Abstract
Objective:
Emblica (Phyllanthus emblica L.), an euphorbiaceous plant, is widely distributed in subtropical and tropical areas of India, China and Indonesia. The fruits possess antimicrobial, antioxidant, anti-inflammatory, analgesic and antipyretic properties. In the current article a new, simple, sensitive, selective, precise, and robust high-performance thin-layer chromatographic (HPTLC) method was developed and validated for the determination of gallic acid in dried fruit powder of Phyllanthus emblica.
Materials and Methods:
The quantitative determination of gallic acid was performed on TLC aluminium plates pre-coated with silica gel 60F-254 as the stationary phase. The linear ascending development was carried out in a twin trough glass chamber saturated with a mobile phase consisting of toluene: ethyl acetate: formic acid: methanol (3:3:0.8:0.2) at room temperature (25 ± 2°C). Camag TLC scanner III was used for spectrodensitometric scanning and analysis, in the absorbance mode, at 278 nm.
Results:
The linear regression analysis data for the calibration plots showed good linear relationship with r2 = 0.99977 in the concentration range of 40 – 240 ng spot—1, with respect to the peak area. According to the guidelines of the International Conference on Harmonization (ICH), the method was validated for precision, accuracy, and recovery.
Conclusion:
Statistical analysis of the data showed that the method was reproducible and selective for the estimation of gallic acid.
Keywords: Phyllanthus emblica, HPTLC fingerprinting, gallic acid
The fruits of Phyllanthus emblica Linn. (Euphorbiaceae), commonly known in India as amla (Sanskrit name amalaki), are consumed as fruit or in the form of food products. This fruit also forms an important constituent of many Ayurvedic preparations such as chyavanprash and triphala and is regarded as ‘one of the best rejuvenating herbs’. It is a medium-sized deciduous tree found throughout India.[1] The fruits are globular, fleshy, smooth, and striated, of yellowish-green color. They contain an obovate-obtusely triangular six-celled nut. Traditionally, the fruit is useful as an astringent, cardiac tonic, diuretic, laxative, livertonic, refrigerant, stomachic, restorative, alterative, antipyretic, anti-inflammatory, hair tonic, and digestive medicine.[1,2] It is used for a variety of ailments such as, anemia, hyperacidity, diarrhea, and eye inflammation, and anomalies of urine, leucorrhea, jaundice, nervine debility, liver complaints, and cough.[3,4] It is reported to have hepatoprotective, antioxidant, antimutagenic, cytoprotective, antitumor, antifungal, antimicrobial, hypolipidemic, and antiatherosclerotic effects.[4,5,6] The fruit contains two hydrolysable tannins Emblicanin A and B, which have antioxidant properties; one on hydrolysis gives gallic acid, ellagic acid, and glucose, whereas the other gives ellagic acid and glucose.[7,8]
The high-performance thin layer chromatographic (HPTLC) method for quantitation of gallic acid from the dried fruit of Phyllanthus emblica has not been reported. The goal of the present article is to validate and determine the content of gallic acid in the dried fruit extract of Phyllanthus emblica by using the HPTLC method. For this purpose, a new, simple, sensitive, precise, and robust HPTLC method was developed, to determine the gallic acid content in the dried fruit extract of Phyllanthus emblica Linn. The method was validated for precision, intraday precision, accuracy, limit of detection, and quantitation.
Materials and Methods
Reagents and materials
Phyllanthus emblica Linn. collected from Vashi (Mumbai) was authenticated by Dr. Vinayak Naik, Senior Research Scientist, Piramal Life Sciences Ltd, Mumbai, India. Analytical grade solvents were used. Standard gallic acid (98% pure) was procured from Sigma Aldrich Chemie (Steinheim, Germany). Silica gel 60F HPTLC pre-coated plates were procured from Merck (Darmstadt, Germany). The dried fruit powder of Phyllanthus emblica Linn. was extracted with methanol in several batches by using the Soxhlet apparatus. The extract thus obtained was concentrated with the help of a rotary vacuum evaporator.
Preparation of standard solution
A stock solution of gallic acid (1 mg/ml) was prepared by dissolving 10 mg of accurately weighed gallic acid in methanol and making up the volume to 10 ml with methanol. The stock solution was further diluted with methanol to give a standard solution of gallic acid (40 μg/ml). This concentration was used as the working standard for the HPTLC method.
Sample preparation
Accurately weigh 100 mg of methanolic extract of dried fruit powder of Phyllanthus emblica Linn. in a 50 ml volumetric flask. Dissolve in 10 ml of methanol by sonication and make up the volume with methanol. Pipette out 2 ml of this solution in a 10 ml volumetric flask and dilute up to the mark with methanol. The stock solution of the sample, having concentration of 0.4 mg/ml (0.4 μg/μl) is prepared thus. This concentration is used for the estimation of gallic acid from the dried fruit powder of the plant material.
All samples were filtered through a 0.22 μ membrane filter from Millipore (Malsheim, France).
Instrumentation and chromatographic conditions
HPTLC aluminium plates pre-coated with silica gel F60254 (10 × 10 cm) with 200 μm thickness (E. Merck, Germany) were used as the stationary phase. The plates were pre-washed with methanol and activated at 110°C for 10 minutes prior to chromatography. The samples were spotted in the form of bands, of 8 mm, with the help of a Camag 100 microliter syringe using a Camag Linomat V (Switzerland) sample applicator. A constant application rate of 100 nL s–1 was employed and the space between two bands was 12 mm. The slit dimension was kept at 6 mm × 0.45 mm, with a scanning speed of 20 mm/second, and a data resolution of 100 μm/step was employed. The composition of the mobile phase was toluene: ethyl acetate: formic acid: methanol (3:3:0.8:0.2). The linear ascending development was carried out in a twin trough glass chamber saturated with the mobile phase. The optimized chamber saturation time for the mobile phase was 30 minutes at room temperature (25 ± 2°C). The length of the chromatogram run was 80 mm. Subsequently, the plate was allowed to dry at room temperature. The separated bands on the HPTLC plates were scanned over the wavelength of 200 – 400 nm. The source of radiation utilized was the tungsten lamp (or deuterium Illumination). The maximum absorbance was found at 278 nm. The images were captured on Camag reprostar 3 with win-CATS software 4.05.
Calibration curve of gallic acid
A stock solution of gallic acid (0.04 μg/μl) was prepared in methanol. Different volumes of standard solution 1, 2, 3, 4, 5, 6 μl were spotted on the HPTLC plate, to obtain concentrations of 40, 80, 120, 160, 200, 240 ng/spot of gallic acid, respectively. The data of the peak areas plotted against the corresponding concentrations were treated by least-square regression analysis.
Method validation[9,10]
Precision
Repeatability of the sample application and measurement of the peak area were carried out using six replicates of the same spot (120 ng spot–1 of gallic acid) and were expressed in terms of percent relative standard deviation (%R.S.D.) and standard error (S.E.). The intra- and inter-day variation for the determination of gallic acid was carried out at three different concentration levels of 80, 120, and 160 ng spot–1.
Robustness of the method
By introducing small changes in the mobile phase composition, mobile phase volume, duration of mobile phase saturation, and activation of the pre-washed TLC plates with methanol, the effects on the results were examined. Robustness of the method was performed in triplicate at a concentration level of 120 ng spot–1 and the %R.S.D and S.E. of peak areas was calculated.
Limit of detection and limit of quantification
In order to estimate the limit of detection (LOD) and limit of quantitation (LOQ), blank methanol was spotted six times and the signal-to-noise ratio was determined. The LOD was considered as 3:1 and LOQ as 10:1. The LOD and LOQ were experimentally verified by diluting the known concentrations of gallic acid until the average responses were approximately thrice or 10 times the standard deviation of the responses for six replicate determinations.
Accuracy
The accuracy of the method was determined by the addition of standard compounds in the samples at three different levels (75, 100, and 125%), and the mixture was analyzed under optimized conditions. The accuracy was calculated from the test result as the percentage of analytes recovered by the assay.
Ruggedness
A solution of concentration 200 ng spot–1 was prepared and analyzed on day 0 and after 6, 12, 24, 48, and 72 hours. Data were treated for %R.S.D., to assess the ruggedness of the method.
Specificity
The specificity of the method was ascertained by analyzing the standard drug and extract. The spot for gallic acid in the sample was confirmed by comparing the Rf values and spectra of the spot with that of the standard. The peak purity of the gallic acid was assessed by comparing the spectra at three different levels, namely, the peak start (S), peak apex (M), and peak end (E) positions of the spot.
Results and Discussion
Development of the optimum mobile phase
The TLC procedure was used to develop the mobile phase of the plant extract. Both the standard and sample were spotted on the TLC plate and different individual solvents as well as a combination of solvents were tried, to get a good separation. Initially the solvent system used was butanol, glacial acetic acid, and water, in the ratio 4:1:1, but the plate was not well resolved. Next, ethyl acetate, formic acid, glacial acetic acid, and water in varying ratios was tried. The mobile phase toluene: ethyl acetate: formic acid: methanol (3:3:0.8:0.2) gave a good resolution with Rf = 0.40 for gallic acid [Figure 1]. Well-defined spots were obtained when the chamber was saturated with the mobile phase for 30 minutes, at room temperature. The Rf value of the plant extract was compared with the standard. The HPTLC plate pictures are shown a little later in the text, with the selected solvent system.
Figure 1.
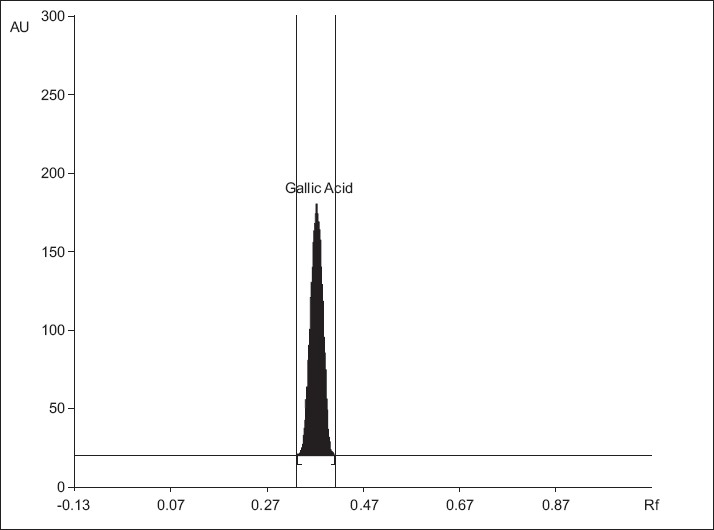
Chromatogram of standard gallic acid 200 ng spot-1, (Rf = 0.40 ± 0.01), mobile phase: toluene: ethyl acetate: formic acid: methanol (3:3:0.8:0.2)
Calibration curves of gallic acid
The calibration curves have been developed for gallic acid at a specific Rf value. The present HPTLC method has shown a calibration curve in the concentration range of 40 – 240 ng/spot for gallic acid. Figure 2 displays a three-dimensional image of the calibration samples at 278 nm. The regression analysis has shown good linear relationship with r2 > 0.99977 for gallic acid. The standard deviation of intercept has been found to be less than 2%. No significant difference has been observed in the slopes of the standard curves.
Figure 2.
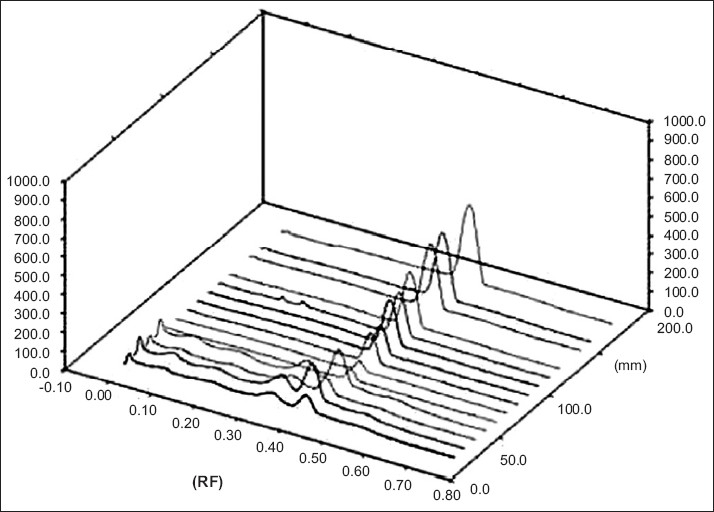
HPTLC three-dimensional image of the calibration curve of gallic acid from amla extract
Method validation
Repeatability of the method was validated in system and method precisions. System precision was measured for six spots of the gallic acid. The results of the precisions were expressed in % R.S.D and are summarized in [Table 1]. System and method precisions were found to be less than 2%. Intraday precision was determined by spotting the three different concentrations of gallic acid, six times a day. Intraday precisions results were expressed in % R.S.D. and dried fruit extract was found to be less than 2%. The accuracy was determined by the standard addition technique. Known amounts of the reference compound in three different levels (75, 100, 125%) were added to the sample (dried fruit extract) and chromatography was conducted under optimized conditions. The accuracy was then calculated from the test results as the percentage of analytes recovered by the assay. The results indicated that the accuracy of the method was very good, as supported by the recovery of 101.1 – 104.58%. [Table 2]. Good correlation was obtained between the standard and the sample overlain spectra of gallic acid Figure 3.
Table 1.
Intra- and inter-day precision of HPTLC method (n = 6)
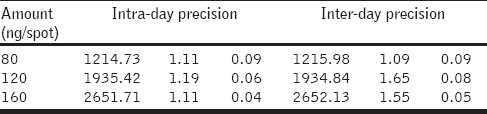
Table 2.
Recoveries study data (n = 6)
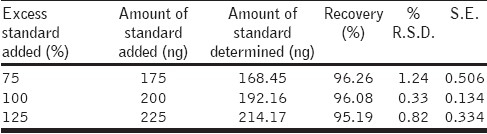
Figure 3.
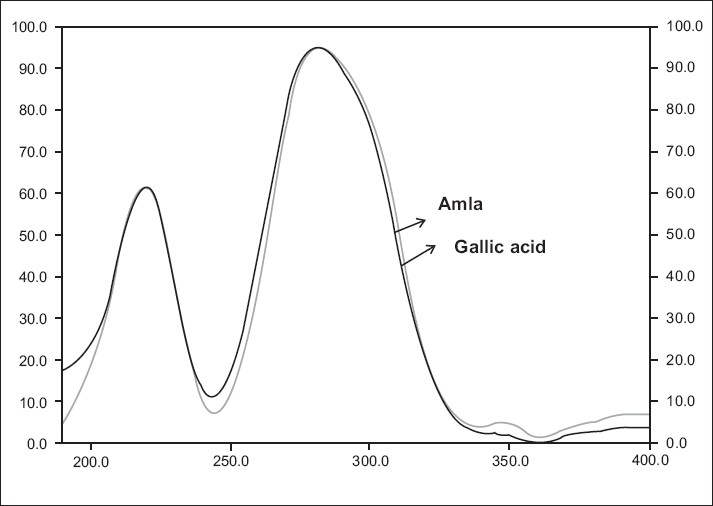
Spectra comparison of standard gallic acid and amla
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Zhang Y, Tanaka T, Iwamoto Y, Yang C, Kouno I. Phyllaemblic acid, a novel highly oxygenated norbisabolane from the roots of Phyllanthus emblica. Tetrahedron Lett. 2000;41:1781–4. [Google Scholar]
- 2.Perianayagam J, Sharma S, Joseph A, Christina A. Evaluation of anti-pyretic and analgesic activity of Emblica officinalis Gaertn. J Ethnopharm. 2004;95:83–5. doi: 10.1016/j.jep.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 3.Calixto JB, Santos ARS, Filho VC, Tunes RA. A review of the plants of the genus Phyllanthus: Their chemistry, pharmacology, and therapeutic potential. J Med Biol. 1998;31:225–8. doi: 10.1002/(sici)1098-1128(199807)18:4<225::aid-med2>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 4.Kroes BH, Van Den Berg JJ, Quarles Van Ufford HC, Van Dijk H, Labadie RP. Anti-inflammatory activity of gallic acid. Planta Med. 1992;58:499–504. doi: 10.1055/s-2006-961535. [DOI] [PubMed] [Google Scholar]
- 5.Ihantola-Vormisto A, Summanen J, Kankaanranta H, Vuorela H, Asmawi MZ, Moilanen E. Anti-inflammatory activity of extracts from leaves of Phyllanthus emblica. Planta Med. 1997;63:518–24. doi: 10.1055/s-2006-957754. [DOI] [PubMed] [Google Scholar]
- 6.Jose JK, Kuttan R. Antioxidant activity of Emblica officinalis. J Clin Biochem Nutr. 1995;19:63–70. [Google Scholar]
- 7.Ghosal S, Tripathi VK, Chauhan S. Active constituent of Emblica officinalis: Part I. The chemistry and antioxidant effects of two new hydrolysable tannins, emblicanin A and B. Indian J Chem. 1996;35:941–8. [Google Scholar]
- 8.Zhang YJ, Tanaka T, Yang CR, Kouno I. New phenolic constituents from the fruit juice of Phyllanthus emblica. Chem Pharm Bull. 2001;49:537–40. doi: 10.1248/cpb.49.537. [DOI] [PubMed] [Google Scholar]
- 9.Cimpoiu C, Jantschi L, Hodisan T. A new method for mobile phase optimization in high-performance thin-layer chromatography (HPTLC) J Planar Chromatogra. 1998;11:191–4. [Google Scholar]
- 10.Sethi PD, Charegaonkar D. Introduction In: Identification of Drugs in Pharamaceutical Formulations by Thin Layer Chromatography. New Delhi: CBS publishers and Distributors; 2008. pp. 1–52. [Google Scholar]


