Abstract
Objective:
In this study efforts have been made to design a drug delivery system based on a superporous hydrogel composite, for floating and sustained delivery of Ranitidine hydrochloride.
Materials and Methods:
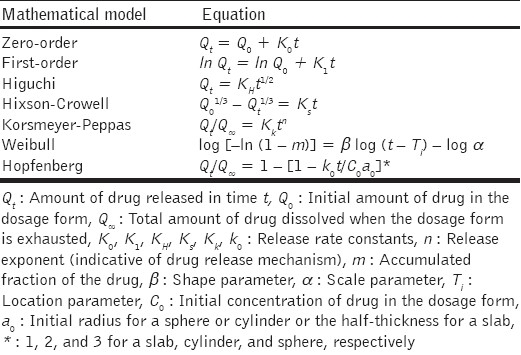
The characterization studies were performed by the measurement of apparent density, porosity, swelling studies, mechanical strength studies, and scanning electron microscopy studies. The prepared formulation was evaluated for buoyant behavior, in vitro drug release, kinetics of drug release, and stability. The release profile of Ranitidine hydrochloride was investigated by changing the release retardant polymer in the formulation. To ascertain the kinetics of drug release, the drug release profiles were fitted to mathematical models that included zero-order, first-order, Higuchi, Hixson-Crowell, Korsmeyer-Peppas, Weibull, and Hopfenberg models.
Results:
Scanning electron microscopy images clearly indicated the formation of interconnected pores and capillary channels, and cross-linked Chitosan molecules were observed around the peripheries of the pores. The prepared drug delivery system floated and delivered the Ranitidine hydrochloride for about 17 hours. The in vitro drug release from the proposed system was best explained by the Korsmeyer-Peppas model. The values of the diffusion exponent in the Korsmeyer-Peppas model ranged between 0.47 ± 0.02 and 0.66 ± 0.02, which appeared to indicate a coupling of the diffusion and erosion mechanisms, anomalous non-Fickian transport.
Conclusion:
It was concluded that the proposed floating drug delivery system, based on the superporous hydrogel composite containing Chitosan as a composite material, is promising for stomach-specific delivery of Ranitidine hydrochloride.
Keywords: Chitosan, floating drug delivery, Ranitidine hydrochloride, superporous hydrogel composite, stomach specific drug delivery
Ranitidine is a competitive, reversible inhibitor of the action of histamine at histamine H2-receptors, including receptors on gastric cells, with a minimal effect on H1-receptors. It is one of the drugs of choice for the treatment of active duodenal ulcers, gastric ulcers, Zollinger-Ellison syndrome, gastroesophageal reflux disease, and erosive esophagitis. The indicated oral dosage of Ranitidine is 150 mg, twice daily, or 300 mg once daily. In the treatment of endoscopically diagnosed erosive esophagitis, the dosage is 150 mg Ranitidine four times a day.[1,2] It has been found that the conventional dose of 150 mg can inhibit gastric acid secretion for up to five hours, but not up to 10 hours, and at the same time, an alternative dose of 300 mg leads to fluctuations in the plasma levels. Taking this into consideration, a sustained release dosage form of Ranitidine hydrochloride (R-HCl) will be very useful.[3] The drug has a short biological half-life of approximately two to three hours, an absolute bioavailability of only 50%, and it is absorbed only in the initial part of the small intestine.[4–6] Colonic metabolism of Ranitidine is also partly responsible for the poor bioavailability of Ranitidine from the colon.[7] A gastroretentive drug delivery system that can be retained in the stomach and increases the local delivery of R-HCl will also be very useful. Local delivery also increases bioavailability of the stomach wall receptor site and increases the efficacy of drugs, to reduce acid secretion.[8] There are a number of approaches that can be used to prolong gastric retention time, such as, swelling and expanding systems, polymeric bioadhesive systems, modified-shape systems, high-density systems, and other delayed gastric emptying devices.[9,10]
Hydrogels are cross-linked hydrophilic polymers with a network structure. They are able to imbibe large amounts of water and are water insoluble.[11–13] For pharmaceutical applications, they are unique carriers for controlled drug delivery; release control can be governed by both swelling and biodegrading properties.[14–16] Owing to their high water affinity and biocompatibility, hydrogels based on poly (acrylic acid) and its derivatives,[17,18] Chitosan,[19] alginate,[20] and collagen[21] have attracted attention. However, these nonporous hydrogels swell slowly and exhibit low loading capacities,[22,23] which restrict their use in effective drug delivery. A new generation of hydrogel, Superporous Hydrogel (SPH), which absorbs water very rapidly and swells to equilibrium size in a short period of time, has been developed.[24–28] SPH synthesis involves copolymerization / cross-linking of co-monomers using multifunctional co-monomer, which acts as cross-linking agent. Chemical initiator initiates the polymerization reaction. Gas blowing techniques are used to synthesize SPHs. The commonly used foaming agents are inorganic carbonates such as sodium carbonate and sodium bicarbonates. The second method involves cross-linking of linear polymers by irradiation or by chemical compounds.[29] Several important properties of SPH, such as fast swelling, large swelling ratio, and surface slipperiness, make it an excellent candidate material to develop gastric retention devices.[30] On account of the poor mechanical strength of SPHs, they are difficult to handle without breaking.[31] Superporous Hydrogel Composites (SPHCs), as the second generation of SPHs, possess improved mechanical properties over SPHs, with composite agents such as, Chitosan,[32,33] Ac-Di-Sol,[34,35] and Carbopol.[36]
The objectives of the investigation were: to synthesize SPHC containing Chitosan as a composite material to improve the characteristics of SPH and to prepare a drug delivery system based on it. Acrylic acid (AA) and Acrylamide (AM) were chosen as the base monomers for their high water affinity and fast copolymerization velocity,[37] while Chitosan was selected as the second polymer component for its biocompatibility.[38] A floating drug delivery system, which was less dense than gastric juice was described.[39] Of late, research has been carried out using R-HCl as an effervescent-type of drug delivery system.[40] Moreover, a new type of multiparticulate floating drug delivery system consisting of a highly porous carrier material (foam powder), a drug, and a polymer involving low density microparticles, has been proposed.[41,42] In the present investigation, preparation of a drug delivery system that delivers R-HCl in the stomach in a sustained manner, as a floating drug delivery system, using a low density SPHCs, has been attempted.
Materials and Methods
Materials
R-HCl was a generous gift from Espee Formulation Pvt. Ltd., Rajkot, Gujarat, India. Chitosan was a generous gift from Mahtani Chitosan Pvt. Ltd., Veraval, Gujarat, India. AM was obtained from Burgoyne Burbidges and Co. Pvt. Ltd., Mumbai, India. AA, N,N’-Methylene-bis-acrylamide (BIS), Span 80, Ammonium Persulfate (APS), N,N,N’,N’-Tetramethylethylenediamine (TEMED), Hydroxypropylmethyl cellulose (HPMC), Carbopol 934P, Ethyl cellulose (EC), and Sodium carboxymethyl cellulose (NaCMC) were purchased from SD Fine Chem Ltd., Mumbai, India. Double distilled water (DDW) was prepared in the laboratory. Simulated gastric fluid (SGF) with pH 1.2 was prepared in the laboratory by dissolving 2 g of sodium chloride, 3.2 g pepsin, and 6.8 ml of hydrochloric acid in DDW to produce 1 L. All other chemicals used were of analytical grade and were used as obtained.
SPHC synthesis
All ingredients except sodium bicarbonate were used as a solution in DDW. For the synthesis of SPHC of poly (AM-co-AA), the following substances were subsequently added into a test tube at 25°C: 300 μl AM 50 %w/v; 200 μl AA 50 %v/v; 70 μl BIS 2.5 %w/v; 30 μl span 80 10 %v/v; 25 μl APS 20 %w/v;25 μl TEMED 20 %v/v; 400 μl Chitosan aqueous solution 6 %w/v, and 200 mg of sodium bicarbonate. The 400 μl of Chitosan aqueous solution was selected based on the primary studies.[33] SPH was prepared by using DDW instead of Chitosan aqueous solution. In this procedure, polymerization was allowed to continue for approximately 10 minutes. After adding each substance to the test tube, the reaction mixture was shaken vigorously. Finally, sodium bicarbonate was added very quickly to the solution and mixed with a spatula. Synthesized SPHCs were removed with a forceps, allowed to dry in oven at 60°C for 48 hours, and cut into pieces of required size. The SPHC was submerged in hexane overnight. This treatment dehydrated the SPHCs rapidly as well as provided drying. Thereafter, the SPHCs were removed with the forceps and put in an oven at 60°C for 48 hours, to ensure that the SPHCs had been dried completely. These SPHCs were stored in an airtight container until further use.
Scanning electron microscopy analysis
The dried SPHCs were cut to expose their inner structure and used for scanning electron microscopy (SEM) studies. The morphology and porous structure of the SPHC was examined using ESEM EDAX XL-30 Scanning Electron Microscope (Philips, Netherlands), with an operating voltage of 30 kV.
Measurement of density, porosity, swelling parameters, and mechanical strength
For density measurement, the solvent displacement method was used.[36] Dried SPHC was used for density measurement, which actually showed the apparent density of the SPHC. A piece of SPHC was taken and weighed in order to determine the mass of the piece. A piece of the polymer was immersed in a predetermined volume of hexane in a graduated cylinder, and the increase in the hexane volume was measured as the volume of the polymer. The density was calculated from equation 1:
![]()
where, VSPHC is the volume of solvent displaced by SPHC and MSPHC is the mass of the SPHC.
For porosity measurement, the dried SPHC was immersed in hexane overnight and weighed after the excess hexane on the surface was blotted. The porosity was calculated from equation 2:
![]()
where, VP (= VT - VSPHC) is the pore volume of SPHC and VT is the total volume of SPHC. Total volume of SPHC can be measured from its dimensions, as it is cylindrical in shape.
The equilibrium swelling ratio can be calculated from equation 3:
![]()
where, Q is the equilibrium swelling ratio, Ms is the mass in the swollen state, and Md is the mass in the dried state. At the beginning of each experiment, the dried gel was measured gravimetrically to obtain Md and then it was immersed in an excess of medium, for swelling. At various time intervals, the hydrogel was removed from the medium and weighed when excessive medium on the surface was blotted to determine Ms.[24]
The penetration pressure of the SPHC was measured using a bench comparator as described by Chen et al., with modifications.[34] The fully swollen hydrogel was put longitudinally under the lower touch and weights were successively applied to the upper touch until the polymer completely fractured. The compressive force could be read from the gauge, and the penetration pressure[35] could be calculated from equation 4:
![]()
where, PP is the penetration pressure, Fu is the ultimate compressive force at complete breakage of the polymer and S is the area of lower touch.
Preparation of the SPHC-based drug delivery system (SPHC-DDS)
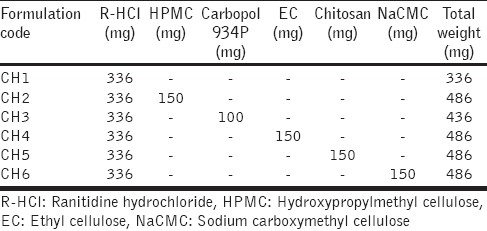
The SPHC-DDS was prepared as a core inside the delivery system (shuttle system) as reported by Dorkoosh et al.,[43] with modifications, as shown in Figure 1, which consisted of two components, (a) conveyor system made of SPHC; (b) core containing a mixture of R-HCl and the polymer. In order to prepare the conveyor system, a hole was made at a certain depth in the prepared SPHC, which was used as the body. The SPHC was allowed to swell in DDW to its maximum and then the excess water from the surface was blotted. A hole of the desired size, which could accommodate core formulation after drying, was made at the center of the SPHC with the help of a borer. The swollen SPHC was then dried in an oven at a temperature below 50°C. To make the core component, R-HCl equivalent to 300 mg of Ranitidine was thoroughly mixed with the release retardant polymers, as shown in Table 1. After preliminary studies (data not shown) the selected amounts of HPMC, Carbopol, EC, Chitosan, and NaCMC, as a release retardant polymer in core formulation, were 150 mg, 100 mg, 150 mg, 150 mg, and 150 mg, respectively. The mixture was sieved through 80# sieve and used as the core formulation. The core component was filled in the hole, prepared in the SPHC. The hole was closed securely with a piece of SPHC, used as a cap, with the help of biodegradable glue. Here a piece of SPHC with the same composition as the body was used as a cap, instead of SPH as reported by Dorkoosh et al.[43] The prepared SPHC-DDS was placed in a hard gelatin capsule (size 000) before in vitro drug release studies. Although the preparation of SPHC-DDS was a bit complex, it provided another aspect of the floating drug delivery system.
Figure 1.
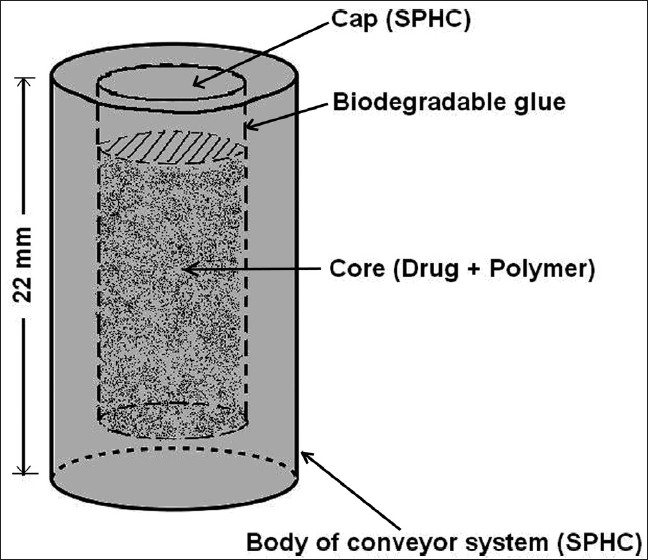
Schematic diagram of SPHC-DDS (Core inside the shuttle system)
Table 1.
CORE composition of Chitosan SPHC-DDSs
Floating behavior of SPHC-DDSs
The SPHC-DDS was placed in a 100 ml beaker containing SGF.[40] The time required for SPHC-DDS to rise to the surface and float was taken as the floating lag time. The total time period for which SPHC-DDS remained buoyant was considered as the total floating time.
In vitro drug release studies
The release rate of R-HCl from SPHC-DDS (n = 3) was determined by using the USP XXIV dissolution testing apparatus II (dissolution tester model TDT-08 L, Electrolab, India). The drug release test was performed using 900 ml SGF, at 37 ± 0.5°C and 75 rpm.[44] Volumes of 10 ml were withdrawn at predetermined interval times (hourly) for 18 hours from the dissolution medium and were replaced immediately with fresh medium. The samples were passed through a 0.45 μm membrane filter and diluted (if needed) to a suitable concentration, with SGF. The absorbance of these solutions was measured at 314 nm by using a UV-1700 UV / VIS double beam spectrophotometer (Shimadzu, Japan). The cumulative percentage of drug release was calculated using an equation obtained from a standard curve.
The in vitro drug release data of all the batches were analyzed by fitting them to different kinetic models, as shown in Table 2,[45–54] in order to evaluate the release mechanism of the drug from SPHCs.
Table 2.
Stability studies
The prepared batches were kept in airtight containers and stored in a stability chamber (TH-90S, Thermolab, India) at 40°C / 75%RH for three months. Results of the in vitro drug release studies obtained after three months were compared with the data obtained at the time of preparation. The similarity factor (f2) and floating behavior were applied to study the effect of storage. The similarity factor can be calculated using equation 5.
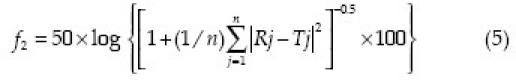
Where, n is the number of dissolution time points and Rj and Tj are the dissolved percent of the reference product and test product at each time point j, respectively.
Results and Discussion
SPHC synthesis
In the synthesis procedure of SPHC, AA and AM are the monomers. BIS is used as a cross-linker, and span 80 is used as a foam stabilizer, which is formed by carbon dioxide originating from sodium bicarbonate. Here span 80 has been used instead of Pluronic F127 as reported by Dorkoosh et al.[55] Span 80 does not contribute to the chemical structure of the polymer, but is very important as a surface-active agent, to create a highly porous polymer structure. APS is used as a polymerization initiator and TEMED as a catalyst. The pH of the AA monomer solution is an important factor that influences the synthesis of the SPHCs. At pH 5.0, SPHCs with well-distributed pores are produced because of the stability and the proper formation rate of the foam.
Synthesis of homogeneous SPHC with a number of pores depended on the amount of Chitosan present. Only when the amount of Chitosan was less than 47.06% of the SPH, homogeneous hydrogels with a number of interconnected pores could be obtained. When the amount was increased to above 47.06% (data not shown), the hydrogel was nonhomogeneous, in that, only a few pores were maintained. As a thickening agent, Chitosan enhanced the viscosity of the stock solution, which efficiently prevented bubbles from escaping from the solution and the residual gas bubbles were able to form interconnected channels. Good solubility of the Chitosan solution at pH values lower than 6,[56] it could be readily blended with the stock solution and well distributed in the SPH, yielding a homogeneous SPHC.
Scanning electron microscopy analysis
Figure 2 shows the SEM pictures of SPHC and SPH. Both SPH and SPHC possessed a numbers of pores, indicating that the formation of a hydrogel would not destroy the superporous structure. White fibers on the peripheries of the inner pores were observed in SPHC, although not in SPH, which were primarily determined to be the Chitosan molecules. The fully swollen SPH was transparent in DDW and lots of bubbles could be seen within the hydrogel. By comparison, white fibers could be observed in the swollen SPHC, which appeared as a netlike distribution. Such differences indicated that the white fibers could be the Chitosan molecules and they were well distributed in the polymer to form a three-dimensional network, which primarily confirmed the formation of SPHC.
Figure 2a.

SEM image of SPH; magnification 1 mm
Figure 2b.
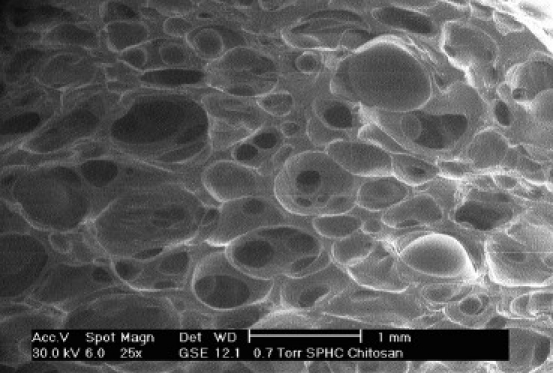
SEM image of SPHC; magnification 1 mm
Measurement of density, porosity, swelling parameters, and mechanical strength
Density, porosity, swelling parameters and mechanical strength of SPH and SPHC are shown in Table 3. Apparent density increased, while the porosity of SPHC decreased in the presence of Chitosan. Chitosan prevented the bubbles from escaping from the solution mixture as well as decreased the pore size of SPHC, due to the accumulation of Chitosan at the periphery of the pores. At higher concentration (data not shown) excessive water was introduced into the system, leading to the collapse of some of the bubbles and a corresponding significantly lower porosity than SPH.
Table 3.
Parameters comparison of SPH and SPHC
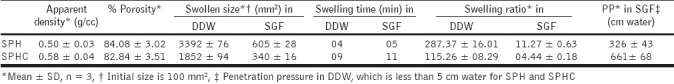
An increase in Chitosan concentration led to slower swelling and decreased the equilibrium swelling ratio of the SPHCs. Through entanglement with the cross-linked Chitosan network, the flexibility of the polymeric chains was greatly restricted. Hydrogen bonds between Chitosan and poly (AM-co-AA) reduced the ability of the polymer to form hydrogen bonds with water molecules, thus limiting its water absorption. Therefore, a dense Chitosan network would further restrict the swelling of the polymer. However, compared to nonporous hydrogels, SPHCs still possessed a significantly faster swelling rate and larger equilibrium swelling ratios, owing to their porous structures.
An SPHC should be able to withstand the pressure expected in the stomach during repeated gastric contractions, especially the housekeeper waves. SPHC when swollen in DDW showed mechanical strength that could withstand the pressure during gastric contraction, but when compared to that in SGF it was less. SPHC showed very good penetration pressure, more than 500 cm water, when swollen in SGF. The maximum pressure during gastric contraction was reported to be in the range of 50 to 130 cm water.[57,58] An increase in the amount of Chitosan helped bring about a denser Chitosan network and a smaller equilibrium swelling ratio, thus enhancing the elasticity of the polymer.
Floating behavior of SPHC-DDSs
The highly porous structure of the SPHC-DDS provides a density that is lower than the density of the release medium. In contrast to the most conventional floating systems including the gas-generating ones, these systems floated immediately upon contact with the release medium, with no lag-time in their floating behavior, because low density was provided from the beginning (t = 0). It was possible to achieve proper in vitro floating behavior for more than 18 hours, in these systems.
In vitro drug release studies
All the formulations were found to be swollen, floating, and retained their physical integrity till the end of the 17-hour drug release study. Batches CH2 to CH6 were formulated to study the effect of various release retardant polymers on the drug release from SPHCs. Batch CH1 was formulated to check the drug release in the absence of a polymer.
The drug release data of the batches was fitted to the zero-order, first-order, Higuchi, Hixson-Crowell, Korsmeyer-Peppas, Weibull, and Hopfenberg models. The in vitro drug release profile of R-HCl in SGF from SPHCs is shown in Figure 3.
Figure 3.
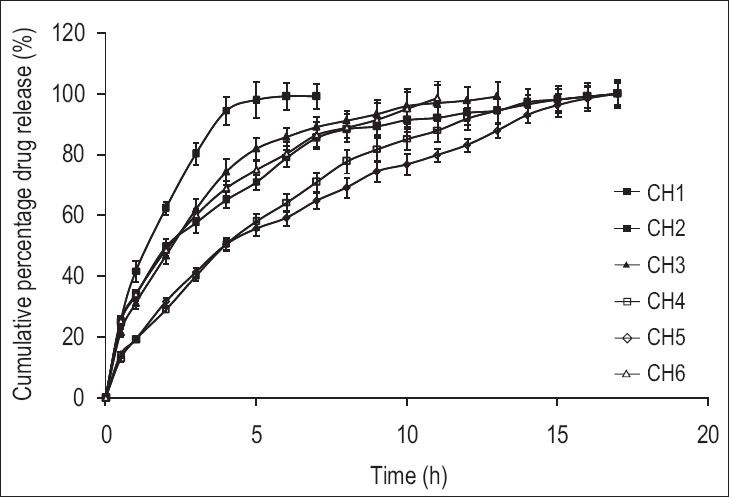
In vitro drug release profile of R-HCl from SPHCs in SGF (Mean, n = 3)
The zero-order rate describes systems where the drug release rate is independent of its concentration. The first-order describes the release from systems where the release rate is concentration-dependent. Higuchi's model describes the release of drugs from an insoluble matrix as a square root of a time-dependent process, based on Fickian diffusion.[48,49] The applicability of the formulation to the Hixson-Crowell cube root law equation might indicate that there is a change in the surface area and diameter of the SPHCs, with progressive dissolution of the formulation as a function of time. Korsmeyer-Peppas model is expected to be valid only up to ~60% cumulative drug release,[51] therefore, the data for analysis are restricted to that range. Korsmeyer-Peppas plots are shown in Figure 4. Weibull plots can describe the drug release curve in terms of applicable parameters. The shape parameter, b, characterizes the curve as either exponential (β = 1), S-shaped with upward curvature followed by a turning point (β > 1), or as one with a steeper initial slope than is consistent with the exponential (β < 1).[52] The Hopfenberg model is applied to ascertain the release of the drug from surface-eroding devices, with a cylinder displaying heterogeneous erosion.
Figure 4.
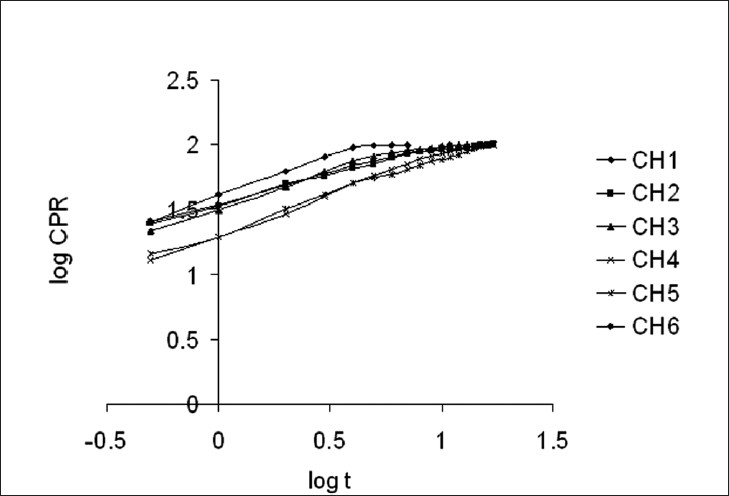
Korsmeyer-Peppas plots for SPHC-DDSs
The curve fitting and plotting was performed on Excel (Microsoft Software Inc., USA) and GraphPad Prism® version 5.02 (GraphPad Software Inc., USA). Linearization of drug release profiles, using the equations in Table 2, was used to characterize the differences found among all batches. The values for the release rate constants, the correlation coefficients (r2), and the release exponent (n) were considered. The correlation coefficient (r2) was used as an indication of the best fit, for each of the models considered. The release constant was calculated from the slope of the appropriate plots, the regression coefficient for individual batches was determined as shown in Table 4. The average r2 of all the batches was used to select the best fit model and this model would indicate the release mechanism of the drug especially from SPHC-DDSs.
Table 4.
Linearization of R-HCl release from SPHC-DDSs
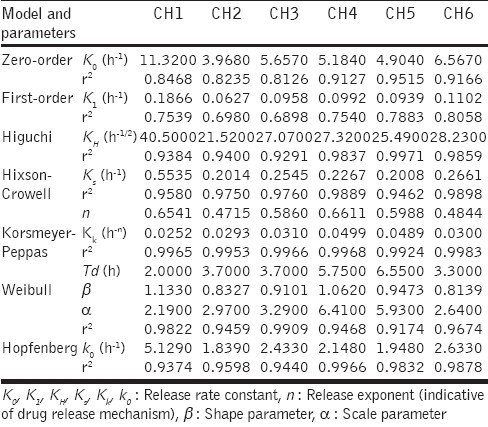
SPHC-DDSs were different from conventional oral dosage forms such as tablets and capsules. It was found that the in vitro drug release of SPHC was best explained by the Korsmeyer-Peppas model, as the plots showed the highest linearity (r2 = 0.9964), followed by Hixson-Crowell (r2 = 0.9723), Hopfenberg (r2 = 0.9681), Higuchi (r2 = 0.9624), and Weibull (r2 = 0.9584). There was less probability of zero-order and first-order release. The magnitude of the exponent n in Korsmeyer-Peppas’ equation indicated that the release mechanism was Fickian diffusion, case II transport, or anomalous transport. In the present study (cylindrical shape) the limits considered were n = 0.45 (indicated a classical Fickian diffusion-controlled drug release) and n = 0.89 (indicated a case II relaxational release transport: polymer relaxation controls drug delivery). Values of n between 0.45 and 0.89 could be regarded as indicators of both phenomena (transport corresponding to coupled drug diffusion in the hydrated matrix and polymer relaxation), commonly called anomalous non-Fickian transport. Values of n greater than 0.89 indicated super case II transport, wherein, a pronounced acceleration in solute release by a film occurred toward the latter stages of release experiments, resulting in a more rapid relaxation-controlled transport.[59] In our studies the values of n ranged between 0.47 ± 0.02 and 0.66 ± 0.02, which appeared to indicate a coupling of the diffusion and polymer relaxation mechanisms, anomalous non-Fickian transport, and might indicate that the drug release from SPHC could be controlled by more than one process.
The prepared SPHC-DDS must be safe as it is retained in the stomach for a long period of time. Dorkoosh et al.,[60] and Risbud and Bhonde[61] have shown the feasibility and safety of similar drug delivery systems. Cytotoxicity assays of the SPH interpenetrating network on RBL-2H3 and Caco-2 cells cause minimal damage to cell viability, lysosomal activity, and metabolic activity, as reported by Yin et al.[62] Therefore, the SPHC-DDS must be preliminarily considered to be biocompatible and may be safe.
Stability studies
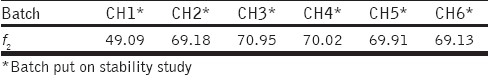
The stability studies showed that there were no significant changes observed in the in vitro drug release studies after three months. Batch CH1 to CH6 were considered as reference for the respective batches (put on stability studies) for similarity factor (f2) calculation. After three months of applied stability conditions, all the batches were seen to be similar to the reference batches, with the exception of Batch CH1. These batches floated immediately upon contact with the release medium, with no lag-times in floating behavior. Table 5 shows f2 values for batches put on stability studies. Although SPHC-DDSs were found to be stable for a period of three months at 40°C / 75%RH, the f2 values were not close to 100, which indicated that some changes in the delivery systems might have occurred during storage. Due to the porous structure of the SPHC-DDS, the drug and / or polymer could have been affected at a constant temperature of 40°C.
Table 5.
f2 values for batches put on stability studies
Conclusion
This study discusses the preparation of a floating stomach-specific drug delivery system of R-HCl. SPHC possesses better characteristics than SPH, especially for sustained release drug delivery systems. The mechanical stability of SPHC in SGF is significantly improved and depends on the Chitosan content. SPHC-DDS is mechanically stable, low dense, has good swelling capacity, and is pH-dependant. The in vitro drug release from SPHC is best explained by the Korsmeyer-Peppas model. The Korsmeyer-Peppas plots have indicated anomalous non-Fickian transport, and hence, the drug release is controlled by more than one process. SPHC-DDSs are found to be stable for a period of three months at 40°C / 75%RH. A sustained release R-HCl drug delivery system has been prepared successfully using a SPHC, with Chitosan as a composite material.
Acknowledgments
Authors are thankful to Espee Formulation Pvt Ltd., Rajkot, and Mahtani Chitosan Pvt. Ltd., Veraval, Gujarat, India for the generous gift of Ranitidine hydrochloride and Chitosan, respectively. Authors acknowledge the advices from Dr. D. M. Patel and Dr. S. T. Prajapati.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Drug facts and comparisons. Louis MO: Facts and Comparisons; 2002. Facts and Comparisons (Firm), Histamine H2 antagonists. In: Facts and Comparisons (Firm), editors; pp. 1192–7. [Google Scholar]
- 2.McCarty-Dawson D, Sue SO, Morrill B, Murdock RH., Jr Ranitidine versus cimetidine in the healing of erosive esophagitis. Clin Ther. 1996;18:1150–60. doi: 10.1016/s0149-2918(96)80069-5. [DOI] [PubMed] [Google Scholar]
- 3.Somade S, Singh K. Comparative evaluation of wet granulation and direct compression methods for preparation of controlled release Ranitidine HCL tablets. Indian J Pharm Sci. 2002;64:285–6. [Google Scholar]
- 4.Lauritsen K, Laursen LS, Rask-Madsen J. Clinical pharmacokinetics of drugs used in the treatment of gastrointestinal diseases. Clin Pharmacokinet. 1990;19:11-31–94-125. doi: 10.2165/00003088-199019020-00002. [DOI] [PubMed] [Google Scholar]
- 5.Grant SM, Langtry HD, Brogden RN. Ranitidine: an updated review of its pharmacodynamic and pharmacokinetic properties and therapeutic use in peptic ulcer and other allied diseases. Drugs. 1989;37:801–70. doi: 10.2165/00003495-198937060-00003. [DOI] [PubMed] [Google Scholar]
- 6.Gramatté T, el Desoky E, Klotz U. Site-dependent small intestinal absorption of Ranitidine. Eur J Clin Pharmacol. 1994;46:253–9. doi: 10.1007/BF00192558. [DOI] [PubMed] [Google Scholar]
- 7.Basit AW, Lacey LF. Colonic metabolism of Ranitidine: implications for its delivery and absorption. Int J Pharm. 2001;227:157–65. doi: 10.1016/s0378-5173(01)00794-3. [DOI] [PubMed] [Google Scholar]
- 8.Coffin M, Parr A. Ranitidine solid dosage form. 1995 US Patent no 5407687. [Google Scholar]
- 9.Singh BN, Kim KH. Floating drug delivery systems: an approach to oral controlled drug delivery via gastric retention. J Control Release. 2000;63:235–59. doi: 10.1016/s0168-3659(99)00204-7. [DOI] [PubMed] [Google Scholar]
- 10.Chawla G, Bansal A. A means to address regional variability in intestinal drug absorption. Pharm Tech. 2003;27:50–68. [Google Scholar]
- 11.English AE, Tanaka T, Edelman ER. Polymer and solution ion shielding in polyampholytic hydrogels. Polymer. 1998;39:5893–7. [Google Scholar]
- 12.Guzman J, Iglesias M, Riande E, Compaii V, Andrio A. Synthesis and polymerization of acrylic monomers with hydrophilic long side groups.Oxygen transport through water swollen membranes prepared from these polymers. Polymer. 1997;38:5227–32. [Google Scholar]
- 13.Sen M, Guven O. Prediction of swelling behaviour of hydrogels containing diprotic acid moieties. Polymer. 1998;39:1165–72. [Google Scholar]
- 14.Chen J, Park H, Park K. Superporous hydrogel as a platform for oral controlled drug delivery. In: Wise DL, editor. Handbook of Pharmaceutical Controlled Release Technology. 2nd ed. New York: Marcel Dekker, Inc; 2005. pp. 211–24. [Google Scholar]
- 15.Hwang SJ, Park H, Park K. Gastric retentive drug delivery systems. Crit Rev Ther Drug Carrier Syst. 1998;15:243–84. [PubMed] [Google Scholar]
- 16.Montheard JP, Chatzopoulos M, Chappard D. 2-hydroxyethyl methacrylate (HEMA): chemical properties and applications in biomedical fields. Polymer Rev. 1992;32:1–34. [Google Scholar]
- 17.Achar L, Peppas NA. Preparation, characterization and mucoadhesive interactions of poly(methacrylic acid) copolymers with rat mucosa. J Control Release. 1994;31:271–6. [Google Scholar]
- 18.Smart JD. An in vitro assessment of some mucoadhesive dosage forms. Int J Pharm. 1991;73:69–74. [Google Scholar]
- 19.Henriksen I, Green KL, Smart JD, Smistad G, Karlsen J. Bioadhesion of hydrated chitosans: an in vitro and in vivo study. Int J Pharm. 1996;145:231–40. [Google Scholar]
- 20.Bertram U, Bodmeier R. In situ gelling, bioadhesive nasal inserts for extended drug delivery: in vitro characterization of a new nasal dosage form. Eur J Pharm Sci. 2006;27:62–71. doi: 10.1016/j.ejps.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 21.Jeyanthi R, Nagarajan B, Rao KP. Solid tumor chemotherapy using implantable collagen-poly(HEMA) hydrogel containing 5-fluorouacil. J Pharm Pharmacol. 1991;43:60–2. doi: 10.1111/j.2042-7158.1991.tb05453.x. [DOI] [PubMed] [Google Scholar]
- 22.Leonard M, De Boisseson MR, Hubert P, Dalençon F, Dellacherie E. Hydrophobically modified alginate hydrogels as protein carriers with specific controlled release properties. J Control Release. 2004;98:395–405. doi: 10.1016/j.jconrel.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 23.Burmania JA, Stevens KR, Kao WJ. Cell interaction with proteinloaded interpenetrating networks containing modified gelatin and poly(ethylene glycol) diacrylate. Biomaterials. 2003;24:3921–30. doi: 10.1016/s0142-9612(03)00270-9. [DOI] [PubMed] [Google Scholar]
- 24.Chen J, Park H, Park K. Synthesis of superporous hydrogels: Hydrogels with fast swelling and superabsorbent properties. J Biomed Mater Res. 1999;44:53–62. doi: 10.1002/(sici)1097-4636(199901)44:1<53::aid-jbm6>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 25.Van Dijk-Wolthuis WNE, Tsang SKY, Kettenes-van den Bosch JJ, Hennink WE. A new class of polymerizable dextrans with hydrolyzable groups: hydroxyethyl methacrylated dextran with and without oligolactate spacer. Polymer. 1997;38:6235–42. [Google Scholar]
- 26.Peniche C, Cohen ME, Vazquez B, Sanroman J. Water sorption of flexible networks based on 2-hydroxyethyl methacrylate-triethylenglycol dimethacrylate copolymers. Polymer. 1997;38:5977–82. [Google Scholar]
- 27.Badiger MV, McNeill ME, Graham NB. Porogens in the preparation of microporous hydrogels based on poly (ethylene oxides) Biomaterials. 1993;4:1059–63. doi: 10.1016/0142-9612(93)90206-h. [DOI] [PubMed] [Google Scholar]
- 28.Lee YM, Kim SS. Hydrogels of poly(ethylene glycol)-co-poly(lactones) diacrylate macromers and β-chitin. Polymer. 1997;38:2415–20. [Google Scholar]
- 29.Satish CS, Satish KP, Shivakumar HG. Hydrogels as controlled drug delivery systems: Synthesis, crosslinking, water and drug transport mechanism. Indian J Pharm Sci. 2006;68:133–40. [Google Scholar]
- 30.Qiu Y, Park K. Superporous IPN hydrogels having enhanced mechanical properties. [cited 2009 Jun 12];AAPS PharmSciTech [serial on the Internet] 2003 4 doi: 10.1208/pt040451. Available from: http://www.aapspharmscitech.org/view.asp?art=pt040451. Article 51[about 7 p.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Omidian H, Rocca JG, Park K. Advances in superporous hydrogels. J Control Release. 2005;102:3–12. doi: 10.1016/j.jconrel.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 32.Yin L, Fei L, Cui F, Tang C, Yin C. Superporous hydrogels containing poly(acrylic acid-co-acrylamide)/O-carboxymethyl Chitosan interpenetrating polymer networks. Biomaterials. 2007;28:1258–66. doi: 10.1016/j.biomaterials.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 33.Chavda HV, Patel CN, Karen HD. Preparation and characterization of Chitosan-based superporous hydrogel composite. J Young Pharmacists. 2009;1:199–204. [Google Scholar]
- 34.Chen J, Park K. Synthesis and characterization of superporous hydrogel composites. J Control Release. 2000;65:73–82. doi: 10.1016/s0168-3659(99)00238-2. [DOI] [PubMed] [Google Scholar]
- 35.Polnok A, Verhoef JC, Borchard G, Sarisuta N, Junginger HE. In vitro evaluation of intestinal absorption of desmopressin using drug delivery systems based on superporous hydrogels. Int J Pharm. 2004;269:303–10. doi: 10.1016/j.ijpharm.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 36.Tang C, Yin CH, Pei YY, Zhang M, Wu LF. New superporous hydrogels composites based on aqueous Carbopols solution (SPHCcs): synthesis, characterization and in vitro bioadhesive force studies. Eur Polymer J. 2005;41:557–62. [Google Scholar]
- 37.Park K, Chen J, Park H. Hydrogel composites and superporous hydrogel composites having fast swelling, high mechanical strength and superabsorbent properties. 2001 US Patent no 6271278. [Google Scholar]
- 38.Ramesh HP, Viswanatha S, Tharanathan RN. Safety evaluation of formulations containing carboxymethyl derivatives of starch and Chitosan in albino rats. Carbohydr Polym. 2004;58:411–35. [Google Scholar]
- 39.Müller W, Anders E. Floating system for oral therapy. 1989 WO Patent no 89/06956. [Google Scholar]
- 40.Dave BS, Amin AF, Patel MM. Gastroretentive drug delivery system of Ranitidine hydrochloride: formulation and in vitro evaluation. [cited 2008 Dec 19];AAPS PharmSciTech [serial on the Internet] 2004 5 doi: 10.1208/pt050234. Available from: http://www.aapspharmscitech. org/articles/pt0502/pt050234/pt050234.pdf. Article 34[about 6 p.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Streubel A, Siepmann J, Bodmeier R. Floating matrix tablets based on low density foam powder: effects of formulation and processing parameters on drug release. Eur J Pharm Sci. 2003;18:37–45. doi: 10.1016/s0928-0987(02)00223-3. [DOI] [PubMed] [Google Scholar]
- 42.Streubel A, Siepmann J, Bodmeier R. Floating microparticles based on low density foam powder. Int J Pharm. 2002;241:279–92. doi: 10.1016/s0378-5173(02)00241-7. [DOI] [PubMed] [Google Scholar]
- 43.Dorkoosh FA, Verhoef JC, Borchard G, Rafiee-Tehrani M, Verheijden JH, Junginger HE. Intestinal absorption of human insulin in pigs using delivery systems based on superporous hydrogel polymers. Int J Pharm. 2002;247:47–55. doi: 10.1016/s0378-5173(02)00361-7. [DOI] [PubMed] [Google Scholar]
- 44.Ranitidine hydrochloride. USA: United States Pharmacopoeial Convention, Inc; 2003. The United States Pharmacopoeia 26 / The National Formulary 21; pp. 1615–9. [Google Scholar]
- 45.Costa P, Sousa Lobo JM. Modeling and comparison of dissolution profiles. Eur J Pharm Sci. 2001;13:123–33. doi: 10.1016/s0928-0987(01)00095-1. [DOI] [PubMed] [Google Scholar]
- 46.Gibaldi M, Feldman S. Establishment of sink conditions in dissolution rate determinations - theoretical considerations and application to nondisintegrating dosage forms. J Pharm Sci. 1967;56:1238–42. doi: 10.1002/jps.2600561005. [DOI] [PubMed] [Google Scholar]
- 47.Wagner JG. Interpretation of percent dissolved-time plots derived from In vitro testing of conventional tablets and capsules. J Pharm Sci. 1969;58:1253–7. doi: 10.1002/jps.2600581021. [DOI] [PubMed] [Google Scholar]
- 48.Higuchi T. Mechanism of sustained-action medication.Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J Pharm Sci. 1963;52:1145–9. doi: 10.1002/jps.2600521210. [DOI] [PubMed] [Google Scholar]
- 49.Sood A, Panchagnula R. Drug release evaluation of diltiazem CR preparations. Int J Pharm. 1998;175:95–107. [Google Scholar]
- 50.Hixson AW, Crowell JH. Dependence of reaction velocity upon surface and agitation. Ind Eng Chem. 1931;23:923–31. [Google Scholar]
- 51.Korsmeyer RW, Gurny R, Doelker E, Buri P, Peppas NA. Mechanisms of solute release from porous hydrophilic polymers. Int J Pharm. 1983;15:25–35. doi: 10.1002/jps.2600721021. [DOI] [PubMed] [Google Scholar]
- 52.Langenbucher F. Linearization of dissolution rate curves by the Weibull distribution. J Pharm Pharmacol. 1972;24:979–81. doi: 10.1111/j.2042-7158.1972.tb08930.x. [DOI] [PubMed] [Google Scholar]
- 53.Hopfenberg HB. Controlled release polymeric formulations. In: Paul DR, Haris FW, editors. Acs Symposium Series. Vol. 33. Washington DC: American Chemical Society; 1976. pp. 26–31. [Google Scholar]
- 54.Katzhendler I, Hoffman A, Goldberger A, Friedman M. Modeling of drug release from erodible tablets. J Pharm Sci. 1997;86:110–5. doi: 10.1021/js9600538. [DOI] [PubMed] [Google Scholar]
- 55.Dorkoosh FA, Brussee J, Verhoef JC, Borchard G, Rafiee-Tehrani M, Junginger H. Preparation and NMR characterization of superporous hydrogels (SPH) and SPH composites. Polymer. 2000;41:8213–20. [Google Scholar]
- 56.Avadi MR, Mahdavinia G, Sadeghi AM, Erfan M, Amini M, Tehrani MR, Shafiee A. Synthesis and characterization of N- diethyl methyl Chitosan. Iranian Polymer J. 2004;13:431–6. [Google Scholar]
- 57.Guyton AC. Basic Human Physiology: Normal Function and Mechanisms of Disease. Vol. 2. Philadelphia: W.B. Saunders Co; 1977. pp. 662–4. [Google Scholar]
- 58.Chen J, Blevins WE, Park H, Park K. Gastric retention properties of superporous hydrogel composites. J Control Release. 2000;64:39–51. doi: 10.1016/s0168-3659(99)00139-x. [DOI] [PubMed] [Google Scholar]
- 59.Jacques CHM, Hopfenberg HB, Stannett V. Super case II transport of organic vapors in glassy polymers. In: Hopfenberg HB, editor. Permeability of plastic films and coatings to gases, vapors, and liquids. New York: Plenum Press; 1974. pp. 73–86. [Google Scholar]
- 60.Dorkoosh FA, Stokkel MP, Blok D, Borchard G, Rafiee-Tehrani M, Verhoef JC, et al. Feasibility study on the retention of superporous hydrogel composite polymer in the intestinal tract of man using scintigraphy. J Control Release. 2004;99:199–206. doi: 10.1016/j.jconrel.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 61.Risbud MV, Bhonde RR. Polyacrylamide-Chitosan hydrogels: In vitro biocompatibility and sustained antibiotic release studies. Drug Deliv. 2000;7:69–75. doi: 10.1080/107175400266623. [DOI] [PubMed] [Google Scholar]
- 62.Yin L, Zhao X, Cui L, Ding J, He M, Tang C, Yin C. Cytotoxicity and genotoxicity of superporous hydrogel containing interpenetrating polymer networks. Food Chem Toxicol. 2009;47:1139–45. doi: 10.1016/j.fct.2009.01.043. [DOI] [PubMed] [Google Scholar]


