twin difference design is a sharp scalpel for dissecting non-shared environmental effects from genetic effects. The strength of the design is that it focuses only on within-pair differences, but it is also a weakness that it ignores between-pair variance and total variance among individuals. Moreover, the design uses only one-quarter of the data available in a twin study—a twin study includes variance within and between identical and fraternal twin pairs. Adding fraternal twin differences to the identical twin differences design provides a replication and a check on the uniqueness of identical twins, since fraternal twins should show non-shared environmental associations with outcomes that are at least as great as those for identical twins. Moreover, genetic contributions can be inferred to the extent that twin difference associations are greater for fraternal twins than for identical twins.
However, adding fraternal twin differences to the identical twin differences design is still limited to the within-pair variance of the twin design. Strong within-pair associations between experiences and outcomes could emerge even though such differential effects account for only a small portion of the total variance, which is comprised of variance between pairs as well as variance within pairs. Multivariate genetic analysis complements the identical twin difference design and incorporates all of the information in the full twin design.86
Instead of estimating genetic and environmental influence on the variance of one phenotype at a time, multivariate genetic analysis investigates the origins of the covariance between phenotypes.87 Traditional univariate genetic analysis compares identical and fraternal twin correlations for one phenotype in order to decompose the variance of that phenotype into genetic and environmental components of variance. In contrast, multivariate genetic analysis compares identical and fraternal twin cross-correlations—i.e. the correlation between variable X for one twin and variable Y for the other twin—in order to decompose the covariance between X and Y into genetic and environmental components of covariance. A genetic contribution to the phenotypic covariance is implied if the identical twin cross-correlation exceeds the fraternal twin cross-correlation.88
Multivariate genetic analysis has been used in hundreds of studies since the 1980s. For example, multivariate genetic analyses have shown a surprising degree of genetic overlap across mental disorders89,90 and across learning abilities and disabilities.91 In addition to the general picture of genetic comorbidity, multivariate genetic analyses have also produced examples of genetic heterogeneity. For example, the triad of symptoms included in diagnoses of autistic spectrum disorder appear to differ genetically.92
However, it was not until the mid-1990s that it was realized that multivariate genetic analysis could be applied to the question of the causality of non-shared environmental effects controlling for genetics. It was not mentioned in the 1987 paper or its commentaries. It took three steps to get to this realization. The first step was to recognize that environmental measures could be treated as phenotypes in univariate quantitative genetic analyses, as discussed in an earlier section on the ‘nature of nurture’.55 The second step was to incorporate environmental measures in multivariate genetic analyses to investigate the aetiology of covariance between environmental measures and outcomes.45 As in most quantitative genetic research, this research highlighted the genetic aspect of these results, often finding substantial genetic contributions to the links between environmental measures and outcomes. The third step was to realise that these same multivariate genetic analyses of environmental measures and outcomes estimate the importance of non-shared environment on an outcome independent of genetics and shared environment, using all of the variance within and between identical and fraternal twins. Unlike other analyses of non-shared environment, structural equation model-fitting estimates non-shared environment as a latent variable free of measurement error if the standard assumption is made that error of measurement does not correlate across measures.
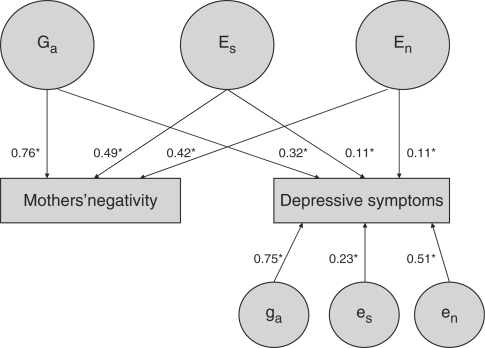
The first analysis of this type was published in 1996 in a multivariate genetic analysis of parental negativity and their adolescents’ depressive symptoms and antisocial behaviour.86 Figure 2 reprints one of the figures from this paper that shows the model-fitting results of the multivariate genetic analysis as depicted in a structural equation path model. The results indicate that maternal negativity is significantly related to adolescent depressive symptoms through non-shared environmental processes, even though genetic factors account for most of the association between maternal negativity and adolescent depressive symptoms. Specifically, the findings indicated that 14% of the phenotypic correlation of 0.33 between maternal negativity and adolescent depressive symptoms can be attributed to non-shared environment (En) independent of genetics (Ga), shared environment (Es) and error. Non-shared environmental influences from maternal negativity explain ~5% of the total non-shared environmental influence on adolescent depressive symptoms. (See legend to Figure 2 for explanation).
Figure 2.
A reprinted figure from the first full multivariate genetic analysis between an environmental measure (mothers’ negativity) and an outcome measure (their adolescent children’s depressive symptoms). (Source: Adapted with permission from Pike et al.58 published by the American Psychological Association).86 Asterisks denote loadings significant at P < 0.05. The latent variables Ga, Es and En represent a genetic factor, a shared environmental factor and a non-shared environmental factor, respectively, that affect the environmental measure (mothers’ negativity) and also affect the outcome measure (adolescent children’s depressive symptoms). The latent variables ga, es and en represent the genetic, shared environmental and non-shared environmental influences, respectively, that are unique to the outcome measure. The path coefficients indicate how the variance of depressive symptoms can be attributed to genetic and environmental components of variance that covary or do not covary with mothers’ negativity. Because the path coefficients are standardized, squaring them indicates the proportion of variance explained by a path. Thus, squaring and summing the six path coefficients leading to depressive symptoms yields 1.0, the total variance of the depressive symptoms measure. Squaring the significant common non-shared environment path of 0.11 indicates that non-shared environmental influences that covary with mothers’ negativity explain only 1.2% of the variance of depressive symptoms. However, the product of the non-shared environmental paths connecting mothers’ negativity and depressive symptoms (i.e. 0.42 × 0.11 = 0.046) indicates that non-shared environmental influences contribute 0.046 to the phenotypic correlation between them. The phenotypic correlation is 0.33, which means that non-shared environment explains 14% of the correlation between mothers’ negativity and depressive symptoms (i.e. 0.046/0.330 = 0.139). The significance of the residual en parameter indicates that there are significant non-shared environmental effects on depressive symptoms that are not explained by non-shared environmental effects due to mothers’ negativity. Squaring the en path coefficient of 0.51 indicates that 26% of the variance of depressive symptoms is due to such residual non-shared environmental influences, which includes error of measurement. Thus, non-shared environmental influences on mothers’ negativity explains 4.6% of the total non-shared environmental variance (including error of measurement) of depressive symptoms (i.e. 0.112/0.512 = 0.012/0.260 = 0.046)
This report86 was part of a 10-year study called the Non-shared Environment in Adolescent Development (NEAD), a project that emerged directly from a conference based on the 1987 paper.31 NEAD used multiple methods to assess parenting and adolescent children’s adjustment over a 3-year interval in 720 families that included identical and fraternal twins, full siblings, half-siblings and genetically unrelated siblings. In general, findings from NEAD were similar to those of the report described above: some significant non-shared environmental contribution can be found between experiences and outcomes but the genetic contribution is more influential.
At least a dozen such multivariate genetic analyses between environmental measures and outcomes have been reported during the past decade, generally yielding results that are similar to those from the NEAD project. Most of these are studies of the effects of negative parenting on children’s antisocial behaviour, such as parental corporal punishment and physical maltreatment,93 parental criticism,94 parental negativity95 and parent–child conflict.96 Other studies have investigated the effects of other aspects of parenting and other outcomes such as parental affection and pro-social behaviour,97 parent–child mutuality and behaviour problems,98 and maternal emotional over-involvement and anxiety.99 In addition, a few studies have begun to look beyond the family, specifically to affiliation with delinquent peers as they influence antisocial behaviour.100–102
In summary, studies using the full multivariate genetic design find some significant—if slight—non-shared environmental effects independent of genetics and shared environment. How does this finding fit with the results from identical twin differences studies which often show significant and apparently substantial non-shared environmental associations between experiences and outcomes? The answer was alluded to earlier: Strong within-pair associations between experiences and outcomes can be found even though such within-family effects account for only a small portion of the total variance. Another reason is that the identical twin differences design focuses exclusively on non-shared environment, whereas the non-shared environmental links between experiences and outcomes are overshadowed by substantial genetic links. As noted earlier, the identical twin differences method is a sharp tool to dissect non-shared environmental effects from genetic and shared environmental influences; the full multivariate genetic design is complementary in that it considers non-shared environmental effects in the context of the total variance between and within families. The two designs should yield complementary results in studies with comparable power.
Conclusion
Where are we now in the search for specific non-shared environmental effects? Some significant but small non-shared environmental effects have been demonstrated, especially on the ‘dark side’ of development—e.g. negative parenting and negative outcomes. However, much of what appears to be differential effects of experience on outcomes turns out to be due to genotype–environment correlation, which has led to excitement about identifying specific genes involved in these correlations.103 The book summarizing the NEAD project is called ‘The Relationship Code’ because genotype–environment correlation can be viewed as restoring to the family some of the influence lost from both non-shared and shared environmental effects in the sense that childhood genetic propensities are expressed in the family environment. Genotype–environment correlation is neither solely genetic effects nor environmental effects—it is both.
Despite the slow progress towards identifying specific sources of non-shared environment, the basic finding of the 1987 paper remains unchallenged: children growing up in the same family are very different. It is rare in a field as complex as the behavioural sciences to discover such clear and consistent evidence for a finding that radically alters the way we think about an issue as basic as how the environment influences development. It was reasonable to assume that the key influences on children’s development are those that are shared by children growing up in the same family: their parents’ personality and family experiences, the quality of their parents’ marital relationship, their parents’ educational background and socioeconomic status, the neighbourhood in which they are raised and their parents’ attitude to school or to discipline. Yet to the extent that these influences are shared environmentally, they cannot account for individual differences in children’s development because the salient environmental influences are non-shared. The message is not that family experiences are unimportant but rather that the relevant experiences are specific to each child in the family, not general to all children in the family. However, my main conclusion has to be that the key question largely remains unanswered: why are children in the same family so different?
Funding
U.K. Medical Research Council (G050079) and the U.S. National Institute of Child Health and Human Development (HD44454, HD46167).
Acknowledgements
The author thanks Claire Haworth and Bonamy Oliver for their suggestions for revising an earlier draft of this paper.
Conflict of interest: None declared.
References
- 1.Plomin R, Daniels D. Why are children in the same family so different from each other? Behav Brain Sci. 1987;10:1–16. [Google Scholar]
- 2.Rowe DC, Plomin R. The importance of nonshared (E1) environmental influences in behavioural development. Dev Psychol. 1981;17:517–31. [Google Scholar]
- 3.Plomin R, Asbury K, Dunn J. Why are children in the same family so different? Nonshared environment a decade later. Can J Psychiat. 2001;46:225–33. doi: 10.1177/070674370104600302. [DOI] [PubMed] [Google Scholar]
- 4.Plomin R, Daniels D. Children in the same family are very different, but why? [response to commentaries] Behav Brain Sci. 1987;10:44–55. [Google Scholar]
- 5.Burt SA. Rethinking environmental contributions to child and adolescent psychopathology: a meta-analysis of shared environmental influences. Psychol Bull. 2009;135:608–37. doi: 10.1037/a0015702. [DOI] [PubMed] [Google Scholar]
- 6.Buchanan JP, McGue M, Keyes M, Iacono WG. Are there shared environmental influences on adolescent behavior? Evidence from a study of adoptive siblings. Behav Genet. 2009;39:532–40. doi: 10.1007/s10519-009-9283-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rhee SH, Waldman ID. Genetic and environmental influences on antisocial behavior: a meta-analysis of twin and adoption studies. Psychol Bull. 2002;128:490–529. [PubMed] [Google Scholar]
- 8.Kovas Y, Haworth CMA, Dale PS, Plomin R. The genetic and environmental origins of learning abilities and disabilities in the early school years. Monogr Soc Res Child Dev. 2007;72:1–144. doi: 10.1111/j.1540-5834.2007.00439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haworth CMA, Wright MJ, Luciano M, et al. The heritability of general cognitive ability increases linearly from childhood to young adulthood. Mol Psychiatry. 2009 doi: 10.1038/mp.2009.55. doi: 10.1038/mp.2009.55 [Epub 2 Jun 2009] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGue M, Bouchard TJ, Jr, Iacono WG, Lykken DT. Behavioral genetics of cognitive ability: a life-span perspective. In: Plomin R, McClearn GE, editors. Nature, Nurture, and Psychology. Washington, DC: American Psychological Association; 1993. pp. 59–76. [Google Scholar]
- 11.Grilo CM, Pogue-Geile MF. The nature of environmental influences on weight and obesity: a behavior genetic analysis. Psychol Bull. 1991;10:520–37. doi: 10.1037/0033-2909.110.3.520. [DOI] [PubMed] [Google Scholar]
- 12.Klump KL, Wonderlich S, Lehoux P, Lilenfeld LR, Bulik CM. Does environment matter? A review of nonshared environment and eating disorders. Int J Eat Disord. 2002;31:118–35. doi: 10.1002/eat.10024. [DOI] [PubMed] [Google Scholar]
- 13.Maes HH, Neale MC, Eaves LJ. Genetic and environmental factors in relative body weight and human adiposity. Behav Genet. 1997;27:325–51. doi: 10.1023/a:1025635913927. [DOI] [PubMed] [Google Scholar]
- 14.Marian AJ. Genetic risk factors for myocardial infarction. Curr Opin Cardiol. 1998;13:171–78. [PubMed] [Google Scholar]
- 15.Lichtenstein P, Holm NV, Verkasalo PK, et al. Environmental and heritable factors in the causation of cancer. N Engl J Med. 2000;343:78–85. doi: 10.1056/NEJM200007133430201. [DOI] [PubMed] [Google Scholar]
- 16.Dunn JF, Plomin R. Separate Lives: Why Siblings Are So Different. New York: Basic Books; 1990. [Google Scholar]
- 17.Herskind AM, McGue M, Holm NV, Sorensen TI, Harvald B, Vaupel JW. The heritability of human longevity: a population-based study of 2872 Danish twin pairs born 1870–1900. Hum Genet. 1996;97:319–23. doi: 10.1007/BF02185763. [DOI] [PubMed] [Google Scholar]
- 18.Hjelmborg JV, Iachine I, Skytthe A, et al. Genetic influence on human lifespan and longevity. Hum Genet. 2006;119:312–21. doi: 10.1007/s00439-006-0144-y. [DOI] [PubMed] [Google Scholar]
- 19.Grjibovski AM, Olsen AO, Magnus P, Harris JR. Psoriasis in Norwegian twins: contribution of genetic and environmental effects. J Eur Acad Dermatol Venereol. 2007;21:1337–43. doi: 10.1111/j.1468-3083.2007.02268.x. [DOI] [PubMed] [Google Scholar]
- 20.Altman D, Forsman M, Falconer C, Lichtenstein P. Genetic influence on stress urinary incontinence and pelvic organ prolapse. Eur Urol. 2008;54:918–22. doi: 10.1016/j.eururo.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 21.Treloar SA, Martin NG, Heath AC. Longitudinal genetic analysis of menstrual flow, pain, and limitation in a sample of Australian twins. Behav Genet. 1998;28:107–16. doi: 10.1023/a:1021419907305. [DOI] [PubMed] [Google Scholar]
- 22.Wahlgren CM, Larsson E, Magnusson PK, Hultgren R, Swedenborg J. Genetic and environmental contributions to abdominal aortic aneurysm development in a twin population. J Vasc Surg. 2010;51:3–7. doi: 10.1016/j.jvs.2009.08.036. [DOI] [PubMed] [Google Scholar]
- 23.Snellman G, Melhus H, Gedeborg R, et al. Seasonal genetic influence on serum 25-hydroxyvitamin D levels: a twin study. PLoS ONE. 2009;4:E7747. doi: 10.1371/journal.pone.0007747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heller DA, Pedersen NL, deFaire U, McClearn GE. Genetic and environmental correlations among serum lipids and apolipoproteins in elderly twins reared together and apart. Am J Hum Genet. 1994;55:1255–67. [PMC free article] [PubMed] [Google Scholar]
- 25.Middelberg RP, Medland SE, Martin NG, Whitfield JB. A longitudinal genetic study of uric acid and liver enzymes in adolescent twins. Twin Res Hum Genet. 2007;10:757–64. doi: 10.1375/twin.10.5.757. [DOI] [PubMed] [Google Scholar]
- 26.Sorensen GL, Hjelmborg JB, Kyvik KO, et al. Genetic and environmental influences of surfactant protein D serum levels. Am J Physiol – Lung C. 2006;290:L1010–17. doi: 10.1152/ajplung.00487.2005. [DOI] [PubMed] [Google Scholar]
- 27.Leder K, Sinclair M, Forbes A, Wain D. Household clustering of gastroenteritis. Epidemiol Infect. 2009;137:1705–12. doi: 10.1017/S0950268809990124. [DOI] [PubMed] [Google Scholar]
- 28.Malaty HM, Engstrand L, Pedersen NL, Graham DY. Helicobacter pylori infection: genetic and environmental influences. A study of twins. Ann Intern Med. 1994;120:982–86. doi: 10.7326/0003-4819-120-12-199406150-00002. [DOI] [PubMed] [Google Scholar]
- 29.Thomsen SF, Stensballe LG, Skytthe A, Kyvik KO, Backer V, Bisgaard H. Increased concordance of severe respiratory syncytial virus infection in identical twins. Pediatrics. 2008;121:493–96. doi: 10.1542/peds.2007-1889. [DOI] [PubMed] [Google Scholar]
- 30.Lawlor DA, Mishra GD. Family Matters: Designing, Analysing and Understanding Family based Studies in Life Course Epidemiology (Life Course Approach to Adult Health) Oxford: Oxford University Press; 2009. [Google Scholar]
- 31.Reiss D, Neiderhiser JM, Hetherington EM, Plomin R. The Relationship Code: Deciphering Genetic and Social Patterns in Adolescent Development. Cambridge, MA: Harvard University Press; 2000. [Google Scholar]
- 32.Hetherington EM, Clingempeel WG. Coping with marital transitions: a family systems perspective. Monogr Soc Res Child Dev. 1992;57:2–3. [Google Scholar]
- 33.Walker SO, Plomin R. Nature, nurture, and perceptions of the classroom environment as they relate to teacher assessed academic achievement: a twin study of 9-year-olds. Educational Psychol. 2006;26:541–61. [Google Scholar]
- 34.Asbury K, Almeida D, Hibel J, Harlaar N, Plomin R. Clones in the classroom: a daily diary study of the nonshared environmental relationship between monozygotic twin differences in school experience and achievement. Twin Res Hum Genet. 2008;11:586–95. doi: 10.1375/twin.11.6.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bergvall N, Cnattingius S. Familial (shared environmental and genetic) factors and the foetal origins of cardiovascular diseases and type 2 diabetes: a review of the literature. J Intern Med. 2008;264:205–23. doi: 10.1111/j.1365-2796.2008.01974.x. [DOI] [PubMed] [Google Scholar]
- 36.Stromswold K. Why aren't identical twins linguistically identical? Genetic, prenatal and postnatal factors. Cognition. 2006;101:333–84. doi: 10.1016/j.cognition.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 37.Neiderhiser JM. Between-litter and within-litter variance in inbred strains of mice as evidence of shared and nonshared environment. Behav Genet. 1989;19:771. [Google Scholar]
- 38.Harris JR. Where is the child's environment – a group-socialization theory of development. Psychol Rev. 1995;102:458–89. [Google Scholar]
- 39.Kendler KS, Baker JH. Genetic influences on measures of the environment: a systematic review. Psychol Med. 2007;37:615–26. doi: 10.1017/S0033291706009524. [DOI] [PubMed] [Google Scholar]
- 40.Asbury K, Dunn J, Plomin R. The use of discordant MZ twins to generate hypotheses regarding non-shared environmental influence on anxiety in middle childhood. Soc Dev. 2006;15:564–70. [Google Scholar]
- 41.Turkheimer E, Waldron M. Nonshared environment: a theoretical, methodological, and quantitative review. Psychol Bull. 2000;126:78–108. doi: 10.1037/0033-2909.126.1.78. [DOI] [PubMed] [Google Scholar]
- 42.Molenaar PCM, Boomsma DI, Dolan CV. A third source of developmental differences. Behav Genet. 1993;6:519–24. doi: 10.1007/BF01068142. [DOI] [PubMed] [Google Scholar]
- 43.Harris JR. The Nurture Assumption: Why Children Turn Out the Way They Do. New York: The Free Press; 1998. [Google Scholar]
- 44.Hetherington EM, Reiss D, Plomin R. Separate Social Worlds of Siblings: Impact of Nonshared Environment on Development. Hillsdale, NJ: Lawrence Erlbaum Assoc. Inc.; 1994. [Google Scholar]
- 45.Plomin R. Genetics and Experience: The Interplay between Nature and Nurture. Thousand Oaks, CA: Sage Publications Inc.; 1994. [Google Scholar]
- 46.Rowe DC. The Limits of Family Influence: Genes Experience, and Behaviour. New York: Guilford Press; 1994. [Google Scholar]
- 47.McCarthy MI, Abecasis GR, Cardon LR, et al. Genome-wide association studies for complex traits: consensus, uncertainty and challenges. Nat Rev Genet. 2008;9:356–69. doi: 10.1038/nrg2344. [DOI] [PubMed] [Google Scholar]
- 48.Maher B. Personal genomes: The case of the missing heritability. Nature. 2008;456:18–21. doi: 10.1038/456018a. [DOI] [PubMed] [Google Scholar]
- 49.Plomin R, Haworth CMA, Davis OSP. Common disorders are quantitative traits. Nat Rev Genet. 2009;10:872–78. doi: 10.1038/nrg2670. [DOI] [PubMed] [Google Scholar]
- 50.Atzaba-Poria N, Pike A. Correlates of parental differential treatment: Parental and contextual factors during middle childhood. Child Dev. 2008;79:217–32. doi: 10.1111/j.1467-8624.2007.01121.x. [DOI] [PubMed] [Google Scholar]
- 51.Boisvert D, Wright JP. Nonshared environmental influences on sibling differences in externalizing problem behavior. Crim Just Behav. 2008;35:863–78. [Google Scholar]
- 52.Bell RQ. A reinterpretation of the direction of effects in socialization. Psychol Rev. 1968;75:81–95. doi: 10.1037/h0025583. [DOI] [PubMed] [Google Scholar]
- 53.Ge XJ, Conger RD, Lorenz FO, Shanahan M, Elder GH. Mutual influences in parent and adolescent psychological distress. Dev Psychol. 1995;31:406–19. [Google Scholar]
- 54.Leung SSK, Stewart SM, Wong JPS, Ho DSY, Fong DYT, Lam TH. The association between adolescents' depressive symptoms, maternal negative affect, and family relationships in Hong Kong: Cross-sectional and longitudinal findings. J Fam Psychol. 2009;23:636–45. doi: 10.1037/a0016379. [DOI] [PubMed] [Google Scholar]
- 55.Plomin R, Bergeman CS. The nature of nurture: Genetic influences on “environmental” measures. Behav Brain Sci. 1991;14:373–427. [Google Scholar]
- 56.Kendler KS, Jacobson KC, Gardner CO, Gillespie N, Aggen SA, Prescott CA. Creating a social world – a developmental twin study of peer-group deviance. Arch Gen Psychiatry. 2007;64:958–65. doi: 10.1001/archpsyc.64.8.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kendler KS, Eaves LJ. Models for the joint effects of genotype and environment on liability to psychiatric illness. Am J Psychiatry. 1986;143:279–89. doi: 10.1176/ajp.143.3.279. [DOI] [PubMed] [Google Scholar]
- 58.Pike A, Reiss D, Hetherington EM, Plomin R. Using MZ differences in the search for nonshared environmental effects. J Child Psychol Psychiat. 1996;37:695–704. doi: 10.1111/j.1469-7610.1996.tb01461.x. [DOI] [PubMed] [Google Scholar]
- 59.Asbury K, Dunn J, Pike A, Plomin R. Nonshared environmental influences on individual differences in early behavioral development: an MZ differences study. Child Dev. 2003;74:933–43. doi: 10.1111/1467-8624.00577. [DOI] [PubMed] [Google Scholar]
- 60.Asbury K, Dunn JF, Plomin R. Birthweight-discordance and differences in early parenting relate to monozygotic twin differences in behaviour problems and academic achievement at age 7. Dev Sci. 2006;9:F22–31. doi: 10.1111/j.1467-7687.2006.00469.x. [DOI] [PubMed] [Google Scholar]
- 61.Beaver KM. Nonshared environmental influences on adolescent delinquent involvement and adult criminal behavior. Criminology. 2008;46:341–69. [Google Scholar]
- 62.Burt SA, McGue M, Iacono WG, Krueger RF. Differential parent-child relationships and adolescent externalizing symptoms: cross-lagged analyses within a monozygotic twin differences design. Dev Psychol. 2006;42:1289–98. doi: 10.1037/0012-1649.42.6.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Burt SA, McGue M, Iacono WG. Nonshared environmental mediation of the association between deviant peer affiliation and adolescent externalizing behaviors over time: results from a cross-lagged monozygotic twin differences design. Dev Psychol. 2009;45:1752–60. doi: 10.1037/a0016687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Caspi A, Moffitt TE, Morgan J, et al. Maternal expressed emotion predicts children's antisocial behavior problems: Using monozygotic-twin differences to identify environmental effects on behavioral development. Dev Psychol. 2004;40:149–61. doi: 10.1037/0012-1649.40.2.149. [DOI] [PubMed] [Google Scholar]
- 65.Deater-Deckard K, Pike A, Petrill SA, Cutting AL, Hughes C, O'Connor TG. Nonshared environmental processes in social-emotional development: an observational study of identical twin differences in the preschool period. Dev Sci. 2001;4:F1–6. [Google Scholar]
- 66.Mullineaux PY, Deater-Deckard K, Petrill SA, Thompson LA. Parenting and child behaviour problems: A longitudinal analysis of non-shared environment. Inf Child Dev. 2009;18:133–48. doi: 10.1002/ICD.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Viding E, Fontaine N, Oliver B, Plomin R. Negative parental discipline, conduct problems and callous-unemotional traits: A monozygotic twin differences study. Br J Psychiatry. 2009;195:414–19. doi: 10.1192/bjp.bp.108.061192. [DOI] [PubMed] [Google Scholar]
- 68.Oliver B, Pike A, Plomin R. Nonshared environmental influences on teacher-reported behaviour problems: Monozygotic twin differences in perceptions of the classroom. J Child Psychol Psychiat. 2008;49:646–53. doi: 10.1111/j.1469-7610.2008.01891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bruder CEG, Piotrowski A, Gijsbers AACJ, et al. Phenotypically concordant and discordant monozygotic twins display different DNA copy-number-variation profiles. Am J Hum Genet. 2008;82:763–71. doi: 10.1016/j.ajhg.2007.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mill J, Petronis A. Molecular studies of major depressive disorder: the epigenetic perspective. Mol Psychiatry. 2007;12:799–814. doi: 10.1038/sj.mp.4001992. [DOI] [PubMed] [Google Scholar]
- 71.Wong AH, Gottesman II, Petronis A. Phenotypic differences in genetically identical organisms: the epigenetic perspective. Hum Mol Genet. 2005;14:R11–18. doi: 10.1093/hmg/ddi116. [DOI] [PubMed] [Google Scholar]
- 72.Petronis A. The origin of schizophrenia: genetic thesis, epigenetic antithesis, and resolving synthesis. Biol Psychiatry. 2004;55:965–70. doi: 10.1016/j.biopsych.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 73.Dolinoy DC, Weidman JR, Jirtle RL. Epigenetic gene regulation: linking early developmental environment to adult disease. Reprod Toxicol. 2007;23:297–307. doi: 10.1016/j.reprotox.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 74.Feil R. Environmental and nutritional effects on the epigenetic regulation of genes. Mutat Res Fund Mol Mech Mut. 2006;600:46–57. doi: 10.1016/j.mrfmmm.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 75.Poulsen P, Esteller M, Vaag A, Fraga MF. The epigenetic basis of twin discordance in age-related diseases. Pediatr Res. 2007;61:38R–42R. doi: 10.1203/pdr.0b013e31803c7b98. [DOI] [PubMed] [Google Scholar]
- 76.Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet. 2002;3:415–28. doi: 10.1038/nrg816. [DOI] [PubMed] [Google Scholar]
- 77.Weidman JR, Dolinoy DC, Murphy SK, Jirtle RL. Cancer susceptibility: Epigenetic manifestation of environmental exposures. Cancer J. 2007;13:9–16. doi: 10.1097/PPO.0b013e31803c71f2. [DOI] [PubMed] [Google Scholar]
- 78.Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Nat Rev Genet. 2007;8:253–62. doi: 10.1038/nrg2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Weaver IC, Cervoni N, Champagne FA, et al. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–54. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 80.Petronis A. Epigenetics and twins: three variations on the theme. Trends Genet. 2006;22:347–50. doi: 10.1016/j.tig.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 81.Kaminsky Z, Petronis A, Wang SC, et al. Epigenetics of personality traits: An illustrative study of identical twins discordant for risk-taking behavior. Twin Res Hum Genet. 2008;11:1–11. doi: 10.1375/twin.11.1.1. [DOI] [PubMed] [Google Scholar]
- 82.Rosa A, Picchioni MM, Kalidindi S, et al. Differential methylation of the X-chromosome is a possible source of discordance for bipolar disorder female monozygotic twins. Am J Med Genet. B Neuropsychiatr Genet. 2008;147:459–62. doi: 10.1002/ajmg.b.30616. [DOI] [PubMed] [Google Scholar]
- 83.Mill J, Dempster E, Caspi A, Williams B, Moffitt T, Craig I. Evidence for monozygotic twin (MZ) discordance in methylation level at two CpG sites in the promoter region of the catechol-O-methyltransferase (COMT) gene. Am J Med Genet. B Neuropsychiatr Genet. 2006;4:421–25. doi: 10.1002/ajmg.b.30316. [DOI] [PubMed] [Google Scholar]
- 84.Petronis A, Gottesman II, Kan P, et al. Monozygotic twins exhibit numerous epigenetic differences: clues to twin discordance? Schizophr Bull. 2003;29:169–78. doi: 10.1093/oxfordjournals.schbul.a006988. [DOI] [PubMed] [Google Scholar]
- 85.Fraga MF, Ballestar E, Paz MF, et al. Epigenetic differences arise during the lifetime of monozygotic twins. Proc Natl Acad Sci USA. 2005;102:10604–9. doi: 10.1073/pnas.0500398102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pike A, McGuire S, Hetherington EM, Reiss D, Plomin R. Family environment and adolescent depressive symptoms and antisocial behavior: a multivariate genetic analysis. Dev Psychol. 1996;32:590–603. [Google Scholar]
- 87.Martin NG, Eaves LJ. The genetical analysis of covariance structure. Heredity. 1977;38:79–95. doi: 10.1038/hdy.1977.9. [DOI] [PubMed] [Google Scholar]
- 88.Plomin R, DeFries JC, McClearn GE, McGuffin P. Behavioral Genetics. 5th edn. New York: Worth Publishers; 2008. [Google Scholar]
- 89.Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Arch Gen Psychiatry. 2003;60:929–37. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- 90.Middeldorp CM, Cath DC, Van Dyck R, et al. The co-morbidity of anxiety and depression in the perspective of genetic epidemiology. A review of twin and family studies. Psychol Med. 2005;35:611–24. doi: 10.1017/s003329170400412x. [DOI] [PubMed] [Google Scholar]
- 91.Plomin R, Kovas Y. Generalist genes and learning disabilities. Psychol Bull. 2005;131:592–617. doi: 10.1037/0033-2909.131.4.592. [DOI] [PubMed] [Google Scholar]
- 92.Happe F, Ronald A, Plomin R. Time to give up on a single explanation for autism. Nature Neurosci. 2006;9:1218–20. doi: 10.1038/nn1770. [DOI] [PubMed] [Google Scholar]
- 93.Jaffee SR, Caspi A, Moffitt TE, Polo-Tomas M, Price TS, Taylor A. The limits of child effects: evidence for genetically mediated child effects on corporal punishment but not on physical maltreatment. Dev Psychol. 2004;40:1047–58. doi: 10.1037/0012-1649.40.6.1047. [DOI] [PubMed] [Google Scholar]
- 94.Narusyte J, Andershed AK, Neiderhiser JM, Lichtenstein P. Aggression as a mediator of genetic contributions to the association between negative parent-child relationships and adolescent antisocial behavior. Eur Child Adolesc Psychiatry. 2007;16:128–37. doi: 10.1007/s00787-006-0582-z. [DOI] [PubMed] [Google Scholar]
- 95.Larsson H, Viding E, Rijsdijk FV, Plomin R. Relationships between parental negativity and childhood antisocial behavior over time: a bidirectional effects model in a longitudinal genetically informative design. J Abnorm Child Psychol. 2008;36:633–45. doi: 10.1007/s10802-007-9151-2. [DOI] [PubMed] [Google Scholar]
- 96.Burt SA, McGue M, Krueger RF, Iacono WG. How are parent-child conflict and childhood externalizing symptoms related over time? Results from a genetically informative cross-lagged study. Dev Psychopathol. 2005;17:145–65. doi: 10.1017/S095457940505008X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Knafo A, Plomin R. Parental discipline and affection, and children's prosocial behavior: genetic and environmental link. J Pers Soc Psychol. 2006;90:147–64. doi: 10.1037/0022-3514.90.1.147. [DOI] [PubMed] [Google Scholar]
- 98.Deater-Deckard K, Petrill SA. Parent-child dyadic mutuality and child behavior problems: an investigation of gene-environment processes. J Child Psychol Psychiat. 2004;45:1171–79. doi: 10.1111/j.1469-7610.2004.00309.x. [DOI] [PubMed] [Google Scholar]
- 99.Narusyte J, Neiderhiser JM, D'Onofrio BM, et al. Testing different types of genotype-environment correlation: an extended children-of-twins model. Dev Psychol. 2008;44:1591–603. doi: 10.1037/a0013911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Button TMM, Corley RP, Rhee SH, Hewitt JK, Young SE, Stallings MC. Delinquent peer affiliation and conduct problems: a twin study. J Abnorm Psychol. 2007;116:554–64. doi: 10.1037/0021-843X.116.3.554. [DOI] [PubMed] [Google Scholar]
- 101.Bullock BM, Deater-Deckard K, Leve LD. Deviant peer affiliation and problem behavior: a test of genetic and environmental influences. J Abnorm Child Psychol. 2006;34:29–41. doi: 10.1007/s10802-005-9004-9. [DOI] [PubMed] [Google Scholar]
- 102.Kendler KS, Jacobson K, Myers JM, Eaves LJ. A genetically informative developmental study of the relationship between conduct disorder and peer deviance in males. Psychol Med. 2008;38:1001–11. doi: 10.1017/S0033291707001821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jaffee SR, Price TS. Gene-environment correlations: a review of the evidence and implications for prevention of mental illness. Mol Psychiatry. 2007;12:432–42. doi: 10.1038/sj.mp.4001950. [DOI] [PMC free article] [PubMed] [Google Scholar]