Abstract
Background A number of prospective cohort studies have examined the association between intelligence in childhood or youth and life expectancy in adulthood; however, the effect size of this association is yet to be quantified and previous reviews require updating.
Methods The systematic review included an electronic search of EMBASE, MEDLINE and PSYCHINFO databases. This yielded 16 unrelated studies that met inclusion criteria, comprising 22 453 deaths among 1 107 022 participants. Heterogeneity was assessed, and fixed effects models were applied to the aggregate data. Publication bias was evaluated, and sensitivity analyses were conducted.
Results A 1-standard deviation (SD) advantage in cognitive test scores was associated with a 24% (95% confidence interval 23–25) lower risk of death, during a 17- to 69-year follow-up. There was little evidence of publication bias (Egger’s intercept = 0.10, P = 0.81), and the intelligence–mortality association was similar for men and women. Adjustment for childhood socio-economic status (SES) in the nine studies containing these data had almost no impact on this relationship, suggesting that this is not a confounder of the intelligence–mortality association. Controlling for adult SES in five studies and for education in six studies attenuated the intelligence–mortality hazard ratios by 34 and 54%, respectively.
Conclusions Future investigations should address the extent to which attenuation of the intelligence–mortality link by adult SES indicators is due to mediation, over-adjustment and/or confounding. The explanation(s) for association between higher early-life intelligence and lower risk of adult mortality require further elucidation.
Keywords: Intelligence, mortality, meta-analysis, socio-economic factors, systematic review
Introduction
Individual differences in intelligence (cognitive ability, mental ability) test scores, as measured by standardized IQ-type tests in childhood, show an inverse association with risk of death from all causes throughout adulthood. That is, higher intelligence appears to confer protection. This finding is replicated in prospective cohorts from several Westernized countries,1 across different ranges of intelligence,2 and in follow-up periods from early through to late adulthood.2–4
Intelligence and somatic health may be inextricably linked throughout the life course. However, longitudinal studies help to establish causal pathway models of the effects of one upon the other. For example, morbidities such as diabetes, cancer, stroke and peripheral atherosclerosis, and/or their treatments, are reported to cause a decline in cognitive function after longitudinal follow-up.5–10 This illness-to-cognitive ability direction of association is a commonplace finding. The reverse direction of association is studied less often, and has only recently come to be recognized under the term ‘cognitive epidemiology’.11,12 That is, mental ability scores from early life associated with later adulthood morbidities, and before any somatic symptoms or risk factors of disease are manifest, provide evidence that cognitive abilities may be predictive of later health outcomes.
The association between premorbid intelligence and adult all-cause mortality was the subject of a systematic review,1 in which all nine studies that met the inclusion criteria demonstrated an inverse relationship between intelligence and risk of dying by the time of follow-up. The review did not quantify the association. Furthermore, there were insufficient studies to address comprehensively a number of pertinent questions from this research domain. One issue is whether or not the association between intelligence and mortality is the same in women as in men. For example, it is possible that sex differences in the incidence, age at onset of health behaviours, and the extent to which these act as risk factors for disease,13,14 could produce sex-specific intelligence–mortality gradients. Data from many more men than women have been included in intelligence–mortality cohort studies to date, mainly due to some studies using military conscript databases. Moreover, when mixed-sex cohorts report mortality risk as predicted by intelligence for men and women separately, they rarely test for statistical difference but, rather, report the observed trend. With more studies now reporting hazard ratios (HRs) for mortality by sex, there is an opportunity to quantify the predictive effects of intelligence on mortality separately for men and women.
A second issue yet to be evaluated systematically is the extent to which intelligence as a predictor of mortality is confounded by early-life environmental influences including socio-economic factors. Socio-economic status (SES) is established as an important determinant of public health inequalities,15–18 including risk of mortality, and it can carry influence in childhood, via factors such as family income and parental education, to predict individual differences in childhood intelligence.19,20 In this context, therefore, intelligence may be considered a mediating variable on the pathway between early-life influences and adult health outcomes. If early social factors substantially confound the link between intelligence and longevity, then adjusting for childhood SES would sizeably attenuate the effect size of the association between intelligence and mortality. In their systematic review, Batty et al.1 identified three out of nine studies that adjusted for childhood SES: one of these showed no change from an unadjusted model, and two had modest attenuating effects, suggesting that intelligence has independent effects on risk of mortality from those of early socio-economic influences. Due to this small number of studies, the role of childhood SES in the intelligence–mortality link requires further investigation.
One explanation why intelligence may exert an influence on life expectancy is its ability to predict educational outcomes21 and occupational class,22 which can both affect health outcomes via a number of mechanisms; for example, the knowledge and living conditions that contribute to better personal health risk assessment, behaviours and management.23 In population studies these adult SES factors are themselves inversely associated with risk of mortality.24–26 Some prospective cohorts take account of the attenuating effects of education and adult SES in estimating the risk of mortality according to intelligence; yet, to date, their influence has not been properly evaluated.
Investigators are giving increasing attention to the issues raised here, with a higher rate of publications reporting risk estimates for all-cause mortality according to differences in intelligence since the first systematic review.1 There is now an opportunity to re-evaluate this augmented literature, this time with a quantitative, meta-analytic approach. The systematic review by Batty et al.1 reported the overall quality of the nine studies as ‘moderate’, which was in part related to the weak validity of some measures of premorbid intelligence. Therefore, one important change to the systematic process reported here is the inclusion of studies in which only valid cognitive assessments were used. Kilgour et al.27 also raised a number of methodological considerations that should be addressed in intelligence–mortality studies, including taking account of ascertainment bias, age, sex and education. In this article we address the influence of these factors using subgroup analyses.
Accordingly, the aims of this report are to (i) quantify the association between premorbid intelligence and all-cause mortality, (ii) determine whether there are sex differences in the association and (iii) conduct subgroup analyses on studies that adjust for early-life SES, adult SES and education, to discover their magnitude of influence as potential confounders or mediators of the intelligence–mortality association.
Methods
Systematic review process
An electronic search was conducted of premorbid intelligence and all-cause mortality in all published articles, letters, abstracts and reviews, using the electronic databases MEDLINE, EMBASE and PSYCHINFO (via Ovid). Searches were limited to articles on humans published in the English language. The databases were searched using a cognitive ability-related term (‘Aptitude or Cognition’* or ‘Cognitive function’* or ‘Cognitive ability’ or ‘Cognitive characteristics’ or ‘Cognitive style’ or ‘intellectual ability’ or ‘Intelligence measures’ or ‘Intelligence quotient’ or ‘Intelligence test’* or ‘Intelligence’* or ‘IQ or Language test’* or ‘Memory’ or ‘Mental ability’* or ‘Mental capacity’ or ‘problem-solving’ or ‘Problem solving’ or ‘Psychological performance’ or ‘Psychometrics’) AND a mortality term (‘Cause of Death’* or ‘Cause of Death trends’ or ‘Death’* or ‘death rate’ or ‘Incidence’ or ‘Morbidity’ or ‘Morbidity trends’ or ‘Mortality Rate’ or ‘Mortality risk’ or ‘Mortality*’ or ‘Mortality trends’), an asterisk allowing the search term to precede a longer word or phrase.
The electronic search, conducted on 5 February 2010, yielded 19 236 articles. Two authors (C.C. and N.L.) independently scanned each title and abstract, retrieving articles on the basis of their relevance to intelligence and mortality. The inclusion criteria listed below were applied to their respective shortlists of papers. The reference lists of the selected articles were then examined, along with review papers on intelligence and mortality, and our own personal files, for articles that the electronic search might have missed. Among the final list of articles, when more than one paper reported intelligence–mortality associations from the same cohort, thereby duplicating data, three authors (C.C., D.B. and I.D.) agreed upon those papers to be retained, according to criteria of the following order: (i) the article reported HRs for mortality per 1-standard deviation (SD) difference in IQ-type score; (ii) the cohort size was larger; (iii) it was the original publication to report the data.
Inclusion criteria
We included published cohort data which fulfilled criteria similar to that of the previous systematic review on intelligence and all-cause mortality:1 (i) to minimize risk of reverse causality, only cohorts where intelligence test score data were collected at a mean age of 24 years or younger were included (the period classified as childhood and youth according to the World Health Organisation Study Group28); (ii) the intelligence and mortality data were collected at the level of the individual; (iii) the relationship between intelligence and all-cause mortality was reported quantitatively. We also stipulated that: (iv) the premorbid test should demonstrate an acceptable degree of validity as a measure of intelligence; and (v) the cohort was not selected from a clinical or unrepresentative population.
Statistical analysis
The HR with 95% confidence intervals (CIs) for all-cause mortality per SD advantage in intelligence test score was the principal outcome variable. For HRs expressed per 1SD disadvantage in intelligence, the reciprocal was used. Reported odds ratios (ORs) were treated as HRs, with the caveat that these effect estimates approximate one another29 when the incidence of an event (i.e. mortality) is low.30 In case–control studies reporting intelligence test means for living and deceased, we converted the standardized mean difference to an OR using formulae by Chinn.31 We contacted authors if we were unable to derive an overall effect size from their published data.
Fixed effects models were assumed for the aggregation of HRs based on evidence of a low degree of heterogeneity (P < 0.10).32 Subgroup analyses were conducted by sex group, for those studies to adjust for SES variables, and by study characteristic groupings for the purposes of sensitivity analysis. MIX 1.5 software33 was used for all analyses and production of plots. The inverse variance method was used to weight studies’ effect sizes.
Sensitivity analyses and publication bias
Sensitivity analyses aggregated effect sizes by the following study characteristics: ascertainment rate at follow-up; age at intelligence testing; cohort size; duration of follow-up; average birth year of the cohort; effect size measure (see Table 2 for group parameters). Ascertainment rate may bias the intelligence–mortality effect size if those who emigrate, and are therefore excluded from follow-up, differ on cognitive ability scores compared with those who remain within geographical regions for census. Follow-up rates were estimated based on the proportion of participants from the original cohort that were followed up and included in the final analyses, regardless of whether or not intelligence test scores were available for them. Studies were grouped on the basis of <80% or 80–100% ascertainment rates. We aggregated studies according to age at cognitive testing, first because the likelihood of an effect of bodily insults on intellectual function increases with age and, with it, the risk of reverse causation bias in the intelligence–mortality link. Conversely, the validity of cognitive testing may be greater in older cohorts, and these may reflect more homogeneous results compared with results of younger children at intelligence testing for whom there is more measurement error. For the duration of follow-up, we divided studies on the basis of a median split of the years traced for mortality. Although there may be stronger grounds for assuming causality as the time period between intelligence testing and mortality increases, there is also evidence that, by older adulthood, the intelligence–mortality association loses significance.34 The reason for aggregating studies that reported HRs or ORs was to ensure that our treatment of these measures of association did not inflate the overall effect size. We also grouped according to cohort size and decade of birth, as these may also have influenced heterogeneity across the studies. We did not aggregate for population representativeness as one of our criteria ensured the exclusion of clinical samples. However, due to two study cohorts being less representative of the general population (twins and gifted children) than all others, we have reported meta-analytic results with and without their effect sizes.35,36
Table 2.
Summary of HRs for all-cause mortality in relation to a 1-SD advantage in intelligence in 16 longitudinal cohort studies
| Heterogeneity |
|||||||
|---|---|---|---|---|---|---|---|
| Subgroups | Studies, n | References | Deaths, n | HR (95% CI) | P | I2 (%)a | Risk attenuation from basic model |
| Basic model | 16 | 2–4, 35, 36, 50–56, 59, 61, 63, 65 | 22 453 | 0.76 (0.75–0.77) | 0.28 | 15.5 | – |
| Mean age at cognitive testing | |||||||
| 7–12 years | 10 | 2, 4, 36, 51–53, 55, 56, 59, 61 | 4424 | 0.80 (0.77–0.83) | 0.61 | 0.0 | – |
| 18–20 years | 6 | 3, 35, 50, 54, 63, 65 | 18 029 | 0.75 (0.74–0.77) | 0.54 | 0.0 | – |
| Percentage ascertainment | |||||||
| <80% | 7 | 3, 4, 52, 55, 59, 61, 65 | 17 148 | 0.76 (0.75–0.78) | 0.39 | 5.4 | – |
| ≥80% | 7 | 2, 36, 50, 51, 53, 63 | 4397 | 0.77 (0.74–0.80) | 0.25 | 23.7 | – |
| Invalidb | 2 | 35, 54 | 908 | – | – | – | – |
| Effect size | |||||||
| HR | 12 | 2–4, 36, 50, 52, 53, 55, 56, 59, 61, 65 | 20 856 | 0.76 (0.75–0.78) | 0.24 | 21.1 | – |
| OR | 4 | 35, 51, 54, 63 | 1597 | 0.71 (0.64–0.79) | 0.57 | 0.0 | – |
| Cohort size | |||||||
| <1000 | 4 | 4, 35, 36, 59 | 1149 | 0.83 (0.76–0.91) | 0.26 | 25.3 | – |
| 1000–10 000 | 7 | 52–56, 61, 65 | 3638 | 0.77 (0.74–0.81) | 0.30 | 17.4 | – |
| >10 000 | 5 | 2, 3, 50, 51, 63 | 17 870 | 0.76 (0.74–0.77) | 0.70 | 0.0 | – |
| Follow-up duration | |||||||
| <40 years | 7 | 3, 50, 51, 54, 61, 63, 65 | 18 495 | 0.75 (0.74–0.77) | 0.71 | 0.0 | – |
| 40–69 years | 9 | 2, 4, 35, 36, 52, 53, 55, 56, 59 | 3958 | 0.80 (0.77–0.83) | 0.50 | 0.0 | – |
| Cohort birth year | |||||||
| 1910–20s | 5 | 4, 35, 36, 53, 55 | 2723 | 0.82 (0.77–0.86) | 0.41 | 0.0 | – |
| 1930–40s | 4 | 52, 56, 59, 65 | 815 | 0.73 (0.65–0.80) | 0.80 | 0.0 | – |
| 1950–60s | 7 | 2, 3, 50, 51, 54, 61, 63 | 14 858 | 0.76 (0.74–0.77) | 0.56 | 0.0 | – |
| Sexc | |||||||
| Female | 7 | 2, 51–53, 55, 59, 63 | 1086 | 0.78 (0.73–0.84) | 0.17 | 33.4 | – |
| Male | 7 | 2, 51–53, 55, 59, 63 | 1771 | 0.80 (0.76–0.85) | 0.88 | 0.0 | – |
| Adjusted for | |||||||
| Childhood SES | 9 | 2, 3, 36, 50, 51, 53, 56, 59, 61 | 18 733 | 0.77 (0.75–0.79) | 0.48 | 0.0 | 4.0%d |
| Adult SES | 5 | 4, 50, 51, 56, 65 | 3070 | 0.84 (0.78–0.90) | 0.52 | 0.0 | 33.5% |
| Education | 6 | 3, 51, 53, 56, 63, 65 | 16 023 | 0.89 (0.86–0.91) | 0.53 | 0.0 | 54.2%d |
Note. All sub-analyses refer to fixed effects models.
aI2 (%) = percentage of variation across studies due to heterogeneity.
bInsufficient data prevented estimation of ascertainment rate at follow-up.
cNumber of deaths reported for men and women exclude data from Jokela et al.,63 which were unreported.
dRemoving the influence of by far the largest cohort by Batty et al.3 gave attenuation effects by childhood SES of 0.0% and by education of 45.8%.
The funnel plot was used to assess publication bias with standard error on the y-axis as recommended by Sterne and Egger.37 Publication bias was further evaluated with Egger’s test of asymmetry, and trim-and-fill adjustment methods.
Results
Systematic retrieval of studies
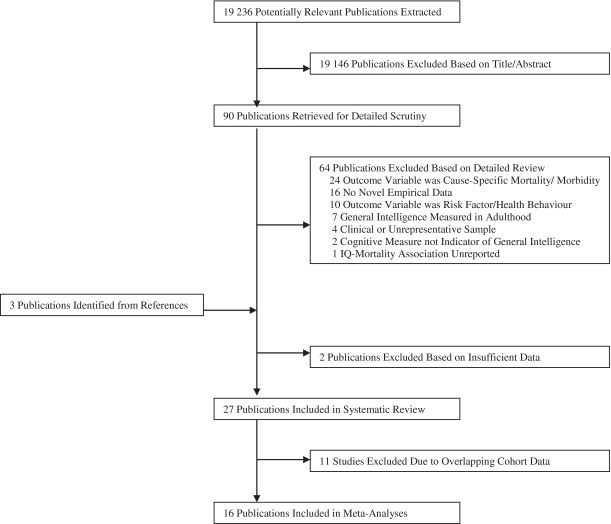
The electronic search resulted in 19 236 publications and, of these, the two reviewers (C.C., N.L.) extracted shortlists of 73 and 69 relevant articles, respectively (<0.4% of total publications), from which 90 non-duplicate publications were retrieved for closer inspection (see Figure 1 for review process). Among these, 64 failed to meet one or more inclusion criteria. A further three studies that met inclusion criteria were identified from the reference lists of the remaining 26 papers, or from review articles or our own records.38–40 Out of 29 studies, 2 were excluded39,40 because we were unable to obtain sufficient data to calculate the intelligence–mortality effect sizes. (Table 1 lists the characteristics of the final 27 studies). We excluded 11 articles from the meta-analysis because they overlapped with cohort data of another report.34,38,41–49 Justification for excluding these data were: (i) the overlapping reports of a sample were generally reported by the same research group resulting in consistent methods of sample selection and data linkage; and (ii) the majority of overlapping cohorts were of similar size and follow-up duration.
Figure 1.
Flow diagram of articles selected for systematic review and meta-analysis
Table 1.
Characteristics of 27 longitudinal cohort studies of premorbid intelligence and all-cause mortality
| Study references | Country, study name | Sex | Birth year(s) | Age at cognitive test, years (mean) | Years of survival follow-up (n years since cognitive test)a | Cohort Size, N | Deaths, n | Intelligence test, cohort type | Model adjustments |
|---|---|---|---|---|---|---|---|---|---|
| *O'Toole et al. (1988)54,b | Australia, Veterans Health Studies | M | 1947–53 (including earlier periods) | ≥18 | 1967–82 (2–17) | 2309 | 523 | AGCT, conscription |
|
| *Whalley and Deary (2001)55 | UK, SMS32 Aberdeen Cohort | M/F | 1921 | 11 | 1932–97 (1–65) | 1153 (M) 1032 (F) | 646 (M) 438 (F) | Moray House No. 12, school | Basic model: age |
| Deary et al. (2003)38 | UK, SMS32 Aberdeen Cohort | M/F | 1921 | 11 | 1932–97 (1–65) | 1139 (M) 1032 (F) | 633 (M) 438 (F) | Moray House No. 12, school | – |
| *Hart et al. (2003)4 | UK, SMS32 and Mid-span studies | M/F | 1921 | 11 | 1970–2001 (38–69) | 922 | 422 | Moray House, school |
|
| *Osler et al. (2003)61 | Denmark, Metropolit2000 | M | 1953 | 12 | 1968–98 (3–33) | 7308 | 522 | Harnquist, school |
|
| Deary et al. (2004)44 | UK, SMS47 Six Day Sample | M/F | 1936 | 11 | 1968–2000 (21–53) | 908 | 125 | Binet test, school | – |
| Kuh et al. (2004)46 | UK, National Survey of Health and Development (British 1946 birth cohort) | M/F | 1946 | 8 | 1971–2000 (17–46) | 2192 (M) 2057 (F) | 133 (M) 96 (F) | NFER tests, school | – |
| Hart et al. (2005)34 | UK, SMS32 and Mid-span studies | M/F | 1921 | 11 | 1970–2001 (38–69) | 938 | 432 | Moray House, school | – |
| *Martin and Kubzansky (2005)36 | USA, Terman Life Cycle Study | M/F | 1903–16 | 6–18 (11) | 1922–86 (1–64) | 862 | 293 | Stanford-Binet, school |
|
| *Hemmingsson et al. (2006)50 | Sweden, Army Conscripts | M | 1949–51 | 18–20 (19) | 1971–2000 (1–31) | 49 262 | 2022 | SEB 1967, conscription |
|
| Vagero et al. (2006)48 | Sweden, Army Conscripts (Stockholm Birth Cohort Study) | M | 1953 | (18) | 1980–2002 (9–31) | 6318 | 204 | SEB, conscription | – |
| *Pearce et al. (2006)59 | UK, Newcastle Thousand Families | M/F | 1947 | 11 | 1959–2003 (1–45) | 357 (M) 360 (F) | 30 (M) 19 (F) | Moray House Nos. 57 & 58, school |
|
| *Holsinger et al. (2007)35,c | USA, NAC-NRC Twin WWII Veterans | M | 1917–27 | 17–21 (19) | 1967–2004 (22–59) | 984 | 385 | AGCT/GCT, conscription | Basic model: unspecified |
| Batty et al. (2008)41 | USA, Vietnam Experience Study | M | 1947 | (19) | 1985–2000 (18–33) | 4157 | 231 | Army General Technical, conscription | – |
| *Batty et al. (2008)65,d | USA, Vietnam Experience Study | M | 1947 | (20) | 1985–2000 (18–33) | 4316 | 241 | Army General Technical, conscription |
|
| *Deary et al. (2008)56 | UK, SMS47 Six Day Sample | M/F | 1936 | 11 | 1968–2003 (21–56) | 1181 | 193 | Binet test, school |
|
| Starr et al. (2008)47 | UK, SMS32 Aberdeen Cohort | M/F | 1921 | 11 | 1932–2007 (1 – 75) | 202 (M) 152 (F) | 102 (M) 56 (F) | Moray House No. 12, school | – |
| Batty et al. (2008)42 | USA, Vietnam Experience Study | M | 1947 | (20) | 1985–2000 (18–33) | 14 437 | 769 | Army General Technical, conscription | – |
| Batty et al. (2008)43 | USA, Vietnam Experience Study | M | 1947 | (20) | 1985–2000 (18–33) | 4166 | 233 | Army General Technical, conscription | – |
| *Batty et al. (2009)3 | Sweden, Army Conscripts | M | 1950–76 | 16–26 (18) | 1971–2001 (1–30) | 994 262 | 14 498 | SEB, conscription |
|
| *Jokela et al. (2009)63 | USA, National Longitudinal Study of Youth | M/F | 1957–64 | 16–23 (19) | 1980–2004 (0–24) | 5682 (M) 5639 (F) | 248 (M) 112 (F) | AFQT, research sample |
|
| *Kuh et al. (2009)52 | UK, National Survey of Health and Development (British 1946 birth cohort) | M/F | 1946 | 8, 11, 15 | 1971–2005 (10–51) | 4128 | 195 (M) 137 (F) | NFER tests, school |
|
| Weiss et al. (2009)49 | USA, Vietnam Experience Study | M | 1947 | (20) | 1985–2000 (18–33) | 4200 | 234 | Army General Technical, conscription | – |
| Hemmingsson et al. (2009)45 | Sweden, Army Conscripts | M | 1949–51 | 18–20 (19) | 1990–2003 (19–36) | 43 834 | Not reported | SEB 1967, conscription | – |
| *Jokela et al. (2009)51,e | UK, National Child Development Survey (British 1958 birth cohort) | M/F | 1958 | 11 | 1969–2004 (1–35) | 14 132 | 213 (M) 116 (F) | NFER tests, school |
|
| *Leon et al. (2009)2 | UK, ACONF | M/F | 1955 | 7 | 1970–2007 (8–45) | 11 603 | 426 (M) 235 (F) | Moray House Picture Tests Nos 1 & 2, school |
|
| *Lager et al. (2009)53 | Sweden, Malmö Longitudinal Study | M/F | 1927–28 | 10 | 1939–2003 (1–65) | 832 (M) 698 (F) | 363 (M) 176 (F) | Hallgren test, school |
|
Studies appear in publication date order; those marked with an asterisk are included in the meta-analysis. ACONF = Aberdeen Children of the 1950s; AGCT = Army General Classification Test; AWOL = absent without leave; FEV1 = forced expiratory volume (1 second); GCT = General Classification Test; NFER = National Foundation for Educational Research; SMS32 = Scottish Mental Surveys of 1932; SMS47 = Scottish Mental Surveys of 1947; SEB = Swedish Enlistment Battery; M = male; F = female.
aIf years of follow-up and/or dates were not reported, these were estimated according to the longest theoretical time period.
bStudy included all deceased and a random sample of survivors from the original cohort, twice as large as the deceased group.
cYears since cognitive testing is an estimate: in correspondence Holsinger et al. reported that the vast majority of conscripts would have been 17–21 years old at testing.
dYears since cognitive testing and birth year are approximations based on the mean age of participants at the beginning of follow-up in 1985–86.
eModels for sex groups and those that adjusted for adult SES and multiple variables are based on a shorter follow-up period (2346 years) and, therefore, a smaller sample size (n = 10 620).
Study descriptions for meta-analysis
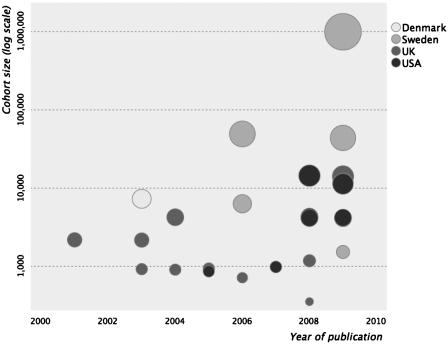
A total of 16 prospective longitudinal cohort studies included 22 453 deaths among 1 107 022 participants. These were from five countries: UK (n = 7), USA (n = 5), Sweden (n = 2), Australia (n = 1) and Denmark (n = 1), ranging in size from 862 to 994 262 participants. Figure 2 illustrates these variables according to year of publication, showing a trend for larger cohorts accumulating in more recent years. Premorbid intelligence test scores were taken from school records (n = 10), military or national service conscription records (n = 5), or a research database (n = 1). The average age at testing ranged from 7 to 20 years, and length of follow-up ranged from 17 to 69 years. Six cohorts were all male (five from conscription databases), and the remainder were mixed sex. A variety of cognitive assessments were used across studies, and we identified evidence for each of them as having validity as standardized measures of intelligence. The concurrent or predictive validity of five tests used across nine of the study cohorts3,4,35,50–55 have been described elsewhere.1 Here we describe evidence for psychometric validity among the seven remaining cohorts.
Figure 2.
Publication rate of longitudinal cohort studies on intelligence in childhood and youth, and all-cause-mortality (n = 27). Circles are shaded to represent country of origin and scaled proportionately to cohort size. One study is missing;54 its publication precedes 2000
The Binet and Stanford-Binet tests used in two studies36,56 are well-established, age-standardized intelligence tests for children. Scores on the original Stanford–Binet test contain a single underlying factor of cognitive ability,57 and the Binet scale has concurrent validity with version 12 of the Moray House intelligence test (r ~ 0.80).56 Two studies included selected tests from the well-validated Moray House series.58 The first study incorporated Moray House tests 57 and 58 in an 11-plus examination that also assessed language and arithmetic.59 On this exam, total scores have shown well-established associations with childhood height at ages 9 and 13 years.60 The second2 used Moray House Picture Tests 1 and 2, which have also shown expected patterns of association with intrauterine and childhood growth.19 The Härnquist test used in the Danish Metropolit study61 has shown concurrent validity: a general intelligence factor extracted from scores on the test at age 13 years strongly positively correlated (r = 0.78) with a military classification intelligence test taken 5 years later.62 The Armed Forces Qualification Test (AFQT) used in another study63 strongly correlates with other well-validated IQ tests (median r with seven tests = 0.81), and scores on the four subtests show high loadings on a single g factor, from 0.81 to 0.87.64 Finally, the Vietnam Experience Study65 used the Army General Technical test, which strongly correlates with verbal reasoning (r = 0.75) and visuospatial (r = 0.51) scores from the Weschler Adult Intelligence Scale (WAIS), a standardized and well-validated cognitive ability test battery.
Records of mortality were ascertained prospectively in seven studies, either by linkage of study members to national register databases4,35,51–52,59 or by individual follow-up with study participants or their families.36,63 In the remaining studies, incidence of death was ascertained retrospectively by access to national death registers—in Swedish cohorts record linkage used personal identification numbers rather than person names3,50,53—with the exception of two studies that did not report methods for extracting death records.54,65
Ten papers estimated the intelligence–mortality effect size as an HR with CIs,2–4,36,50,53,55,56,59,65 two used ORs or logistic regression coefficients,51,63 and two reported means and SDs that we converted to ORs.35,54 Authors of the two remaining papers provided HRs52,61 in response to email requests, which were unreported in their original publications.
Intelligence–mortality meta-analysis: basic model
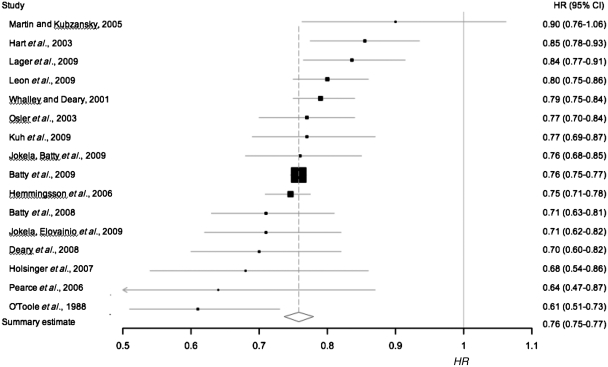
In the basic model, the HR from each of the 16 studies was either: unadjusted (n = 2), adjusted for age (n = 3), sex (n = 3), age and sex (n = 3), or was unspecified (n = 5) (Table 1). However, there was a low degree of heterogeneity between the effect sizes of these models (Q = 17.7, I2 = 15.5%, P = 0.28). In a fixed effects model, a 1-SD advantage in intelligence was associated with the lower risk of all-cause mortality (HR 0.76, 95% CI 0.75–0.77) (Figure 3). The exclusion of two studies35,36 based on selected samples (twins and gifted children) did not alter this estimate; neither did the exclusion of two studies that reported ORs and where incidence of death was between 20 and 40%35,54 (data not shown). The statistical weight of the largest study3 was 70.5%; excluding this cohort from the model made a negligible change to the effect of intelligence on risk of mortality (HR 0.77, 95% CI 0.75–0.80).
Figure 3.
Risk of all-cause-mortality per 1-SD advantage in intelligence test scores (n = 16), in a basic model. Squares mark cohort-specific effect sizes, which are proportional to the statistical weight (i.e. inverse variance), and the diamond indicates the aggregate effect size. Horizontal lines represent 95% CIs. ORs from four studies35,51,54,63 are treated as HRs; excluding the two studies with 20–40% risk of death35,54 made no change to the summary estimate
Sensitivity analyses results are presented in Table 2. Age at intelligence testing may have had a small effect in predicting the risk of mortality. Aggregation of studies in which premorbid intelligence was tested at an average age of between 7 and 12 years resulted in a small attenuation (16%) of the risk of mortality (HR 0.79, 95% CI 0.76–0.82) compared with that of 18- to 20-year olds (HR 0.75, 95% CI 0.74–0.77). Studies of longer follow-up (40–69 years) showed a 20% attenuation of the risk of mortality as predicted by a 1-SD advantage in intelligence (HR 0.80, 95% CI 0.76–0.83), compared with those cohorts of shorter follow-up (HR 0.75, 95% CI 0.74–0.77). Furthermore, there was a trend for cohorts born in the 1910s and 1920s to show an attenuated effect size compared with those cohorts born in the 1930–60s. However, these older age cohorts were also those with a longer duration of follow-up.
Ascertainment bias was unlikely to have affected the total aggregate HR. That is, studies of low ascertainment (62–79%) showed a similar aggregate effect size (HR 0.76, 95% CI 0.75–0.78) to that of studies with 80–100% ascertainment (HR 0.77, 95% CI 0.74–0.80). There was also no observable effect of cohort size on the magnitude of the intelligence–mortality association.
The aggregate effect size for studies reporting ORs resulted in a higher risk of mortality as predicted by intelligence (0.71, 95% CI 0.64–0.79) compared with the aggregate effect size from studies reporting HRs (0.76, 95% CI 0.75–0.78). However, the four studies reporting ORs had among the lowest weightings of the 16 cohorts (0.42–1.08%), which may explain why their inclusion in the basic model was less likely to have incurred statistical bias.
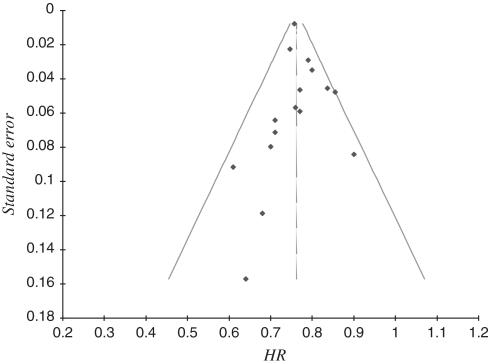
Publication bias was first addressed by examination of the funnel plot, which revealed one study4 on the outside of 95% CI parameters (Figure 4). Egger’s test of asymmetry supported a low risk of publication bias (intercept = 0.10, 95% CI 0.72–0.91, P = 0.81), as did application of trim and fill adjustments in which only one missing study was estimated, and its imputation made no difference to the magnitude of the risk estimate (HR 0.76, 95% CI 0.75–0.78).
Figure 4.
Funnel plot of HRs and standard errors to assess publication bias. Diagonal lines indicate 95% CIs of the aggregate HR (shown by vertical line) from all studies combined. One study4 is an outlier of the CI parameters
Stratification by sex
Seven studies reported intelligence–mortality effect sizes for men and women separately, and their follow-up spanned 24–65 years.2,51–53,55,59,63 During this period the absolute risk of death was 5.6% for women and 8.2% for men. Four out of the seven studies reported negligible sex differences2,51,52,63 (two of these formally tested intelligence × sex interaction effects), two reported a stronger effect for men (one reported a null effect in women with an intelligence × sex interaction effect),53,59 and one reported a stronger effect in women.55 However, fixed effects models were applied to aggregate the sex-specific HRs, given the evidence for low heterogeneity (Table 2). A 1-SD advantage in intelligence among women was associated with a 22% lower risk of all-cause mortality (HR 0.78, 95% CI 0.73–0.84), whereas among men there was a 20% reduced risk of mortality per 1-SD advantage in intelligence (HR 0.80, 95% CI 0.76–0.85). Nevertheless, there was a high degree of overlap in the CIs of these respective effect sizes. Egger’s test of asymmetry supported a lack of publication bias among sex-specific cohorts.
Adjustment for childhood SES
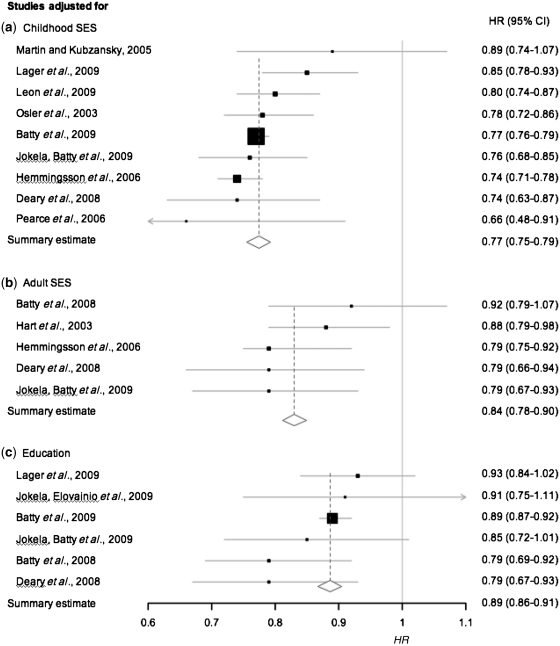
Nine studies that included 18 733 deaths, reported effect-size models adjusted for childhood SES, measured either by father’s occupation or income,2,36,50,51,56,59,61 the highest socio-economic index recorded for either parent,3 or father’s education.53 Heterogeneity was very low in unadjusted (Q = 8.56, I2 = 6.6%, P = 0.38) and adjusted models (Q = 7.49, I2 = 0.0%, P = 0.48). In a fixed effects basic model the HR for this subgroup of papers did not deviate from the HR for the 16 studies (HR 0.76, 95% CI 0.75–0.77). However, even after adjustment for childhood SES there was a very small attenuation (by 4%) of the effect size (HR 0.77, 95% CI 0.75–0.79) (Figure 5). Excluding the large study of over one million Swedish men had no effect on the aggregate effect size of the childhood SES-adjusted model, except to slightly widen the 95% CI parameters (HR 0.77, 95% CI 0.74–0.80). Compared with the unadjusted model of this smaller group of studies in which there were 4608 deaths (HR 0.77, 95% CI 0.74–0.80), controlling for childhood SES had no effect on the intelligence–mortality gradient when the influence of this largest weighted study was removed.
Figure 5.
Risk of all-cause-mortality per 1-SD advantage in intelligence test scores after adjustment for: (a) childhood SES, (b) adult SES and (c) education. Squares mark cohort-specific effect sizes, which are proportional to the statistical weight (i.e. inverse variance), and diamonds indicate the aggregate effect sizes for studies adjusted for each covariate. Horizontal lines represent 95% CIs
A 10th publication46 from the systematic review, which could not be included in meta-analysis, reported data consistent with this finding: early-life socio-economic inequalities do little to explain the inverse association between intelligence and all-cause-mortality.
Controlling for adult SES and education
There was no evidence for publication bias among studies that controlled for adult SES or education. In five studies that adjusted for adult SES, there were 3070 deaths among 66 301 participants. SES was measured either by occupational social class,4,50,51,56 or income.65 The unadjusted effect size for this subgroup of studies (HR 0.76, 95% CI 0.72–0.79) matched that of all 16 studies. After adjustment for adulthood SES, the lower risk of mortality predicted by higher intelligence was attenuated by 33.5% from the basic model (HR 0.84, 95% CI 0.78–0.90) (Figure 5).
Among the six studies that adjusted for educational attainment, there were 16 023 deaths out of 1026 742 participants.3,51,53,56,63,65 Again, the aggregate effect size for this subgroup of studies in an unadjusted model (HR 0.76, 95% CI 0.74–0.77) was no different from that for all 16 studies. After adjustment for education (HR 0.89, 95% CI 0.86–0.91), the effect of intelligence on mortality was reduced by 54.2% (Figure 5). Exclusion of the large Swedish cohort3 from the model, as expected, widened the CI parameters (HR 0.87, 95% CI 0.81–0.93), but still reduced the intelligence–mortality gradient by 45.8% from the unadjusted model.
Two further studies from the systematic review,45,46 excluded from meta-analysis due to the type of statistics reported, are consistent with our result. They observed attenuation effects by education of over one third, of the intelligence–mortality association.
Multiple covariates
Eleven studies, including 15 148 deaths, reported effect sizes for the risk of mortality according to intelligence while adjusting for multiple variables2–4,50–52,54,56,61,63,65 (see Table 1 for covariates). Among these cohorts, four showed entire attenuation of the intelligence–mortality effect size from unadjusted (or basic) models.51,52,63,65 These studies tended to adjust for adult SES variables with the addition of other important covariates, including education63 or smoking52 among other cardiovascular disease risk factors.51,65 The remaining studies reported a smaller degree of attenuation from unadjusted models. Due to the varying number and nature of covariates across the studies it was not appropriate to aggregate their effect sizes in meta-analyses.
Discussion
The present meta-analysis of 16 published prospective cohort studies, comprising over 1.1 million participants and 22 453 deaths, demonstrates and quantifies the consistently-reported association between higher premorbid intelligence and lower mortality risk. A 1-SD advantage in intelligence in childhood and youth was associated with a 24% lower risk of mortality. The effect was similar in men and women, and was not explained by socio-economic differences in early life, as indicated by parental occupation or income. The association was attenuated by approximately a third after adjusting for adult SES and by approximately a half after adjusting for educational experience. Intelligence remained a predictor of mortality after these attenuating effects, and removal of one study that carried by far the largest weighting in the models3 did little to change the magnitude of these effects.
This is the first meta-analysis of studies examining the relationship between premorbid intelligence and all-cause mortality. A recent systematic review, which was based on nine identified at that time, reported the inverse association.1 Since then the number of publications of the intelligence–mortality association has grown, and the 16 unrelated cohorts we identified represent more than four times as many deaths. We found little evidence of publication bias, and so the estimated risk of mortality according to a 1-SD advantage in intelligence may be generalized to cohorts beyond those included in this meta-analysis, at least to those of the five countries included in the analyses. Our treatment of ORs as HRs in two studies where the absolute risk of death was >5%, which could have incurred statistical error, was not found to inflate the aggregate effect size.
Heterogeneity was not apparent across the studies despite most using different assessments of premorbid intelligence. This may be because most omnibus intelligence tests of the types used in the identified studies show strong loadings on general intelligence, g.66 The intelligence–mortality association was, however, slightly weaker among cohorts of younger ages at cognitive testing, and those of longer follow-up duration. As it was the same cohorts that were followed up beyond 40 years who were the youngest at intelligence testing, it is difficult to establish which factor would make the larger contribution to attenuating the intelligence–mortality association. However, it seems less likely to have been due to differences in the validity of intelligence tests taken at younger and older ages, given the equally low heterogeneity among these two cohort groupings. It may be that older cohorts at cognitive testing show a steeper intelligence–mortality gradient because of the increased likelihood of bodily insults, or, it is still possible that the association varies according to age at mortality, most likely due to cause of death.
Lack of confounding by sex and early-life SES
Our observation of negligible differences between men and women in the relative risk of mortality as predicted by intelligence, may be surprising given well-documented sex differences in patterns of risk factors, onset and prevalence of specific diseases and life expectancies.67 However, there were exceptions in individual studies, with differences between men and women reported, although there seem to be cohort-specific explanations for these. In one study55 the lower relative risk among men was probably due to the rise in deaths of higher intelligence servicemen during World War II.68 In another, the lower relative risk among women could have resulted from a lack of statistical power due to the small number of female deaths.59 The result from an older birth cohort study53 of a null association among women, could have been influenced by a relatively higher incidence of smoking among well-educated women during an era before the health hazards of smoking were widely known. In general, however, data from large post-war birth cohort studies show negligible sex differences in the effects of intelligence in relation to risk of mortality, and results from our meta-analyses support this. Equivalent effect sizes by sex still do not mean that the mechanisms that explain the intelligence–mortality association act in equal measure for men and women, and it continues to be of interest to study sex differences in cognitive epidemiology. Differences in health behaviours, risk patterns and medical interventions should also be considered when comparing ethnic groups or diverse countries. However, there is currently a lack of cohort data to evaluate how such group differences influence the risk of all-cause mortality as predicted by premorbid intelligence.
Socio-economic conditions in early life, determined by parental occupation or income, were also unlikely confounders. Individual differences in cognitive ability appear to act independently of childhood social inequalities in predicting all-cause mortality. There may of course be alternative early-life factors contributing to confounding that were not covariates of the cohorts we reviewed. Among three studies that adjusted for birth weight in multivariate-adjusted models, one reported no change from unadjusted models,2 and two reported a risk attenuation of 1 and 4%, respectively, compared with models that adjusted for childhood SES61 and education.51 However, recent evidence suggests that birth weight may not be the ideal indicator for exposures in the intrauterine environment, which carry their most critical influence on neurological and physiological development during the early prenatal period.69 Other qualitative characteristics in early childhood may further explain the relationship between premorbid intelligence and longevity,27 including style of parenting and cognitive stimulation at home,70 or the effects of diet. However, so far, the potential confounding of these early-life factors have not been demonstrated, and these other suggested variables are likely to be associated with parental intelligence.
Attenuation of the intelligence–mortality association
Education and adult SES were found partially to attenuate the risk of mortality according to a 1-SD advantage in intelligence. Premorbid cognitive ability may act via occupational status and wealth to reduce the risk of mortality, by providing a less hazardous work environment, a safer and more comfortable home environment, and the material means to access better and more immediate medical care. Furthermore, intelligence may be mediated by education to reduce the likelihood of death, perhaps by increasing a person’s receptivity to health education messages (thereby reducing negative behaviours such as smoking and excess alcohol consumption, and promoting exercise and healthy eating), and by improving comprehension of medical terminology and instruction that impacts on disease management and prevention. Nevertheless, the results to date cannot tell us for certain whether education and adult SES are simply partial mediators of the association between intelligence and mortality, or whether the results reflect over-adjustments if both factors are partial surrogates for intelligence, or if these variables confound intelligence–mortality associations.71 Structural equation modelling can examine for statistical mediation, and one study to employ this technique reported that the effect of a general intelligence factor on mortality was entirely mediated by income, education and poor physical health in adulthood.49 However, in this study, with cognitive ability measured at age 20 years, the association between intelligence and mortality could also have been partially confounded by education. In our meta-analyses, two out of five studies that adjusted for adult SES,50,65 and three out of six studies adjusting for education,3,63,65 had intelligence test scores measured in later youth (19–20 years of age), when most people have completed education. There is evidence for a causal association from childhood intelligence scores to later educational achievement in longitudinal studies, and it is also likely educational experience can boost cognitive test scores to some extent.72 Therefore reciprocal dynamic pathways between intelligence, education and adult SES need to be considered.
Few studies in the meta-analysis adjusted for both education and adult SES in the same model. It is suggested that both factors may overlap in their attenuation effects on the intelligence–mortality association,40 but there is also evidence to show that they are not interchangeable, and have independent effects on health outcomes.73,74 Among three studies to control simultaneously for adult SES and education, the relative risk of mortality was entirely attenuated.51,52,63 Interpretation of these findings should also consider the likelihood of over-adjustment. In studies that reported complete attenuation effects of the intelligence–mortality gradient after multivariate adjustments, in addition to controlling for socio-economic and educational variables, it was noted that three studies adjusted for smoking,51,52,65 two adjusted for alcohol consumption,51,65 and there were further adjustments made for psychiatric illness,65 parental interest in a child’s education,51 or the quality and care of a household.52 These potential explanatory factors are worthy of further investigation, particularly as two of these (smoking and alcohol consumption) are important risk factors for various chronic diseases.
Future directions
The present meta-analysis was unable to consider cause of death in the intelligence–mortality association, but this would seem an important area for future systematic review, particularly as it was likely to have driven the stronger effect sizes of cohorts followed to younger ages in adulthood. For example, it may be that intelligence has a stronger relation to mortality caused by external events such as accidents,54 more prevalent among younger adults, than cause-specific mortalities more typical in later life.4 Studies have already replicated the inverse association between premorbid intelligence and cardiovascular disease-related mortality, with increased effect size magnitudes for coronary heart disease-related deaths3,75–78 compared with stroke-related deaths.75,77,78 The relationship between childhood cognitive ability and risk of cancer mortality is also likely to vary by type.4 For example, smoking-related cancers might carry a stronger association with intelligence4,79,80 than other cancer types.79 Specific causes of death are therefore likely to be crucial in providing explanations as to why intelligence predicts life expectancy, and larger cohorts with increased numbers of cause-specific mortalities will help to clarify this issue.
In the present study we found that education and social position in adulthood are factors that may help to account for the intelligence–all-cause mortality association. However, the extent to which these SES indicators act as partial surrogates for intelligence, or mediators and/or confounders of the intelligence–mortality association requires formal testing. Future longitudinal studies of mortality risk with repeated measures of intelligence, education, and adult SES, spanning childhood to adulthood could contribute to do this. Twin studies to determine the extent to which intelligence shares genetic and environmental causes with health, education, and social class, in predicting mortality, will also help to inform this issue. With evidence of associations between cognitive performance and education showing substantial heritability,81,82 it is possible that these variables may share some genetic effects in predicting death.
Although early-life SES did not help to explain the intelligence–mortality association and birthweight is another unlikely confounder, future studies could explore alternative early-life variables, in particular the intrauterine environment, and how these might simultaneously determine neurological and physiological integrity, in interaction with genetic influences, leading to lifelong effects on cognition and health.
Funding
The work was supported by The University of Edinburgh Centre for Cognitive Ageing and Cognitive Epidemiology, part of the cross council Lifelong Health and Wellbeing Initiative (G0700704/84698). Funding from the Biotechnology and Biological Sciences Research Council (BBSRC); Engineering and Physical Sciences Research Council (EPSRC); Economic and Social Research Council (ESRC); and Medical Research Council (MRC) is gratefully acknowledged; Wellcome Trust Fellowship (WBS Code U.1300.00.006.00012.01) to D.B. The Medical Research Council (MRC) Social and Public Health Sciences Unit receives funding from the MRC and the Chief Scientist Office at the Scottish Government Health Directorates.
Acknowledgements
The authors thank the following contributors for providing additional analyses of their data: Tomas Hemmingsson, Tracey Holsinger, Diana Kuh, Anton Lager, Laurie Martin, Merete Osler, Brian O’Toole and Denny Vågerö.
Conflict of interest: None declared.
KEY MESSAGES.
Higher intelligence test scores measured in youth are associated with the reduced risk of mortality by mid-to-late adulthood.
The intelligence–mortality association does not appear to be confounded by gender or early-life socio-economic inequalities.
Adult SES and education attenuate the intelligence–mortality association by a third and a half, respectively. Improved study design can contribute to a better understanding of the mechanisms involved in this effect, including: lifetime repeated measures of intelligence and SES indicators; detailed early-life and adult covariate data, and specific causes of mortality; twin studies that estimate the environmental and genetic contributions to intelligence–SES–mortality associations.
References
- 1.Batty GD, Deary IJ, Gottfredson LS. Premorbid (early life) IQ, and later mortality risk: systematic review. Ann Epidemiol. 2007;17:278–88. doi: 10.1016/j.annepidem.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 2.Leon DA, Lawlor DA, Clark H, Batty GD, Macintyre S. The association of childhood intelligence with mortality risk from adolescence to middle age: findings from the Aberdeen Children of the 1950s cohort study. Intelligence. 2009;37:520–28. [Google Scholar]
- 3.Batty GD, Wennerstad KM, Smith GD, et al. IQ in early adulthood and mortality by middle age: Cohort study of 1 million Swedish men. Epidemiology. 2009;20:100–9. doi: 10.1097/EDE.0b013e31818ba076. [DOI] [PubMed] [Google Scholar]
- 4.Hart CL, Taylor MD, Davey Smith G, et al. Childhood IQ, social class, deprivation, and their relationships with mortality and morbidity risk in later life: Prospective observational study linking the Scottish Mental Survey 1932 and the Midspan studies. Psychom Med. 2003;65:877–83. doi: 10.1097/01.psy.0000088584.82822.86. [DOI] [PubMed] [Google Scholar]
- 5.Arvanitakis Z, Wilson RS, Bennett DA. Diabetes mellitus, dementia, and cognitive function in older persons. J Nutr Health Aging. 2006;10:287–91. [PubMed] [Google Scholar]
- 6.Comijs HC, Kriegsman DM, Dik MG, Deeg DJ, Jonker C, Stalman WA. Somatic chronic diseases and 6-year change in cognitive functioning among older persons. Arch Gerontol Geriatr. 2009;48:191–96. doi: 10.1016/j.archger.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Kivipelto M, Nganda T, Fratiglioni L, et al. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol. 2005;62:1556–60. doi: 10.1001/archneur.62.10.1556. [DOI] [PubMed] [Google Scholar]
- 8.Okereke OI, Kang JH, Cook NR, et al. Type 2 diabetes mellitus and cognitive decline in two large cohorts of community-dwelling older adults. J Am Geriatr Soc. 2008;56:1028–36. doi: 10.1111/j.1532-5415.2008.01686.x. [DOI] [PubMed] [Google Scholar]
- 9.Rafnsson SB, Deary IJ, Smith FB, Whiteman MC, Fowkes FGR. Cardiovascular diseases and decline in cognitive function in an elderly community population: the Edinburgh Artery study. Psychom Med. 2007;69:425–34. doi: 10.1097/psy.0b013e318068fce4. [DOI] [PubMed] [Google Scholar]
- 10.Yaffe K, Kanaya A, Lindquist K, et al. The metabolic syndrome, inflammation, and risk of cognitive decline. J Am Med Assoc. 2004;292:2237–42. doi: 10.1001/jama.292.18.2237. [DOI] [PubMed] [Google Scholar]
- 11.Deary IJ. Introduction to the special issue on cognitive epidemiology. Intelligence. 2009;37:517–19. [Google Scholar]
- 12.Deary IJ, Batty GD. Cognitive epidemiology. J Epidemiol Community Health. 2007;61:378–84. doi: 10.1136/jech.2005.039206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anand SS, Islam S, Rosengren A, et al. Risk factors for myocardial infarction in women and men: Insights from the INTERHEART study. Eur Heart J. 2008;29:932–40. doi: 10.1093/eurheartj/ehn018. [DOI] [PubMed] [Google Scholar]
- 14.Schenck-Gustafsson K. Risk factors for cardiovascular disease in women. Maturitas. 2009;63:186–90. doi: 10.1016/j.maturitas.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 15.Davey Smith G, Lynch J. Life course approaches to socioeconomic differentials in health. In: Kuh D, Shlomo YB, editors. A Life Course Approach to Chronic Disease Epidemiology. Oxford: Oxford University Press; 2004. pp. 77–115. [Google Scholar]
- 16.Gallo LC, Espinosa de los Monteros K, Shivpuri S. Socioeconomic status and health: What is the role of reserve capacity? Curr Dir Psychol Sci. 2009;18:269–74. doi: 10.1111/j.1467-8721.2009.01650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Macintyre S. Social inequalities in health in the contemporary world: comparative overview. In: Strickland SS, Shetty PS, editors. Human Biology and Social Inequality: 39th Symposium Volume of the Society for the Study of Human Biology. Cambridge: Cambridge University Press; 1998. pp. 20–35. [Google Scholar]
- 18.Marmot M. The Marmot review. Strategic review of health inequalities in England post-2010. (11 February 2010, date last accessed) http://www.marmot-review.org.uk. [Google Scholar]
- 19.Lawlor DA, Batty GD, Morton SMB, et al. Early life predictors of childhood intelligence: evidence from the Aberdeen children of the 1950s study. J Epidemiol Community Health. 2005;59:656–63. doi: 10.1136/jech.2004.030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McLoyd VC. Socioeconomic disadvantage and child development. Am Psychol. 1998;53:185–204. doi: 10.1037//0003-066x.53.2.185. [DOI] [PubMed] [Google Scholar]
- 21.Deary IJ, Strand S, Smith P, Fernandes C. Intelligence and educational achievement. Intelligence. 2007;35:13–21. [Google Scholar]
- 22.Schmidt FL, Hunter J. General mental ability in the world of work: Occupational attainment and job performance. J Pers Soc Psychol. 2004;86:162–73. doi: 10.1037/0022-3514.86.1.162. [DOI] [PubMed] [Google Scholar]
- 23.Torssander J, Erikson R. Stratification and mortality – a comparison of education, class, status, and income. European Sociological Review. 2009;26:465–74. [Google Scholar]
- 24.DeWalt DA, Berkman ND, Sheridan S, Lohr KA, Pignone MP. Literacy and health outcomes: a review of the literature. J Gen Intern Med. 2004;19:1228–39. doi: 10.1111/j.1525-1497.2004.40153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huisman M, Kunst AE, Bopp M, et al. Educational inequalities in cause-specific mortality in middle-aged and older men and women in eight western European populations. Lancet. 2005;365:493–500. doi: 10.1016/S0140-6736(05)17867-2. [DOI] [PubMed] [Google Scholar]
- 26.Lleras-Muney A. The relationship between education and adult mortality in the United States. Rev Econ Stud. 2005;72:189–221. [Google Scholar]
- 27.Kilgour AH, Starr JM, Whalley LJ. Associations between childhood intelligence (IQ), adult morbidity and mortality. Maturitas. 2009;65:98–105. doi: 10.1016/j.maturitas.2009.09.021. [DOI] [PubMed] [Google Scholar]
- 28.WHO Study Group. Prevention in Childhood and Youth of Adult Cardiovascular Diseases: Time for Action (Technical Report Series 792) Geneva: World Health Organization; 1990. [PubMed] [Google Scholar]
- 29.Greenland S. Interpretation and choice of effect measures in epidemiologic analyses. Am J Epidemiol. 1987;125:761–8. doi: 10.1093/oxfordjournals.aje.a114593. [DOI] [PubMed] [Google Scholar]
- 30.Symons MJ, Moore DT. Hazard rate ratio and epidemiological studies. J Clin Epidemiol. 2002;55:893–99. doi: 10.1016/s0895-4356(02)00443-2. [DOI] [PubMed] [Google Scholar]
- 31.Chinn S. A simple method for converting an odds ratio to effect size for use in meta-analysis. Stat Med. 2000;19:3127–31. doi: 10.1002/1097-0258(20001130)19:22<3127::aid-sim784>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 32.Sutton AJ, Abrams KR, Jones DR, Sheldon TA, Song F. Methods for Meta-analysis in Medical Research. Chichester, UK: John Wiley & Sons; 2000. [Google Scholar]
- 33.Bax L, Yu LM, Ikeda N, Tsurata N, Moons KGM. MIX: Comprehensive Free Software for Meta-analysis of Causal Research Data. Version 1.5, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hart CL, Taylor MD, Smith GD, et al. Childhood IQ and all-cause mortality before and after age 65: prospective observational study linking the Scottish Mental Survey 1932 and the Midspan studies. Br J Health Psychol. 2005;10:153–65. doi: 10.1348/135910704X14591. [DOI] [PubMed] [Google Scholar]
- 35.Holsinger T, Helms M, Plassman B. Intelligence in early adulthood and life span up to 65 years later in male elderly twins. Age Ageing. 2007;36:286–291. doi: 10.1093/ageing/afm016. [DOI] [PubMed] [Google Scholar]
- 36.Martin LT, Kubzansky LD. Childhood cognitive performance and risk of mortality: A prospective cohort study of gifted individuals. Am J Epidemiol. 2005;162:887–90. doi: 10.1093/aje/kwi300. [DOI] [PubMed] [Google Scholar]
- 37.Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: Guidelines on choice of axis. J Clin Epidemiol. 2001;54:1046–55. doi: 10.1016/s0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 38.Deary IJ, Whalley LJ, Starr JM. IQ at Age 11 and Longevity: Results from a Follow-up of the Scottish Mental Survey 1932. Berlin: Springer; 2003. [Google Scholar]
- 39.Furu M, Lingarde F, Ljung B-O, Munck I, Kristenson H. Premature Death, Cognitive Ability and Socioeconomic Background. Stockholm: Stockholm Institute of Education; 1984. [Google Scholar]
- 40.Link BG, Phelan JC, Miech R, Westin EL. The resources that matter: Fundamental social causes of health disparities and the challenge of intelligence. J Health Soc Behav. 2008;49:72–91. doi: 10.1177/002214650804900106. [DOI] [PubMed] [Google Scholar]
- 41.Batty GD, Gale CR, Mortensen LH, Langenberg C, Shipley MJ, Deary IJ. Pre-morbid intelligence, the metabolic syndrome and mortality: The Vietnam Experience Study. Diabetologia. 2008;51:436–43. doi: 10.1007/s00125-007-0908-5. [DOI] [PubMed] [Google Scholar]
- 42.Batty GD, Mortensen LH, Gale CR, Deary IJ. Is low IQ related to risk of death by homicide? Testing a hypothesis using data from the Vietnam Experience Study. Psychiatr Res. 2008;161:112–15. doi: 10.1016/j.psychres.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 43.Batty GD, Shipley MJ, Gale CR, Mortensen LH, Deary IJ. Does IQ predict total and cardiovascular disease mortality as strongly as other risk factors? Comparison of effect estimates using the Vietnam Experience Study. Heart. 2008;94:1541–44. doi: 10.1136/hrt.2008.149567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deary IJ, Whiteman MC, Starr JM, Whalley LJ, Fox HC. The impact of childhood intelligence on later life: Following up the Scottish mental surveys of 1932 and 1947. J Pers Soc Psychol. 2004;86:130–47. doi: 10.1037/0022-3514.86.1.130. [DOI] [PubMed] [Google Scholar]
- 45.Hemmingsson T, Melin B, Allebeck P, Lundberg I. Cognitive ability in adolescence and mortality in middle age: A prospective life course study. J Epidemiol Community Health. 2009;63:697–702. doi: 10.1136/jech.2008.079160. [DOI] [PubMed] [Google Scholar]
- 46.Kuh D, Richards M, Hardy R, Butterworth S, Wadsworth ME. Childhood cognitive ability and deaths up until middle age: A post-war birth cohort study. Int J Epidemiol. 2004;33:408–13. doi: 10.1093/ije/dyh043. [DOI] [PubMed] [Google Scholar]
- 47.Starr JM, Deary IJ, Whalley LJ. All-cause mortality in the Aberdeen 1921 birth cohort: Effects of socio-demographic, physical and cognitive factors. BMC Pub Health. 2008;8:307. doi: 10.1186/1471-2458-8-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vagero D, Modin B. Commentary: The associations between height, cognition, and education and their relevance for health studies. Int J Epidemiol. 2006;35:663–64. doi: 10.1093/ije/dyl087. [DOI] [PubMed] [Google Scholar]
- 49.Weiss A, Gale CR, Batty GD, Deary IJ. Emotionally stable, intelligent men live longer: The Vietnam Experience Study cohort. Psychom Med. 2009;71:385–94. doi: 10.1097/PSY.0b013e318198de78. [DOI] [PubMed] [Google Scholar]
- 50.Hemmingsson T, Melin B, Allebeck P, Lundberg I. The association between cognitive ability measured at ages 18–20 and mortality during 30 years of follow-up–a prospective observational study among Swedish males born 1949–51. Int J Epidemiol. 2006;35:665–70. doi: 10.1093/ije/dyi321. [DOI] [PubMed] [Google Scholar]
- 51.Jokela M, Batty GD, Deary IJ, Gale CR, Kivimaki M. Low childhood IQ and early adult mortality: the role of explanatory factors in the 1958 British birth cohort. Pediatrics. 2009;124:e380–88. doi: 10.1542/peds.2009-0334. [DOI] [PubMed] [Google Scholar]
- 52.Kuh D, Shah I, Richards M, Mishra G, Wadsworth M, Hardy R. Do childhood cognitive ability or smoking behaviour explain the influence of lifetime socio-economic conditions on premature adult mortality in a British post war birth cohort? Soc Sci Med. 2009;68:1565–73. doi: 10.1016/j.socscimed.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lager A, Bremberg S, Vagero D. The association of early IQ and education with mortality: 65 year longitudinal study in Malmo, Sweden. BMJ. 2009;339:b5282. doi: 10.1136/bmj.b5282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O'Toole BI, Adena MA, Jones MP. Risk factors for mortality in Australian Vietnam-era national servicemen: A case-control study. Community Health Stud. 1988;12:408–17. doi: 10.1111/j.1753-6405.1988.tb00607.x. [DOI] [PubMed] [Google Scholar]
- 55.Whalley LJ, Deary IJ. Longitudinal cohort study of childhood IQ and survival up to age 76. BMJ. 2001;322:819. doi: 10.1136/bmj.322.7290.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Deary IJ, Batty GD, Pattie A, Gale CR. More intelligent, more dependable children live longer: A 55-year longitudinal study of a representative sample of the Scottish nation. Psychol Sci. 2008;19:874–80. doi: 10.1111/j.1467-9280.2008.02171.x. [DOI] [PubMed] [Google Scholar]
- 57.Wright RE. A factor analysis of the original Stanford-Binet scale. Psychometrika. 1939;4:209–20. [Google Scholar]
- 58.Thomson GH. What are Moray House Tests? London, UK: University of London Press; 1940. [Google Scholar]
- 59.Pearce MS, Deary IJ, Young AH, Parker L. Childhood IQ and deaths up to middle age: The Newcastle Thousand Families Study. Public Health. 2006;120:1020–26. doi: 10.1016/j.puhe.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 60.Pearce MS, Deary IJ, Young AH, Parker L. Growth in early life and childhood IQ at age 11 years: The Newcastle Thousand Families Study. Int J Epidemiol. 2005;34:673–77. doi: 10.1093/ije/dyi038. [DOI] [PubMed] [Google Scholar]
- 61.Osler M, Andersen AM, Due P, Lund R, Damsgaard MT, Holstein BE. Socioeconomic position in early life, birth weight, childhood cognitive function, and adult mortality: A longitudinal study of Danish men born in 1953. J Epidemiol Community Health. 2003;57:681–86. doi: 10.1136/jech.57.9.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Härnqvist K. Relative changes in intelligence from 13 to 18: II Results. Scand J Psychol. 1968;9:65–82. doi: 10.1111/j.1467-9450.1968.tb00519.x. [DOI] [PubMed] [Google Scholar]
- 63.Jokela M, Elovainio M, Singh-Manoux A, Kivimaki M. IQ, socioeconomic status, and early death: The US National Longitudinal Survey of Youth. Psychom Med. 2009;71:322–28. doi: 10.1097/PSY.0b013e31819b69f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Herrnstein RJ, Murray C. The Bell Curve. New York: The Free Press; 1994. [Google Scholar]
- 65.Batty GD, Shipley MJ, Mortensen LH, et al. IQ in late adolescence/early adulthood, risk factors in middle age and later all-cause mortality in men: the Vietnam Experience Study. J Epidemiol Community Health. 2008;62:522–31. doi: 10.1136/jech.2007.064881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Carroll JB. Human Cognitive Abilities: A Survey of Factor-analytic Studies. Cambridge, New York: Cambridge University Press; 1993. [Google Scholar]
- 67.Wingard DL. The sex differential in morbidity, mortality, and lifestyle. Ann Rev Pub Health. 1984;5:433–58. doi: 10.1146/annurev.pu.05.050184.002245. [DOI] [PubMed] [Google Scholar]
- 68.Corley J, Crang JA, Deary IJ. Childhood IQ and in-service mortality in Scottish army personnel during World War II. Intelligence. 2009;37:238–42. [Google Scholar]
- 69.Kelly E. The Scourge of Asian Flu: In Utero Exposure to Pandemic Influenza and the Development of a Cohort of British Children. Institute for Fiscal Studies, Working Paper 09/17, Sep. 2009, University College London. [Google Scholar]
- 70.Guo G, Harris KM. The mechanisms mediating the effects of poverty on children’s intellectual development. Demography. 2000;37:431–47. doi: 10.1353/dem.2000.0005. [DOI] [PubMed] [Google Scholar]
- 71.Batty GD, Deary IJ. Education and mortality: the role of intelligence. Lancet. 2005;365:1765–66. doi: 10.1016/S0140-6736(05)66573-7. [DOI] [PubMed] [Google Scholar]
- 72.Deary IJ, Johnson W. Intelligence and education: causal perceptions drive analytic processes and therefore conclusions. Int J Epidemiol. 2010;39:1362–69. doi: 10.1093/ije/dyq072. [DOI] [PubMed] [Google Scholar]
- 73.Geyer S, Hemstrom O, Peter R, Vågerö D. Education, income, and occupational class cannot be used interchangeably in social epidemiology. Empirical evidence against a common practice. J Epidemiol Community Health. 2006;60:804–10. doi: 10.1136/jech.2005.041319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Martikainen P, Blomgren J, Valkonen T. Change in the total and independent effects of education and occupational social class on mortality: analyses of all Finnish men and women in the period 1971–2000. J Epidemiol Community Health. 2007;61:499–505. doi: 10.1136/jech.2006.049940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Batty GD, Mortensen EL, Andersen AN, Osler M. Childhood intelligence in relation to adult coronary heart disease and stroke risk: Evidence from a Danish birth cohort study. Paediatr Perinat Epidemiol. 2005;19:452–59. doi: 10.1111/j.1365-3016.2005.00671.x. [DOI] [PubMed] [Google Scholar]
- 76.Batty GD, Shipley MJ, Mortensen LH, Gale CR, Deary IJ. IQ in late adolescence/early adulthood, risk factors in middle-age and later coronary heart disease mortality in men: the Vietnam Experience Study. Eur J Cardiovasc Prev Rehabil. 2008;15:359–61. doi: 10.1097/HJR.0b013e3282f738a6. [DOI] [PubMed] [Google Scholar]
- 77.Hemmingsson T, Essen JV, Melin B, Allebeck P, Lundberg I. The association between cognitive ability measured at ages 18-20 and coronary heart disease in middle age among men: a prospective study using the Swedish 1969 conscription cohort. Soc Sci Med. 2007;65:1410–19. doi: 10.1016/j.socscimed.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 78.Lawlor DA, Batty GD, Clark H, McIntyre S, Leon DA. Association of childhood intelligence with risk of coronary heart disease and stroke: findings from the Aberdeen Children of the 1950s cohort study. Eur J Epidemiol. 2008;23:695–706. doi: 10.1007/s10654-008-9281-z. [DOI] [PubMed] [Google Scholar]
- 79.Batty GD, Mortensen LH, Gale CR, Shipley M, Roberts B, Deary IJ. IQ in early adulthood, risk factors in middle age, and later cancer mortality in men: the Vietnam Experience Study. Psycho-Oncology. 2009;18:1122–26. doi: 10.1002/pon.1521. [DOI] [PubMed] [Google Scholar]
- 80.Taylor MD, Hart CL, Davey Smith G, et al. Childhood IQ and social factors on smoking behaviour, lung function and smoking-related outcomes in adulthood: Linking the Scottish Mental Survey 1932 and the Midspan studies. Br J Health Psychol. 2005;10:399–410. doi: 10.1348/135910705X25075. [DOI] [PubMed] [Google Scholar]
- 81.Bartels M, Rietveld MJH, Van Baal GCM, Boomsma DI. Heritability of educational achievement in 12-year-olds and the overlap with cognitive ability. Twin Res. 2002;5:544–53. doi: 10.1375/136905202762342017. [DOI] [PubMed] [Google Scholar]
- 82.Johnson W, McGue M, Iacono WG. Genetic and environmental influences on academic achievement trajectories during adolescence. Dev Psychology. 2006;42:514–32. doi: 10.1037/0012-1649.42.3.514. [DOI] [PubMed] [Google Scholar]