Abstract
Background Proteinuria has been recognized as a marker for an increased risk of chronic renal disease. It is unclear whether arsenic (As) exposure from drinking water is associated with proteinuria.
Methods We evaluated the association between As exposure from drinking water and proteinuria in 11 122 participants in the Health Effects of Arsenic Longitudinal Study (HEALS). Proteinuria was detected by urinary dipstick tests at baseline and at 2-year intervals. As exposure variables included baseline well As and changes in urinary As during follow-up modelled as time-dependent variables in the analyses.
Results At baseline, well As was positively related to prevalence of proteinuria; prevalence odds ratios (PORs) for proteinuria in increasing quintiles of well As (≤7, 8–39, 40–91, 92–179 and 180–864 µg/l) were 1.00 (ref), POR 0.99 [95% confidence interval (CI) 0.77–1.27], POR 1.23 (95% CI 0.97–1.57), POR 1.50 (95% CI 1.18–1.89) and POR 1.59 (95% CI 1.26–2.00) (P for trend <0.01). Hazard ratios for incidence of proteinuria were POR 0.83 (95% CI 0.67–1.03) and POR 0.91 (95% CI 0.74–1.12) for participants with a decreasing level of >70 and 17–70 µg/l in urinary As over time, respectively, and were POR 1.17 (95% CI 0.97–1.42) and POR 1.42 (95% CI 1.16–1.73) for participants with an increasing level of 16–68 and >68 µg/l in urinary As over time, respectively, compared with the group with relatively little changes in urinary As as the reference group (urinary As −16 to 15 µg/l).
Conclusion The findings suggest that there are adverse effects of As exposure on the risk of proteinuria and the effects are modifiable by recent changes in As exposure.
Keywords: Arsenic exposure, Bangladesh, proteinuria, environmental epidemiology
Introduction
Inorganic arsenic (As) exposure from drinking water has been linked to an elevated risk of internal cancers,1–6 diabetes7–9 and cardiovascular disease (CVD).10–14 Several studies with As exposure measured ecologically have reported a positive association between As exposure, non-malignant chronic kidney diseases, and microvascular diseases such as renal dysfunction.15–18 However, the evidence has not been well established because the studies have been limited to using ecological measures of the exposure and/or the disease. In addition, neither a biological mechanism nor the effect of low to moderate levels of exposure is clear.
Proteinuria, or albuminuria, is the presence of an excess of serum proteins in the urine due to damaged glomeruli, the tiny tufts of capillaries that filter blood in the kidney. Proteinuria has long been recognized as a marker for renal disease. Proteinuria is also a well-known predictor of poor renal outcomes in patients with type 2 diabetes and essential hypertension.19,20 Risk factors including glucose and cholesterol levels, body mass index (BMI) and blood pressure that are related to renal disease have also been independently associated with incident proteinuria.21 Studies of As exposure and markers of chronic kidney disease such as proteinuria in populations exposed to a wide range of As levels may extend our knowledge on the biological basis of the relationship between As exposure and renal disease.
It has been estimated that 13 million Americans have been exposed to 10–50 µg/l of As.22,23 In Bangladesh, more than 50 million people have been chronically exposed to drinking groundwater with As concentrations exceeding the WHO standard (10 µg/l).24 In 2000, we established a prospective cohort study of 11 746 individuals in Araihazar, Bangladesh, to assess As-related health effects. The average duration of exposure to well As levels assessed at baseline was 8 years.25 In addition, at baseline, we implemented an As mitigation program in the study area to promote well switching to safe wells, i.e. wells yielding water with an As concentration less than the Bangladesh standard of 50 µg/l. Within 2 years since baseline, a total of 58% of the 6512 participants with unsafe wells (As ≥50 µg) at baseline had responded by switching to other wells.26 The implementation of As mitigation interventions in the cohort provides a unique opportunity to evaluate the health effects related to changes in As exposure over time. With chronic and continuing exposure, steady-state concentrations of As in urine are achieved, and urinary As can serve as a good biomarker for long-term continuing exposure and for tracking changes in exposure due to intervention over time. In the present study, we conducted cross-sectional and cohort analyses to evaluate the associations of As exposure, measured both in water and repeated urinary samples, with the risk of proteinuria detected by urine dipstick.
Methods
Study participants
The Health Effects of Arsenic Longitudinal Study (HEALS) is an ongoing prospective cohort study in Araihazar, Bangladesh. Details of the study methodologies have been presented elsewhere.27,28 Briefly, prior to subject recruitment, water samples and geographic positional system (GPS) data were collected for 5966 contiguous wells in a well-defined geographical area of 25 km2 in Araihazar. Between October 2000 and May 2002, 11 746 men and women were recruited, with a participation rate of 97.5%.27 Information on demographic and lifestyle variables was collected using a standardized questionnaire at baseline and follow-up visits. Blood pressure was measured by trained clinicians using an automatic sphygmomanometer as previously described.29 Participants who had a self-reported physician diagnosis of diabetes prior to baseline were retrospectively identified from data collected at the first follow-up, as previously described.30 The comparison between self-reported diabetes status and test results for blood glycosylated haemoglobin (HbA1c) and glucosuria suggested validity of the questionnaire data.30
The cohort is being followed with in-person visits at 2-year intervals. The first 2-yearly follow-up visit took place between June 2002 and June 2004, and the second took place between September 2004 and November 2006. Verbal consent was obtained from study participants and the study procedures were approved by the Institutional Review Boards of Columbia University and University of Chicago and also the Ethical Committee of the Bangladesh Medical Research Council. The present study utilized data collected up to the second follow-up visit. A total of 888 participants had either died (n = 219), moved out of the study area (n = 504) or were lost (n = 165) during the follow-up.
A spot urine sample was collected in 50-ml acid-washed tubes from 95.6, 94.5 and 91.2% of the total cohort participants at baseline, first follow-up and second follow-up visits, respectively. Urine samples were kept in portable coolers immediately after collection. Within 2–8 h, urine samples were processed and transferred to −20°C freezers in the study office located in Dhaka city. All samples were kept frozen and shipped to Columbia University on dry ice within 1–2 months.
Proteinuria
At the time of baseline and each of the follow-up visits, dipstick urinalysis was performed by a trained physician on freshly evacuated spot urine samples collected from the participants using the Chemstrip Micral Test Strips (Roche Diagnostics, USA). The study physicians were blinded to urinary As and well As level.25 The results of the urine test were based on a colour scale that quantified proteinuria as absent, trace, ≥30, >100 and >500 mg/dl. In the present study, proteinuria was defined as a dipstick finding with trace, >30, >100 or >500 mg/dl protein in the urine.
As exposure measurements
Prior to baseline subject recruitment, water samples from all 5966 tube wells in the study area serving the source population were collected in 50-ml acid-washed tubes following well pumping for 5 min.31,32 Total As concentration was determined by graphite furnace atomic-absorption spectrometry (GFAA) with a Hitachi Z-8200 system at the Lamont–Doherty Earth observatory of Columbia University.31 Samples (21%) that fell below the detection limit of GFAA (5 μg/l) were subsequently analysed by inductively coupled plasma mass spectrometry (ICP-MS), which has a detection limit of 0.1 μg/l.33 All the samples were detectable by ICP-MS. Analyses for time-series samples collected from a sample of 20 tube wells in the study area showed that the As concentration in well water is relatively stable over time.33
Total urinary As concentration in urine samples collected at baseline and all the follow-up visits were measured by GFAA, using a Perkin-Elmer Analyst 600 graphite furnace system, as previously described.34 Urinary creatinine was analysed using a method based on the Jaffe reaction for adjustment of urinary total As concentration.35 In a random 10% of participants, inorganic As (AsV and AsIII) and its metabolites monomethylarsonate (MMA) to dimethylarsinate (DMA) accounted for 96% of total urinary As, whereas arsenobetaine and arsenocholine, derived mainly from dietary intakes of certain marine fish, together accounted for 3%. The correlation of well water As with total urinary As, DMA, and MMA concentrations were 0.70, 0.61 and 0.57, respectively, indicating a good relative validity of total urinary As as a reflection of As exposure from well water.
Statistical analyses
We first conducted descriptive analyses to compare participants with and without proteinuria at baseline, as well as participants who never had proteinuria at any of the follow-up visits, with respect to baseline characteristics. Pair-wise correlations for urinary As at each visit were calculated using urinary creatinine-adjusted As.
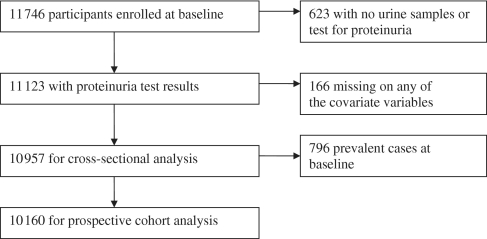
Unconditional logistic regression was used to evaluate the cross-sectional relationships of baseline well As and urinary As with the prevalence of proteinuria at baseline. We estimated prevalence odds ratios (PORs) for proteinuria in relation to As exposure levels, adjusting for potential confounding variables including age, gender, educational attainment, smoking status, BMI, systolic blood pressure (SBP) and diastolic blood pressure (DBP) and diabetes status.30 Effect estimates in relation to urinary As were additionally adjusted for urinary creatinine level. Urinary creatinine was adjusted by including it as a separate independent variable in the model, as recommended by Barr et al.36 and Gamble and Liu,37 to allow the statistical significance of other variables in the model to be independent of effects of creatinine concentration. There were 11 122 participants with a valid urine dipstick test at baseline (99.1% of those who gave a urine sample), of which 166 subjects were excluded because they were missing on at least one of the covariates of interest (Figure 1).
Figure 1.
Health effects of arsenic longitudinal study (HEALS). Covariate variables include urinary creatinine, age, gender, BMI, cigarette smoking status, education length, systolic blood pressure, diastolic blood pressure and diabetes status
Proteinuria is a well-known predictor of poor renal outcomes in patients with type 2 diabetes and essential hypertension19,20 and As exposure has been associated with both of these conditions. Therefore, in addition to control for diabetes status and both SBP and DBP, analyses were conducted with exclusion of individuals with high blood pressure (SBP ≥140 mmHg or DBP ≥90 mmHg) or individuals with history of diabetes at baseline. Among the first 2100 cohort participants, we measured HbA1c, a long-term marker of plasma glucose concentration. Among these individuals, we estimated PORs for proteinuria in relation to As exposure levels with adjustment for HbA1c and also with exclusion of participants with a high HbA1c level (>5.5%).
The prospective cohort analyses included 10 160 participants with a negative urine dipstick test at baseline and non-missing values on the covariates of interest (Figure 1). We considered a positive finding for proteinuria at any of the two follow-up visits as our primary outcome. Cox proportional hazard models, which have been used in other cohort studies with repeated dipstick tests over time,21 were used to compare the incidence of proteinuria across different levels of baseline well As, baseline urinary As, and change in urinary As since last visit. We estimated hazard ratios (HRs) for the association between arsenic exposure variables and proteinuria adjusting for the above-mentioned potential confounders. Specifically, change in urinary As since the last visit was modelled as a time-dependent variable because the value varied visit by visit. Baseline age, blood pressure, educational attainment, BMI and smoking status were treated as time-invariate variables.
Proteinuria status at follow-up was considered censored for those who died, moved, lost to follow-up or did not give urine samples at follow-up. We calculated person-years of observation from the date of baseline visit to the date of follow-up visit with the first positive testing for participants with proteinuria; to death date for those who had died; to the date of move (reported by close relatives or neighbours) for those who moved; and to the date of last follow-up visit for those who tested negative on all the dipstick tests. For the 165 subjects who were lost to follow-up, person-years of observation were considered from baseline to the midpoint between last visit and follow-up visit. We repeated the analyses excluding these subjects and the results did not change appreciably (data not shown). Separate dummy variables for the time-dependent urinary As variables were created for participants who did not give urine samples at first (n = 515) or second follow-up visit (n = 534). Analyses were also conducted for the 9096 participants free of proteinuria at baseline with urinary As values available at all three visits; since results were similar they are not shown. Seafood intakes were not related to urinary As in our population (Hall M, unpublished results). Additional analyses were conducted to control seafood intakes measured by a validated food frequency questionnaire designed for the study population.38 However, results were similar and therefore are not shown. Sensitivity analyses were also conducted using As measures that incorporated duration of well usages and amount of water consumption such as the time-weighted As concentration and the cumulative As index, as described previously.25 These measures were highly correlated with well As in the study population; the analysis results using these measures were similar and therefore are not presented. All analyses were conducted using the SAS 9.1.3 statistical package for Windows (SAS Institute Inc., Cary, NC, USA).
Results
The prevalence of proteinuria at baseline was 7.25%, toward the high end of prevalence that has been reported in the literature, ranging from 2.4 to 10% in different populations.39 The comparison of prevalent cases of proteinuria and non-cases at baseline showed that cases were older, had a higher level of BMI, educational attainment and blood pressure (Table 1). Compared with participants who did not have proteinuria during the follow-up period, incident cases were older and had a higher level of baseline blood pressure.
Table 1.
Baseline characteristics by proteinuria status
| Study population for cross-sectional analysis |
Study population for cohort analysis |
|||||
|---|---|---|---|---|---|---|
| Baseline variables | Non-cases of proteinuria at baseline (n = 10 160) | Prevalent cases of proteinuria (n = 796) | P-valuea | Never cases of proteinuria (n = 9130) | Incident cases of proteinuria (n = 1030) | P-valuea |
| Age in years, mean (SD) | 37.0 (10.0) | 39.2 (11.3) | <0.01 | 36.8 (10.0) | 38.4 (10.3) | 0.01 |
| BMI in kg/m2, mean (SD) | 19.7 (3.1) | 20.1 (3.7) | 0.01 | 19.7 (3.1) | 19.8 (3.4) | 0.32 |
| Education in years, mean (SD) | 3.5 (3.8) | 4.0 (4.1) | <0.01 | 3.5 (3.8) | 3.4 (3.8) | 0.71 |
| Male (%) | 43.4 | 43.1 | 0.88 | 43.6 | 41.3 | 0.15 |
| SBP in mmHg, mean (SD) | 114.5 (17.5) | 117.8 (22.4) | <0.01 | 114.4 (17.5) | 115.5 (17.6) | 0.05 |
| DBP in mmHg, mean (SD) | 73.8 (11.7) | 76.3 (13.4) | <0.01 | 73.7 (11.7) | 74.8 (12.0) | <0.01 |
| Urinary creatinine in mg/dl, mean (SD) | 56.6 (43.7) | 82.2 (59.5) | <0.01 | 56.0 (43.3) | 62.6 (46.3) | <0.01 |
| Smoking status | ||||||
| Past smokers (%) | 6.6 | 7.3 | 0.77 | 6.5 | 7.6 | 0.15 |
| Current smokers (%) | 29.2 | 28.8 | 29.4 | 27.6 | ||
| History of diabetes (%) | 1.6 | 2.6 | 0.04 | 1.4 | 3.5 | <0.01 |
| HbA1c (%)b | 4.9 | 5.3 | 0.02 | 4.9 | 5.0 | 0.14 |
aP-values for χ2 test or t-tests.
bBased on a subgroup of 2044 subjects.
In cross-sectional analyses, we found a dose–response relationship between baseline well As and proteinuria (Table 2); the PORs for proteinuria increased with increasing quintiles of baseline well As (P-value for trend <0.01). The ORs for proteinuria comparing the higher four quintiles with the bottom quintile of total urinary As were also elevated. However, there appears to be a threshold effect since the magnitude of the ORs was similar. Multivariate analysis was also performed in participants with no history of diabetes and in the subpopulation with longer term As exposure from the baseline well, defined as those consumed water from the baseline well for >5 years. The positive associations remained similar in these subgroup analyses (data not shown). For instance, among participants free of diabetes (n = 10936), the adjusted PORs for proteinuria were 1.00 (ref.), POR 1.03 (95% CI 0.80–1.33), POR 1.29 [95% confidence interval (CI) 1.01–1.66], POR 1.48 (95% CI 1.17–1.89) and POR 1.59 (95% CI 1.25–2.01) in increasing quintiles of well As. Among the 2100 cohort members with HbA1c data available, we estimated PORs for proteinuria with additional adjustment for HbA1c. Due to the limited sample size, we treated As exposure variable as a continuous variable and estimated PORs in relation to every 100-µg/l increase in baseline well As concentration. The POR for proteinuria was 1.18 (95% CI 1.03–1.36) before the adjustment and was 1.19 (95% CI 1.04–1.37) after controlling for HbA1c. Excluding participants with high level of HbA1c (>5.5%) did not make a difference in the estimate POR = 1.20 (95% CI 1.04–1.38). Exclusion of participants with high blood pressure also did not change the results appreciably (data not shown).
Table 2.
Cross-sectional analysis of associations between As exposure and proteinuria at baseline
| Overall population |
Subpopulation with ≥5 years of use of current well |
||||
|---|---|---|---|---|---|
| As exposure variablesa | Prevalence of proteinuria in %, n (Yes/No) | Creatinine- adjusted OR (95% CI) for proteinuriab | Adjusted OR (95% CI) for proteinuriac | Prevalence of proteinuria in %, n (Yes/No) | Adjusted OR (95% CI) for proteinuriac |
| Baseline well As (μg/l) | |||||
| 0.1–7d | 5.87 (129/2067) | 1.00 (Ref) | 1.00 (Ref) | 5.87 (79/1267) | 1.00 (Ref) |
| 8–39 | 5.82 (130/2105) | 0.99 (0.77–1.27) | 1.01 (0.79–1.31) | 5.32 (75/1334) | 0.94 (0.67–1.31) |
| 40–91 | 7.13 (155/2019) | 1.23 (0.97–1.57) | 1.33 (1.04–1.70) | 7.63 (100/1211) | 1.45 (1.06-1–0.99) |
| 92–179 | 8.54 (184/1971) | 1.50 (1.18–1.89) | 1.54 (1.22–1.96) | 9.82 (135/1240) | 1.87 (1.40–2.52) |
| 180–864 | 9.02 (198/1998) | 1.59 (1.26–2.00) | 1.65 (1.31–2.09) | 9.63 (129/1210) | 1.82 (1.35–2.44) |
| P-value for trend | <0.01 | <0.01 | <0.01 | ||
| Baseline urinary As (μg/l) | |||||
| 1–36d | 3.83 (86/2160) | 1.00 (Ref) | 1.00 (Ref) | 3.20 (44/1329) | 1.00 (Ref) |
| 37–66 | 6.49 (139/2004) | 1.46 (1.11–1.93) | 1.48 (1.12–1.96) | 6.76 (89/1228) | 1.87 (1.28–2.72) |
| 67–114 | 7.82 (171/2017) | 1.59 (1.22–2.10) | 1.65 (1.25–2.16) | 8.18 (109/1223) | 2.07 (1.43–2.99) |
| 115–205 | 8.09 (178/2022) | 1.47 (1.11–1.94) | 1.53 (1.16–2.02) | 8.58 (117/1246) | 1.94 (1.34–2.82) |
| ≥206 | 10.19 (222/1957) | 1.52 (1.15–2.01) | 1.65 (1.24–2.20) | 11.40 (159/1236) | 2.36 (1.62–3.44) |
| P-value for trend | 0.11 | 0.02 | 0.03 | ||
aCut points were determined by quintiles of overall study population.
bORs were adjusted for urinary creatinine.
cORs were adjusted for urinary creatinine, age, gender, BMI, cigarette smoking status, education length, SBP, DBP and diabetes status.
dReference group.
CI, confidence interval.
Over the 4-year follow-up period, among those who were free of proteinuria and who gave a urine sample at all three visits (n = 9224), the correlation of baseline urinary As with urinary As at follow-up visits decreased from 0.66 for the first follow-up to 0.60 for the second follow-up. Baseline well As was strongly correlated with baseline urinary As (r = 0.70, P < 0.01), and moderately correlated with urinary As at the first follow-up (r = 0.49, P < 0.01) and urinary As at the second follow-up (r = 0.45, P < 0.01). Baseline well As was inversely associated with the changes in urinary As (r = −0.18, P < 0.01) between baseline first follow-up visit (r = 0.18, P < 0.01) but was not related to the changes in urinary As between first and second follow-up (r = −0.04, P = 0.18).
There was no apparent association of either baseline well As or baseline urinary As with the incidence of proteinuria (Table 3). We calculated the difference in total urinary As between visits. Cut points in the analyses were determined using the quintile of changes in urinary As from baseline to the first follow-up visit. There was a positive association between changes in urinary As since last visit and incidence of proteinuria. Participants with an increase of > 68 µg/l in urinary As since last visit had a 42% increase in risk of having a positive dipstick testing for proteinuria (HR = 1.42; 95% CI 1.16–1.73), compared with those with a relatively stable urinary As level. Participants with a decrease of >70 µg/l in urinary As since last visit, on the other hand, had a 17% reduction in risk of proteinuria (HR = 0.83; 95% CI 0.67–1.03). We also conducted the same analyses among those consumed water from the baseline well for >5 years. The results were similar and therefore are not shown.
Table 3.
Prospective analysis of associations between As exposure and incident proteinuria at follow-up
| As variables | n/person- years | HR for incident proteinuriaa |
|---|---|---|
| Baseline well As (μg/l) | ||
| 0.1–7 | 250/7106 | 1.00 (ref) |
| 8–39 | 209/7177 | 0.84 (0.70–1.01) |
| 40–91 | 187/7023 | 0.79 (0.65–1.07) |
| 92–179 | 197/7159 | 0.85 (0.70–1.04) |
| 180–864 | 187/7049 | 0.84 (0.69–1.06) |
| P-value for trend | 0.15 | |
| Baseline urinary As (μg/l) | ||
| 1–36 | 193/6850 | 1.00 (ref) |
| 37–66 | 196/6338 | 1.00 (0.81–1.22) |
| 67–114 | 200/6607 | 0.94 (0.76–1.15) |
| 115–205 | 207/6970 | 0.90 (0.72–1.12) |
| ≥206 | 234/8749 | 0.88 (0.69–1.08) |
| P-value for trend | 0.12 | |
| Change in urinary As since last visit (μg/l) | ||
| <−70 | 170/6991 | 0.84 (0.67–1.04) |
| −70 to −17 | 168/6716 | 0.91 (0.74–1.12) |
| −16 to 15 | 211/7862 | 1.00 (ref) |
| 16 to 68 | 231/7016 | 1.17 (0.97–1.42) |
| ≥69 | 250/6929 | 1.43 (1.17–1.74) |
| P-value for trend | <0.01 |
aHRs were adjusted for age, gender, BMI, cigarette smoking status, education length, SBP, DBP, diabetes status and urinary creatinine measured at each visit in the model. HRs associated with baseline well and urinary As were adjusted for change in urinary As since last visit. HRs associated with change in urinary As since last visit were adjusted for baseline well As.
Discussion
To our knowledge, the present study is the first large epidemiological study that has examined the relationship between As exposure from drinking water and proteinuria. We found a positive relationship between baseline As exposure and the prevalence of proteinuria in cross-sectional analyses of baseline data. In prospective cohort analyses, we found positive associations between changes in total urinary As over time and incidence of proteinuria.
The concentration of total As in urine has previously been shown to be an excellent biomarker of As exposure in this cohort.40 The correlation between total urinary As and water As was >0.7040 and our previous work has shown that urinary As is as strongly predictive as well As of As-induced skin lesions,27,41 a hallmark of chronic As poisoning. Therefore, urinary As changes were used as a measure of the changes in As exposure status over time.26 The cross-sectional analyses showed that the PORs for proteinuria in relation to As exposure did not differ in the subpopulation with water consumption from the baseline well for ≥5 years (Table 2), suggesting an effect of long-term exposure to baseline wells. As previously described, an As mitigation program with health education, well labelling and installations of deep wells was initiated in the study area since baseline, which led to changes in urinary As among some cohort participants.26 The absence of an association between baseline As exposure status and proteinuria incidence during follow-up and the positive association between changes in urinary As and proteinuria incidence together suggest that the effect of recent changes in As exposure overrode the effect of As exposure from the more distant past (>4 years ago). The findings indicate that there may be an adverse effect of As exposure on renal function indicated by proteinuria status, and that the effect is modifiable by changes in As exposure status.
Several studies have reported a positive association between As exposure and non-malignant renal disease.15–17,42. For instance, a standardized mortality ratio (SMR) analysis in a six-county study area of southeastern Michigan, found a positive association between county-level mean As concentrations and kidney disease.15 SMR analyses in an area in Taiwan with high As exposure showed that mortality from renal disease declined gradually after provision of As-free drinking water. However, the ecological study design and the group level measures of both exposure and the disease limit the interpretation of these results.16 It is not clear whether the findings were affected by the measurement error of the ecological measure of As exposure or confounding factors not controlled in the study. In addition, previous studies did not exclude the possibility that the observed association is due to the effect of As exposure on blood pressure or type 2 diabetes. In our analyses, the association remained similar with the adjustment for blood pressure status and HbA1c, suggesting that the effect of As on proteinuria is independent of these factors. With the use of proteinuria as a biomarker of renal function, our study provides some evidence of the possible adverse effect of As exposure on renal disease and calls for future research on the topic.
Strengths of the study include the availability of multiple As exposure measures at the individual level, the use of both cross-sectional and prospective cohort analyses, and the large variation in exposure levels in the study population. The study also has several limitations. Using a simple urine dipstick screening method may lead to misclassification of proteinuria. Conditions such as alkaline urine, gross blood in the urine, accidental introduction of detergents to the urine collection and fever may lead to some false positive results.15 It is unlikely that these states are related to As exposure and therefore the misclassification should be non-differential. By regarding trace proteinuria as positives, the sensitivity of the urine protein dipstick test for micro- and macro-albuminuria can be improved, while its specificity is not changed.43 The use of multiple dipstick tests over time should also improve the overall sensitivity.
In conclusion, we found positive associations between long-term As exposure and the prevalence of proteinuria and between changes in urinary As since baseline and incidence of proteinuria. Proteinuria may be an early and easily detectable sign for renal impairment induced by As exposure. Future studies are needed to investigate the relationship between As exposure and renal dysfunction and its biomarkers such as β-2 microglobulin.
Funding
This research was funded by US National Institute of Health grants, P42ES010349, R01ES017541, R01CA102484, R01CA107431, R01ES017541, R01ESO11601, P30ES09089, CA016087, ES000260 and CA014599.
Conflict of interest: None declared.
KEY MESSAGES.
The relationship between As exposure from drinking water and the risk of proteinuria has not been well characterized.
In a Bangladeshi population with low to moderate levels of As exposure, a dose–response relationship between baseline well As concentration and the prevalence of proteinuria was observed.
During follow-up, increasing levels of urinary As were associated with an increased risk of proteinuria, and decreasing levels of urinary As were related to a reduced risk of proteinuria.
References
- 1.Chen CJ, Kuo TL, Wu MM. Arsenic and cancers. Lancet. 1988;1:414–15. doi: 10.1016/s0140-6736(88)91207-x. [DOI] [PubMed] [Google Scholar]
- 2.Chen CL, Hsu LI, Chiou HY, et al. Ingested arsenic, cigarette smoking, and lung cancer risk: a follow-up study in arseniasis-endemic areas in Taiwan. JAMA. 2004;292:2984–90. doi: 10.1001/jama.292.24.2984. [DOI] [PubMed] [Google Scholar]
- 3.Morales KH, Ryan L, Kuo TL, Wu MM, Chen CJ. Risk of internal cancers from arsenic in drinking water. Environ Health Perspect. 2000;108:655–61. doi: 10.1289/ehp.00108655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albert CM, Cook NR, Gaziano JM, et al. Effect of folic acid and B vitamins on risk of cardiovascular events and total mortality among women at high risk for cardiovascular disease: a randomized trial. JAMA. 2008;299:2027–36. doi: 10.1001/jama.299.17.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tseng CH, Chong CK, Chen CJ, Tai TY. Lipid profile and peripheral vascular disease in arseniasis-hyperendemic villages in Taiwan. Angiology. 1997;48:321–35. doi: 10.1177/000331979704800405. [DOI] [PubMed] [Google Scholar]
- 6.Simeonova PP, Hulderman T, Harki D, Luster MI. Arsenic exposure accelerates atherogenesis in apolipoprotein E(-/-) mice. Environ Health Perspect. 2003;111:1744–48. doi: 10.1289/ehp.6332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang SL, Chiou JM, Chen CJ, et al. Prevalence of non-insulin-dependent diabetes mellitus and related vascular diseases in southwestern arseniasis-endemic and nonendemic areas in Taiwan. Environ Health Perspect. 2003;111:155–60. doi: 10.1289/ehp.5457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tseng CH, Tseng CP, Chiou HY, Hsueh YM, Chong CK, Chen CJ. Epidemiologic evidence of diabetogenic effect of arsenic. Toxicol Lett. 2002;133:69–76. doi: 10.1016/s0378-4274(02)00085-1. [DOI] [PubMed] [Google Scholar]
- 9.Ebbing M, Bleie O, Ueland PM, et al. Mortality and cardiovascular events in patients treated with homocysteine-lowering B vitamins after coronary angiography: a randomized controlled trial. JAMA. 2008;300:795–804. doi: 10.1001/jama.300.7.795. [DOI] [PubMed] [Google Scholar]
- 10.Chen CJ, Chiou HY, Chiang MH, Lin LJ, Tai TY. Dose-response relationship between ischemic heart disease mortality and long-term arsenic exposure. Arterioscler Thromb Vasc Biol. 1996;16:504–10. doi: 10.1161/01.atv.16.4.504. [DOI] [PubMed] [Google Scholar]
- 11.Tseng CH, Chong CK, Tseng CP, et al. Long-term arsenic exposure and ischemic heart disease in arseniasis-hyperendemic villages in Taiwan. Toxicol Lett. 2003;137:15–21. doi: 10.1016/s0378-4274(02)00377-6. [DOI] [PubMed] [Google Scholar]
- 12.Wang CH, Jeng JS, Yip PK, et al. Biological gradient between long-term arsenic exposure and carotid atherosclerosis. Circulation. 2002;105:1804–09. doi: 10.1161/01.cir.0000015862.64816.b2. [DOI] [PubMed] [Google Scholar]
- 13.Chiou HY, Huang WI, Su CL, Chang SF, Hsu YH, Chen CJ. Dose-response relationship between prevalence of cerebrovascular disease and ingested inorganic arsenic. Stroke. 1997;28:1717–23. doi: 10.1161/01.str.28.9.1717. [DOI] [PubMed] [Google Scholar]
- 14.Liao YT, Li WF, Chen CJ, et al. Synergistic effect of polymorphisms of paraoxonase gene cluster and arsenic exposure on electrocardiogram abnormality. Toxicol Appl Pharmacol. 2009;239:178–83. doi: 10.1016/j.taap.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 15.Meliker JR, Wahl RL, Cameron LL, Nriagu JO. Arsenic in drinking water and cerebrovascular disease, diabetes mellitus, and kidney disease in Michigan: a standardized mortality ratio analysis. Environ Health. 2007;6:4. doi: 10.1186/1476-069X-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chiu HF, Yang CY. Decreasing trend in renal disease mortality after cessation from arsenic exposure in a previous arseniasis-endemic area in southwestern Taiwan. J Toxicol Environ Health A. 2005;68:319–27. doi: 10.1080/15287390590900804. [DOI] [PubMed] [Google Scholar]
- 17.Lewis DR, Southwick JW, Ouellet–Hellstrom R, Rench J, Calderon RL. Drinking water arsenic in Utah: a cohort mortality study. Environ Health Perspect. 1999;107:359–65. doi: 10.1289/ehp.99107359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lau AT, He QY, Chiu JF. A proteome analysis of the arsenite response in cultured lung cells: evidence for in vitro oxidative stress-induced apoptosis. Biochem J. 2004;382:641–50. doi: 10.1042/BJ20040224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mogensen CE. Microalbuminuria predicts clinical proteinuria and early mortality in maturity-onset diabetes. N Engl J Med. 1984;310:356–60. doi: 10.1056/NEJM198402093100605. [DOI] [PubMed] [Google Scholar]
- 20.Berrut G, Bouhanick B, Fabbri P, et al. Microalbuminuria as a predictor of a drop in glomerular filtration rate in subjects with non-insulin-dependent diabetes mellitus and hypertension. Clin Nephrol. 1997;48:92–97. [PubMed] [Google Scholar]
- 21.Jee SH, Boulware LE, Guallar E, Suh I, Appel LJ, Miller ER III. Direct, progressive association of cardiovascular risk factors with incident proteinuria: results from the Korea Medical Insurance Corporation (KMIC) study. Arch Intern Med. 2005;165:2299–304. doi: 10.1001/archinte.165.19.2299. [DOI] [PubMed] [Google Scholar]
- 22.Fact sheet about the January 2001 arsenic rule (EPA 815-F-00-015) http://www.epa.gov/safewater/arsenic/regulations_factsheet.html (3 January 2010, date last accessed)
- 23.Arsenic Occurrence in Public Drinking Water Supplies (EPA-815-R-00-023/December 2000) http://www.epa.gov/safewater/arsenic/pdfs/occurrence.pdf (3 January 2009, date last accessed)
- 24.British Geological Survey. Groundwater studies for arsenic contamination in Bangladesh-Phase 1 findings. 2007 http://www.bgs.ac.uk/arsenic/ (3 January 2010, date last accessed) [Google Scholar]
- 25.Ahsan H, Chen Y, Parvez F, et al. Arsenic exposure from drinking water and risk of premalignant skin lesions in Bangladesh: baseline results from the Health Effects of Arsenic Longitudinal Study. Am J Epidemiol. 2006;163:1138–48. doi: 10.1093/aje/kwj154. [DOI] [PubMed] [Google Scholar]
- 26.Chen Y, van Geen A, Graziano JH, et al. Reduction in urinary arsenic levels in response to arsenic mitigation efforts in Araihazar, Bangladesh. Environ Health Perspect. 2007;115:917–23. doi: 10.1289/ehp.9833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahsan H, Chen Y, Parvez F, et al. Health Effects of Arsenic Longitudinal Study (HEALS): description of a multidisciplinary epidemiologic investigation. J Expo Sci Environ Epidemiol. 2006;16:191–205. doi: 10.1038/sj.jea.7500449. [DOI] [PubMed] [Google Scholar]
- 28.Parvez F, Chen Y, Argos M, et al. Prevalence of arsenic exposure from drinking water and awareness of its health risks in a Bangladeshi population: results from a large population-based study. Environ Health Perspect. 2006;114:355–59. doi: 10.1289/ehp.7903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Y, Factor-Litvak P, Howe GR, et al. Arsenic exposure from drinking water, dietary intakes of B vitamins and folate, and risk of high blood pressure in Bangladesh: a population-based, cross-sectional study. Am J Epidemiol. 2007;165:541–52. doi: 10.1093/aje/kwk037. [DOI] [PubMed] [Google Scholar]
- 30.Bunderson M, Brooks DM, Walker DL, Rosenfeld ME, Coffin JD, Beall HD. Arsenic exposure exacerbates atherosclerotic plaque formation and increases nitrotyrosine and leukotriene biosynthesis. Toxicol Appl Pharmacol. 2004;201:32–39. doi: 10.1016/j.taap.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 31.van Geen A, Ahsan H, Horneman AH, et al. Promotion of well-switching to mitigate the current arsenic crisis in Bangladesh. Bull World Health Organ. 2002;80:732–77. [PMC free article] [PubMed] [Google Scholar]
- 32.van Geen A, Zheng Y, Versteeg R, et al. Spatial variability of arsenic in 6000 tube wells in a 25 km2 area of Bangladesh. Water Resour Res. 2003;39:1140. [Google Scholar]
- 33.Cheng Z, van Geen A, Seddique AA, Ahmed KM. Limited temporal variability of arsenic concentrations in 20 wells monitored for 3 years in Araihazar, Bangladesh. Environ Sci Technol. 2005;39:4759–66. doi: 10.1021/es048065f. [DOI] [PubMed] [Google Scholar]
- 34.Nixon DE, Mussmann GV, Eckdahl SJ, Moyer TP. Total arsenic in urine: palladium-persulfate vs nickel as a matrix modifier for graphite furnace atomic absorption spectrophotometry. Clin Chem. 1991;37:1575–79. [PubMed] [Google Scholar]
- 35.Slot C. Plasma creatinine determination. A new and specific Jaffe reaction method. Scand J Clin Lab Invest. 1965;17:381–87. doi: 10.3109/00365516509077065. [DOI] [PubMed] [Google Scholar]
- 36.Barr DB, Wilder LC, Caudill SP, Gonzalez AJ, Needham LL, Pirkle JL. Urinary creatinine concentrations in the US population: implications for urinary biologic monitoring measurements. Environ Health Perspect. 2005;113:192–200. doi: 10.1289/ehp.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gamble MV, Liu X. Urinary creatinine and arsenic metabolism. Environ Health Perspect. 2005;113:A442–43. doi: 10.1289/ehp.113-a442a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen Y, Ahsan H, Parvez F, Howe GR. Validity of a food-frequency questionnaire for a large prospective cohort study in Bangladesh. Br J Nutr. 2004;92:851–59. doi: 10.1079/bjn20041277. [DOI] [PubMed] [Google Scholar]
- 39.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 40.Hall M, Chen Y, Ahsan H, et al. Blood arsenic as a biomarker of arsenic exposure: results from a prospective study. Toxicology. 2006;225:225–33. doi: 10.1016/j.tox.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 41.Ahsan H, Chen Y, Kibriya MG, et al. Arsenic metabolism, genetic susceptibility, and risk of premalignant skin lesions in Bangladesh. Cancer Epidemiol Biomarkers Prev. 2007;16:1270–78. doi: 10.1158/1055-9965.EPI-06-0676. [DOI] [PubMed] [Google Scholar]
- 42.Hsueh YM, Chung CJ, Shiue HS, et al. Urinary arsenic species and CKD in a Taiwanese population: a case-control study. Am J Kidney Dis. 2009;54:859–70. doi: 10.1053/j.ajkd.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 43.Konta T, Hao Z, Takasaki S, et al. Clinical utility of trace proteinuria for microalbuminuria screening in the general population. Clin Exp Nephrol. 2007;11:51–55. doi: 10.1007/s10157-006-0458-z. [DOI] [PubMed] [Google Scholar]