Abstract
Background:
Pain is an unpleasant and subjective sensation that results from a harmful sensorial stimulation, which alerts the body about current or potential damage to its tissues and organs. Fever is a complex physiological response triggered by infections or aseptic stimuli. Elevation in body temperature occurs when the concentration of prostaglandin E2 (PGE2) increases within parts of the brain. Triazole derivatives have been found to possess various pharmacological and biological activities, such as, anti-inflammatory, analgesics, antipyretic, and antifungal.
Materials and Methods:
Various 4-[{1-(aryl)methylidene}-amino]-3-(4-pyridyl)-5-mercapto-4H-1,2,4-triazole derivatives were synthesized by a sequence of reactions starting from isonicotinic acid hydrazide. The synthesized compounds were screened for in-vivo analgesic by the tail-flick method and anti-pyretic activities at a dose of 25 and 100 mg/kg body weight respectively. The antipyretic activity was evaluated using Brewer's yeast induced pyrexia in rats. Fever was induced by subcutaneously injecting 20 ml/kg of 20% aqueous suspension of Brewer's yeast in normal saline.
Results and Discussion:
The analgesic screening results revealed that the compounds 3b, 3c, and 3d exhibited excellent analgesic activity at 60 and 90 minutes compared to the standard drug (Analgin). Results revealed that the compounds 3a, 3e, and 3f significantly decreased the temperature of pyretic (P<0.001) rats at one, three and six hours after compound administration as compared to Aspirin (standard drug).
Conclusion:
Compounds 3b, 3c, and 3d exhibited significant analgesic activity comparable with the standard drug analgin, using the tail flick model. Compounds 3a, 3e, and 3f showed significant anti-pyretic activities comparable with the standard drug aspirin using the yeast-induced pyrexia model.
Keywords: Isonicotinic acid hydrazide; 1,2,4-triazole; analgesic; antipyretic activity
Pain is an unpleasant and subjective sensation that results from a harmful sensorial stimulation, which alerts the body about current or potential damage to its tissues and organs.[1]It is estimated that more than 75 million people refer to health services annually, presenting with some form of recurrent or persistent pain.[2] In spite of the painful sensation that can be solved most efficiently by removal of the underlying cause, the pain-causing stimulus cannot always be either easily defined or quickly removed. Therefore, the health professionals are usually faced with the necessity to manage the symptomatology of the pain. Fever is a complex physiological response triggered by infections or aseptic stimuli. Elevation in body temperature occurs when the concentration of prostaglandin E2 (PGE2) increases within parts of the brain. Such an elevation contributes to a considerable alteration in the firing rate of the neurons that control the thermoregulation process in the hypothalamus. It is now evident that most anti-pyretics exert their action by inhibiting the enzymatic activity of cyclooxygenase and consequently reducing the levels of PGE2 within the hypothalamic region. In order to combat these diseases caused by pathogens, it is usual that chemotherapeutics, analgesics, and anti-pyretic agents are prescribed separately in clinical practices.[3] Triazole derivatives have been found to possess various pharmacological and biological activities, such as, anticonvulsant, anti-inflammatory, analgesics, antipyretic, and antifungal.[4–11]
Materials and Methods
Chemistry
The chemicals were supplied by E. Merck (Germany) and S.D Fine chemicals (India). The melting points were determined by the open tube capillary method and were uncorrected. The purity of the compounds was checked on thin layer chromatography (TLC) plates (silica gel G) in the solvent system toluene-ethyl formate-formic acid (5:4:1) and benzene-methanol (8:2); the spots were located under iodine vapors and UV light. The infrared (IR) spectra were obtained on a Perkin-Elmer 1720 FT-IR spectrometer (KBr pellets). 1H NMR spectra were recorded on a Bruker AC 400 MHz spectrometer using TMS as the internal standard in DMSO-d6/ CDCl3. Mass spectra, under fast atom bombardment conditions (FAB) were recorded at 70 ev ionizing voltage with a VG Prospec instrument and were presented as m/z. UV spectra were recorded on a UV-Visible Spectrophotometer Pharma Spec-1700 (SHIMADZU). Elemental analysis was carried out on CHNS Elementar (Vario EL III) using sulfanilic acid as a standard and tungsten (VI) oxide as a combusting agent and analyses for C, H, and N were within ± 0.4% of the theoretical values.
Synthesis of 4-amino-3-(4-pyridyl)-5-mercapto-4H-1,2,4-triazole (2)
Isonicotinic acid hydrazide 13.7 g (0.1 mol) was transferred in a 1000 mL round bottom flask and dissolved in 200 mL of absolute ethanol containing potassium hydroxide 11.2 g (0.1 mol) at room temperature. To this 12.5 mL carbon disulfide was added in parts and the reaction mixture was stirred for 16 hours at room temperature. After the reaction was completion, 100 ml of diethyl ether was added and the reaction mixture was stirred for a further three hours. After this 10.3 g (0.1 mol, 99%) hydrazine hydrate was added gradually to the potassium dithiocarbazinate salt and dissolved in 100 mL water with stirring and the content was refluxed for eight hours, during which hydrogen sulfide gas evolved and the color of the reaction mixture changed to deep green. It was then cooled and acidified with hydrochloric acid to pH 1. The yellow-colored solid separated out and was filtered and purified by recrystallization from ethanol to give compound (2) .
General procedure for synthesis of 4-[{1-(aryl)methylidene}-amino]-3-(4-pyridyl)-5-mercapto-4H-1,2,4-triazoles (3a- 3f)
Few drops of glacial acetic acid were added to a solution of 0.01 mol of compound 2 (4-amino-3-(4-pyridyl)-5-mercapto-4H-1,2,4-triazole) in dimethylformamide (20 mL) and 0.01 mol of various benzaldehyde derivatives were added and refluxed for nine hours. The reaction mixture was cooled and the precipitate obtained was filtered, dried in a vacuum, and recrystallized from ethanol to give the final compound. The compounds were synthesized as per (scheme 1) [Figure 1].
Figure 1.
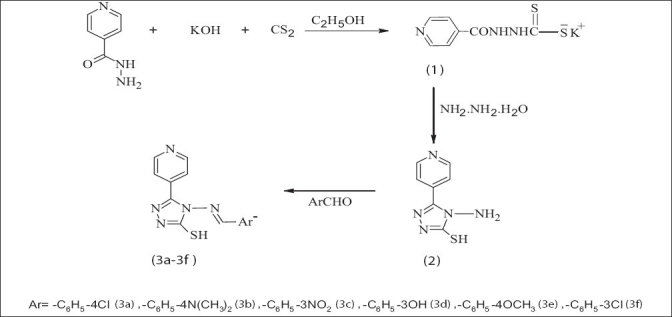
Schematic diagram for the synthesis of 4-[{1-(aryl)methylidene}-amino]-3-(4-pyridyl)-5-mercapto-4H-1,2,4-triazole derivatives (3a-3f)
4-{[(E)-(4-chlorophenyl)methylidene]amino}-5-(pyridin-4-yl)-4H-1,2,4-triazole-3-thiol 3a
Colorless solid, Yield 44%, mp 276 - 278°C, Rf 0.76, UV λmax (DMF): 245.8 nm. FTIR (KBr) n cm-1: 3013 (Ar C-H stretching), 2563 (S-H stretching), 1603, 1485 (Ar C=C stretching) 1563 (C=N stretching), 1313 (C-N stretching), 828 (C-Cl stretching), 688 (C-S stretching). 1H NMR (400 MHz DMSO-d6 δ ppm): 6.06 (s, 1H, N=CH), 7.59-8.76 (m, 8H, Ar-H), 12.59 (s, 1H, S-H). Anal. Calcd. for C14H10ClN5S : C, 53.25; H, 3.19; N, 22.18. Found: C, 53.18; H, 3.16; N, 22.12. FABMS (m/z): 315/317 (M+/M+ +2).
4-({(E)-[4-(dimethylamino)phenyl]methylidene}amino)-5-(pyridin-4-yl)-4H-1,2,4-triazole-3-thiol 3b
Colorless solid, Yield 31%, mp 224 - 226°C, Rf 0.82, UV λmax (DMF): 260.7 nm. FTIR (KBr) n cm-1: 3132 (Ar C-H stretching), 2544 (S-H stretching), 1523 (C=N stretching), 1344 (C-N stretching), 671 cm-1 (C-S stretching). 1H NMR (400 MHz DMSO- d6 δ ppm): 3.20 [s, 6H, N(CH3)2], 8.08 (s, 1H, N=CH), 7.59-8.78 (m, 8H, Ar-H), 12.32 (s, 1H, SH). Anal. Calcd. for C16H16N6S : C, 57.67; H, 5.16; N, 26.90. Found: C, 59.24; H, 4.97; N, 25.91. FABMS (m/z): 324/325 (M+/M++1).
4-{[(E)-(3-nitrophenyl)methylidene]amino}-5-(pyridin-4-yl)-4H-1,2,4-triazole-3-thiol 3c
Colorless solid, Yield 66%, mp 288 - 290°C, Rf 0.79, UV λmax (DMF): 221.9 nm. FTIR (KBr) n cm-1: 3033 (Ar C-H stretching), 2579 (S-H stretching), 1609, 1585, 1452 (Ar C=C stretching), 1520 (C=N stretching), 1353 (Ar-NO2), 1315 (C-N stretching), 698 cm-1 (C-S stretching). 1H NMR (400 MHz DMSO- d6 δ ppm): 5.58 (s, 1H, N=CH), 7.78-8.71 (m, 8H, Ar-H), 14.02 (s, 1H , S-H). Anal. Calcd. for C14H10N6O2S : C, 51.53; H, 3.09; N, 25.75. Found: C, 51.50; H, 3.02; N, 25.68. FABMS (m/z): 325/326 (M+/M+ +1).
3-[(E)-{[3-(pyridin-4-yl)-5-sulfanyl-4H-1,2,4-triazole-4-yl]imino}methyl]phenol 3d
Colorless solid, Yield 62%, mp 282 - 284°C, Rf 0.66, UV λmax (DMF): 234.6 nm. FTIR (KBr) n cm -1 : 3450 (O-H stretching), 3032 (Ar C-H stretching), 2579 (S-H stretching), 1609, 1585, 1453 (Ar C=C stretching), 1546 (C=N stretching), 1315 (C-N stretching), 699 (C-S stretching).1H NMR (400 MHz DMSO-d6 δ ppm): 5.64 (s, 1H, N=CH), 7.82 (s, 1H, O-H), 7.86-8.72 (m, 8H, Ar-H), 13.96 (s, 1H, S-H). Anal. Calcd. for C14H11N5SO : C, 56.55; H, 3.73; N, 23.55. Found: C, 56.48; H, 3.64; N, 23.46. FABMS (m/z): 297/298 (M+/M+ +1).
4-{[(E)-(3-methoxyphenyl)methylidene]amino}-5-(pyridin-4-yl)-4H-1,2,4-triazole-3-thiol 3e
Colorless solid, Yield 50.8%, mp 272 - 274°C, Rf 0.72, UV λmax (DMF) 212.4 nm. FTIR (KBr) n cm-1: 3051 (Ar C-H stretching), 2872 (C-O-CH3), 2621 (S-H stretching), 1606 (Ar C=C stretching), 1541 (C=N stretching), 1377 (C-N stretching), 678 (C-S stretching). 1H NMR (400 MHz DMSO-d6 δ ppm): 3.79 (s, 3H, -O-CH3), 7.67 (s, 1H, N=CH), 7.25-7.80 (m, 8H, Ar-H), 11.02 (s, 1H, SH). Anal. Calcd. for C15H13N5OS: C, 57.86; H, 4.21; N, 22.49. Found: C, 57.74; H, 4.16; N, 22.42. FABMS (m/z): 311/312 (M+/M+ +1).
4-{[(E)-(3-chlorophenyl)methylidene]amino}-5-(pyridin-4-yl)-4H-1,2,4-triazole-3-thiol 3f
Colorless solid, Yield 51%, mp 296 - 298°C, Rf 0.81, UV lmax (DMF): 240.6 nm. FTIR (KBr) n cm-1: 2975 (Ar C-H stretching), 2648 (S-H stretching), 1664 (Ar C=C), 1523 (C=N stretching), 1365 (C-N stretching), 815 (C-Cl stretching), 682 (C-S stretching). 1H NMR (400 MHz DMSO-d6 δ ppm): 5.69 (s, 1H, N=CH), 7.72- 7.90 (m, 4H, Ar-H) 8.01-8.13 (m, 4H, Ar-H), 12.49 (s, 1H, S-H). Anal. Calcd. for C14H10N5SC : C, 53.25; H, 3.19; N, 22.18. Found: C, 53.18; H, 3.10; N, 22.06. FABMS (m/z): 315/317 (M+/M++2).
Pharmacology
Animals
Albino-Swiss mice weighing 20 - 25 g and albino-Wistar rats weighing 150 - 200 g were used for studying in-vivo analgesic and anti-pyretic activities. The animals were maintained under standard laboratory conditions (24 ± 2°C and relative humidity 60 - 70%).
Analgesic activity
The animals were divided into eight groups containing six rats in each group as shown in Table 1. The reaction time was measured at the end of 0, 30, 60 and 90 minutes after the administration of the compound. The drugs were administered orally. The tail-flick latency was assessed by the time taken by the rat to withdraw its tail from the organ bath containing hot water (temperature 55 ± 0.5 °C). The tail-flick latency of treated animals was compared with the control and standard.
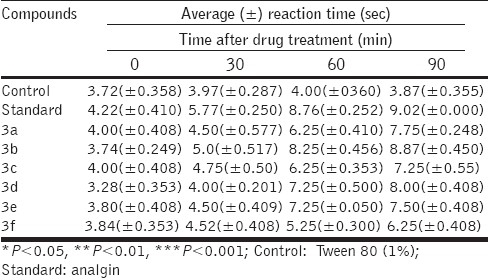
Table 1.
Analgesic activity evaluated by the tail-flick method in rats (dose = 25 mg/kg, mean±SEM, n= 6)
Anti-pyretic activity
The antipyretic activity was evaluated using Brewer's yeast-induced pyrexia in rats. Fever was induced by subcutaneously injecting 20 ml/kg of 20% aqueous suspension of Brewer's yeast in normal saline, below the nape of the neck and rectal temperature was recorded with a clinical thermometer immediately before (-18 hours) and 18 hours after (0 hour) the Brewers’ yeast injection. Prior to the experiment, the rats were maintained in separate cages for seven days and the animals with approximately constant rectal temperature were selected for the study. Aspirin (300 mg/kg, p.o.) was used as standard drug for comparing the antipyretic action of compounds. The experimental rats showed a mean increase of about 0.86 °C in rectal temperature, 18 hours after Brewer's yeast injection. Compounds at 100 mg/kg produced significant (P <0.05 and P <0.01, respectively) antipyretic activity at one, three and six hours after drug administration.
Statistical analysis
Statistical analysis was performed by one-way analysis of variance (ANOVA) followed by the Dunnett's t-test for multiple comparisons of all compounds in various pharmacological assays. Data were expressed as mean ± SEM.
Results and Discussion
Analgesic activity
All the synthesized compounds were screened for analgesic activity by the tail-flick method used by D’Amour and Smith.[12] The analgesic screening results revealed that the compounds 3b, 3c, and 3d exhibited excellent analgesic activity at 60 and 90 minutes compared to the standard drug, as shown in Table 1. However, compounds 3a, 3e, and 3f showed nearly comparable activity to that of the standard drug analgin in peripheral analgesic activity.
Anti-pyretic activity
All the synthesized compoundswere screened for anti-pyretic activity by using the Brewer's yeast-induced pyrexia method[13]. Aspirin was used as a reference drug. The anti-pyretic screening results depicted in Table 2 revealed thatthe compounds 3a, 3e, and 3f significantly decreased the temperature of pyretic (P <0.001) rats at one, three and six hours after compound administration as compared to aspirin (standard drug). The maximum mean rectal temperatures produced by Brewer's yeast, in the presence of compounds 3a, 3e, and 3f were 32.31, 32.45 and 31.84°C, respectively. In addition, compounds 3b, 3c, and 3d showed a decrease in the rectal temperature, after three hours, of 32.64, 32.61, and 32.50°C, respectively, compared to 34.68°C in the control group.
Table 2.
Anti-pyretic activity of the synthesized compounds (3a-3f) on Brewers’ yeast-induced pyrexia in rats

Conclusion
A new series of 4-[{1-(aryl)methylidene}-amino]-3-(4-pyridyl)-5-mercapto-4H-1,2,4-triazolederivative (3a-3f) analogs were designed, synthesized, and characterized. The synthesized compounds were screened for their in-vivo analgesic and anti-pyretic activity. Some of the synthesized compounds 3b, 3c, and 3d exhibited significant analgesic activity and the remaining compounds showed good-to-moderate analgesic activity comparable to that of the standard drug analgin in the tail flick model at 25 mg/kg body weight of the animals. Compounds 3a, 3e, and 3f had a significant anti-pyretic activity comparable with the standard drug aspirin in the yeast-induced pyrexia model at 100 mg/kg body weight.
Acknowledgments
The authors are thankful to the Krupanidhi College of Pharmacy, Bangalore-560034 for providing the necessary facility, and IISC, Bangalore for recording the H 1 NMR and FAB-MS spectral data.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Ruoff G, Lema M. Strategies in pain management: New and potential indications for COX-2 specific inhibitors. J Pain Symptom Manage. 2003;25:21–31. doi: 10.1016/s0885-3924(02)00628-0. [DOI] [PubMed] [Google Scholar]
- 2.Caudill MA, Holman GH, Turk D. Effective ways to manage chronic pain. Patient Care. 1996;30:154–67. [Google Scholar]
- 3.Flower RJ, Vane JR. Inhibition of prostaglandin synthetase in brain explains the antipyretic activity of paracetamol (4-Acetamidophenol) Nature. 1972;240:410–11. doi: 10.1038/240410a0. [DOI] [PubMed] [Google Scholar]
- 4.Dawood KM, Abdel GH, Mohammad HA. Synthesis anticonvulsant and anti-inflammatory evalution of benzotriazole and benzofuran based heterocycles. Bioorg Med Chem. 2006;14:3672–80. doi: 10.1016/j.bmc.2006.01.033. [DOI] [PubMed] [Google Scholar]
- 5.Grossi G, Roma G, Braccio M, Calcina F, Barocelli Substituted 4H-[1,2,4]- triazole-[4,3-a] [1,5]-benzodiazepine-5-amine as analgesic, anti-inflammatory and/ or antipyretic agent with low acute toxicity. Eur J Med Chem. 2002;7:155–65. doi: 10.1016/s0223-5234(02)01400-9. [DOI] [PubMed] [Google Scholar]
- 6.Kamotra P, Gupta AK, Gupta R. Microwave assisted synthesis and biological activity of 3-alkyl/aryl-6-(1-chloro-3,4-dihydronaphth-2-yl)-5,6-dihydro-s-triazolo [3,4-b] [1,3,4] thiadiazoles. Ind J Chem. 2007;46:980–84. [Google Scholar]
- 7.Labanauskas L, Udrenaite E, Gaidelis P, Brukštus A. Synthesis of 5,2,3 and 4-methoxyphenyl-4H-1,2,4-triazole-3-thiol derivatives exhibiting anti-inflammatory activity. II Farmaco. 2004;59:255–59. doi: 10.1016/j.farmac.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Lin TS, Liu MC. Synthesis of 1-(2-hydroxyl(hydroxymethyl)(ethoxy)methyl]-1,2,4-triazole-3-and 5-carboxamide. Tetrahedron Lett. 1984;25:11–12. [Google Scholar]
- 9.Mathew V, Keshavayya J, Vaidy VP. Synthesis and pharmacological activities of some substituted 1,2,4-triazolo(3,4-b)1,3,4-thiadiazoles. Eur J Med Chem. 2006;41:1048–58. doi: 10.1016/j.ejmech.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 10.Mohamed BG, Abdel A. Hussein MA.Synthesis of acyl-2-alkylthio-1,2,4-triazolobenzimidazole with antifungal, anti- inflammatory effect. Acta Pharma. 2006;56:31–48. [PubMed] [Google Scholar]
- 11.Mulwad VV, Mhaskar MD. Synthesis of 1,2,3-triazole derivative from 6-azido-2H-benzopyran-2-ones. Ind J Heterocycl Chem. 2007;17:41–44. [Google Scholar]
- 12.Turner RA. Screening Methods in Pharmacology. New York & London: Academic Press; 1965. p. 223. [Google Scholar]
- 13.Loux JJ, Depalma PD, Yankell SL. Antipyretic testing of aspirin in rats. Toxicol Appl Pharmacol. 1972;22:672–75. doi: 10.1016/0041-008x(72)90295-5. [DOI] [PubMed] [Google Scholar]


