Abstract
Antimalarial drugs constitute a major part of antiprotozoal drugs and have been in practice for a long time. Antimalarial agents generally belong to the class of quinoline which acts by interfering with heme metabolism. The recent increase in development of chloroquine-resistant strains of Plasmodium falciparum and failure of vaccination program against malaria have fuelled the drug discovery program against this old and widespread disease. Quinoline and its related derivative comprise a class of heterocycles, which has been exploited immensely than any other nucleus for the development of potent antimalarial agents. Various chemical modifications of quinoline have been attempted to achieve analogs with potent antimalarial properties against sensitive as well as resistant strains of Plasmodium sp., together with minimal potential undesirable side effects. This review outlines essentially some of the recent chemical modifications undertaken for the development of potent antimalarial agents based on quinoline.
Keywords: Antimalarial activity, chemical modifications, quinoline
Malaria is one of the most widespread diseases in the world besides tuberculosis and AIDS. It is estimated by World Health Organization (WHO) that about 40% of the world's population presently lives under malarial threat and approximately 300–500 million cases of malaria occur annually, leading to 1–3 million deaths. Mortality is particularly high for children under the age of 5 years, accounting for about 25% of childhood deaths in Africa.[1–3] Malaria is caused by different species of Plasmodium, of which Plasmodium falciparum is the most virulent human malarial parasite and others include Plasmodium vivax, Plasmodium malariae and Plasmodium ovale. A major reason for the continued severity of the worldwide malarial problem is the increasing resistance of malarial parasites to the available antimalarial drugs such as chloroquine (CQ). Although continued attempts to develop a vaccine for malaria are ongoing, drugs continue to be the only treatment option available.
Quinoline containing compounds have long been used for the treatment of malaria, beginning with quinine. Systematic modification of quinine led to the potent and inexpensive 4-aminoquinoline drug, CQ, and other related drugs [Figure 1]. After the worldwide development of drug resistance to CQ, rational approach in chemistry and screening efforts produced mefloquine, another quinoline containing compound that was highly active against the CQ-resistant (CQR) strains of P. falciparum. Since the development of mefloquine, there have been several reports of new potent quinoline compounds. Most of these contain the 7-chloroquinoline nucleus of CQ and vary in the length and nature of their basic amine side chain. Currently, compounds such as amodiaquine (AQ) and AQ-13 are promising leads for the development of new drugs.[4,5]
Figure 1.
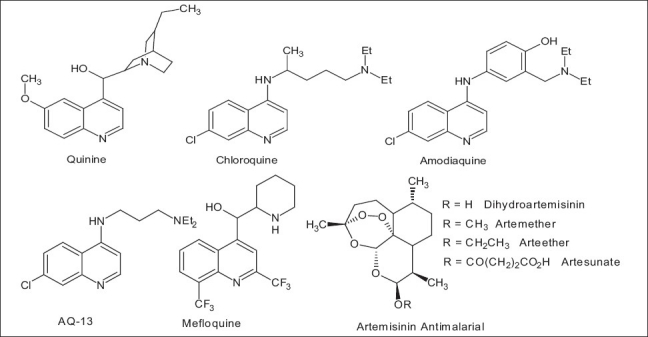
Chemical structures of some antimalarial drugs
With a chemical structure significantly different from that of quinoline-based drugs, the natural product artemisinin and its derivatives have attracted the attention of many different groups. The mechanism of action of artemisinin antimalarials is reported to be devoid of significant clinical resistance, with its 1,2,4-trioxane as active motif. 1,2,4-trioxane as active motif of artemisinin has served as a template for the design of synthetic peroxide-containing drugs.[6,7]
In drug designing programs, an essential component of the search for new lead is the synthesis of molecules which are novel, yet resemble known biologically active molecules by virtue of the presence of critical structural features essential for desired pharmacologic effect. In this review, we have highlighted some of the antimalarial drug discovery efforts related to quinoline nucleus, which are currently being developed at universities, research institutions, and pharmaceutical companies worldwide.
Quinoline Chalcones
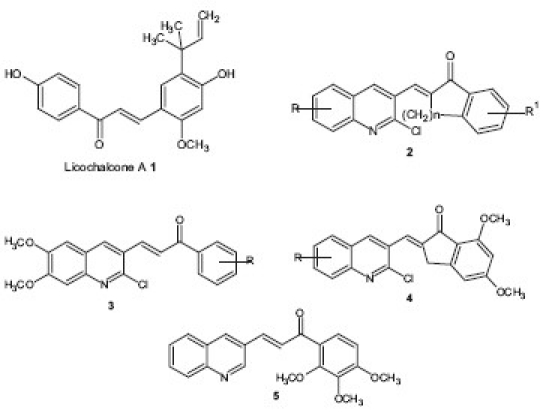
The antimalarial activity of chalcones was first noted when licochalcone A 1 , a natural product isolated from Chinese liquorice roots, was reported to exhibit potent antimalarial activity. Later on, it was observed that good antimalarial effects were exerted by alkoxylated chalcones with polar B rings, in particular, those substituted with electron withdrawing groups or those incorporating quinoline rings.[8] Since then, a number of chalcone derivatives containing quinoline and other heteroaryl moiety have been synthesized and evaluated for potential antimalarial activity. Charris et al.[9,10] have reported a series of E-2-quinolinylbenzocycloalcanones 2 and evaluated their activity to inhibit β-hematin formation and the hydrolysis of hemoglobin in vitro and their efficacy in rodent Plasmodium berghei. Inhibition of β-hematin formation was minimal when a hydrogen or methoxy groups were present on the position 8 of the quinoline and position 4’ of the indanone ring. The study was further elaborated by synthesizing quinolinyl chalcones having dimethoxy group 3, 4.[11] Similarly, Liu et al.[12] have synthesized multi alkoxylated chalcones 5 on ring B and evaluated their in vitro action against P. falciparum (K1) in a [3H] hypoxanthine uptake assay. Trimethoxy, dimethoxy and methoxy analogs showed good in vitro activities (IC50 < 5 μM).
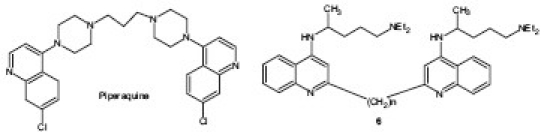
Bisquinoline Derivatives
Antimalarial drug containing bisquinoline was first synthesized in the 1960s (piperaquine) and used extensively in China as a prophylactic and for treatment against malaria during that era. With the development of piperaquine-resistant strains of P. falciparum and the emergence of the artemisinin derivatives, its use declined during the 1980s.[13] Recently, various laboratories all over the world have again focused on developing potential antimalarial agents having bisquinoline structure. Ayad et al.[14] synthesized a series of novel bisquinoline compounds 6 comprising 4-(4-diethylamino-1-methylbutyl)aminoquinoline units joined through the 2-position by a (CH2)n linker and evaluated their ability to inhibit the growth of both CQ-sensitive (CQS) (D10) and CQR (K1) strains of P. falciparum, the hydrogen peroxide-mediated pathway for decomposition of heme and the conversion of heme to β-hematin. Results of the study revealed that the most active compound (n = 12) exhibited effects similar to CQ in three of the assays. However, it was even more active against the resistant strain [IC50, 17 nM (K1); 43 nM (D10)], much superior to CQ (IC50, 540 nM) and slightly better than mefloquine (IC50, 30 nM).
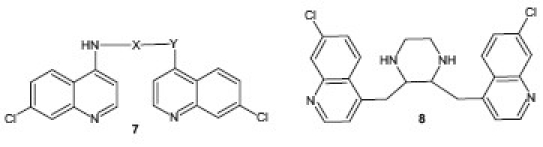
A series of bisquinoline derivatives 7 have been synthesized and screened in vitro for methemoglobin (MetHb) formation and methemoglobin reductase activity by Srivastava et al.,[15] with promising antimalarial activity. N,N-bis(7-chloroquinolin-4-yl)heteroalkanediamines 8 were synthesized and screened in vitro against P. falciparum and P. berghei in vivo by Vennerstrom et al.[16] These bisquinolines showed IC50 values in the range of 1–100 nM against P. falciparum (in vitro) while they were potent inhibitors of hematin polymerization with IC50 values falling in the narrow range of 5–20 μM.
Chloroquine Analogs
CQ, N’-(7-chloroquinolin-4-yl)-N,N-diethyl-pentane-1,4-diamine was discovered in 1934 by Hans Andersag and coworkers at the Bayer laboratories, who named it “Resochin”. It was ignored for a decade because it was considered too toxic for human use. During World War II, the United States government-sponsored clinical trials for antimalarial drug development showed unequivocally that CQ has a significant therapeutic value as an antimalarial drug. It was introduced into clinical practice in 1947 for the prophylactic treatment of malaria. Until the emergence of CQR strains of P. falciparum, this drug was treated as panacea against the malaria disease. In the last few decades, there has been a tremendous amount of research activity directed toward the development of potent antimalarials based on chloroquine.
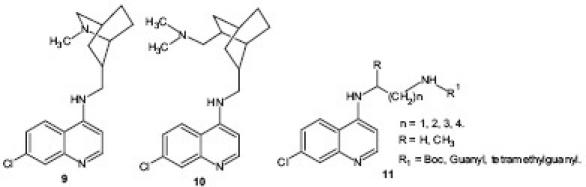
Faruk Khan et al.[17] reported a semirigid analog of the antimalarial drug CQ by incorporating isoquinuclidine (2-azabicyclo[2.2.2]octane) 9 , 10 ring system which may be viewed as a semirigid boat form of the piperidine ring and, when properly substituted, a scaffold for rigid analogs of biologically active ethanolamines and propanolamines. All the analogs were tested in vitro against P. falciparum strains and Leishmania donovani promastigote cultures and some of them displayed potent antimalarial activity against both CQ-susceptible D6 and the CQR W2 strains of P. falciparum. Similarly, Solomon et al.[18] reported a series of CQ analogs containing guanyl and tetramethylguanyl moieties 11 and tested in vitro against CQS strain of P. falciparum and CQR N-67 strain of P. yoelii in vivo. All the analogs were found to form strong complex with hematin and inhibit the β-hematin formation in vitro and this suggests that these compounds act on heme polymerization.
They also synthesized a new series of side chain modified, i.e., 4-aminoquinolines 2-(substituted phenyl)-2,3-dihydro-4H-1,3-benzothiazin-4-one incorporated, CQ derivatives 12 with potent antimalarial activity against P. falciparum in vitro and P. yoelli in vivo.[19] A series of (trifluoromethyl-1H-pyrazol-1-yl) analogs incorporating CQ motif were designed and synthesized by Cunico et al.[20] and compound 13 showed significant activity in vitro and emerged to be a promising new class of antimalarial drug. The transformation of freely rotatable N,N-diethyl-pentane-1,4-diamine chain of CQ into semirigid structure using pyrrolizidinylalkyl 14 motif has been reported by Sparatore et al.[21] and tested in vitro against CQS and CQR strains of P. falciparum and in vivo in a P. berghei mouse model of infection. Both the compounds exhibited excellent activity in all tests and low toxicity against mammalian cells. Development of novel metal-based chemotherapeutic agent has become an area of interest against many tropical diseases. Navarro et al.[22] prepared a number of new Au(I) and Au(III) complexes of CQ 15 , 16 and evaluated them in vitro against several strains of P. falciparum. All the complexes displayed in vitro activity against CQS and CQR strains of P. falciparum. The highest activity for this series was obtained for complex Au(I), which is 5 times more active than chloroquine diphosphate (CQDP) against the CQR strain FcB1. Ray and coworkers designed two lead molecules, 17 and 18 , with promising in vitro therapeutic efficacy, improved Absorption Distribution Metabolism Excretion and Toxicity (ADMET) properties, low risk for drug—drug interactions, and desirable pharmacokinetic profiles. Both the derivatives exhibited highly potent antimalarial activity, with IC50 values of 5.6 and 17.3 nM, respectively, against the W2 (CQR) strain of P. falciparum (for CQ, IC50 = 382 nM).[23]
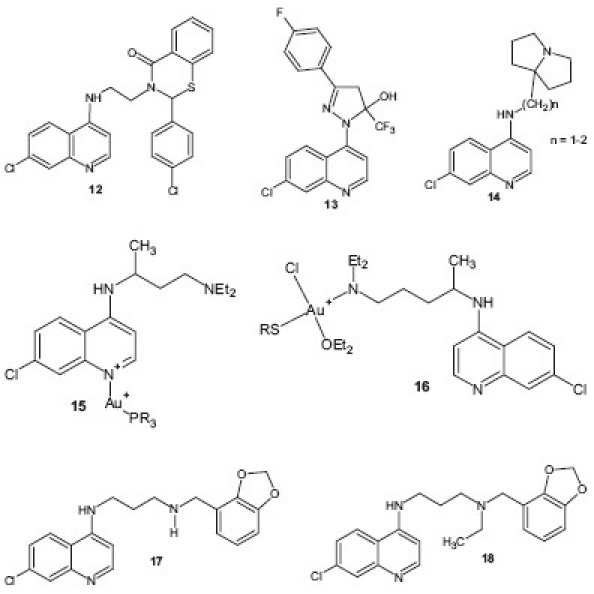
Based on the fact that incorporation of an intermolecular hydrogen-bonding motif in the side chain of 4-aminoquinolines enhanced the activity against drug-resistant P. falciparum, a series of 116 compounds 19 , 20 containing four different alkyl linkers and various aromatic substitutions with hydrogen bond accepting capability were synthesized by Madrid et al.[24] Compounds showed broad potency against the drug-resistant W2 strain of P. falciparum and, in particular, a novel series containing variations of the α-aminocresol motif gave compounds with IC50 values more potent than 5 nM against the W2 strain.
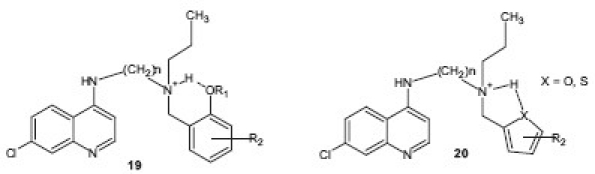
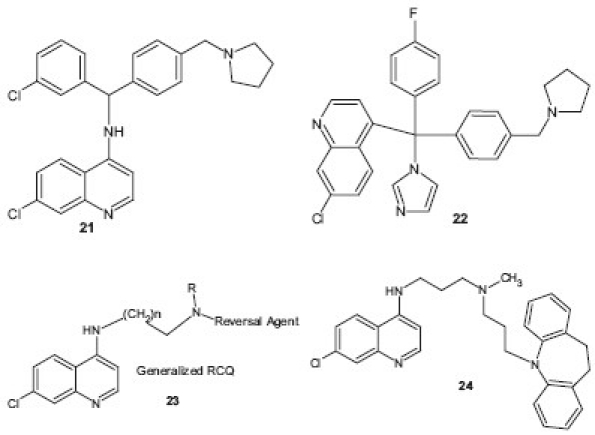
Design and synthesis of antimalarials based on novel structural pharmacophores has become an important approach for developing potential antimalarial agent in recent times. Antimalarials based on a polyaromatic pharmacophore were designed by Gemma et al.[25,26] using hybrid molecules with clotrimazole-like pharmacophore with a polyarylmethyl group 21 , 22 . These compounds were found to be selective in interacting with free heme and interfering with P. falciparum heme metabolism. The reason for this interaction appeared to be a combination of the polyarylmethyl system, which enables to form and stabilize radical intermediates, with the iron-complexing and conjugation-mediated electron transfer properties of the 4(9)-aminoquinoline(acridine). Among the compounds tested, 21 exhibited in vivo potent activity against P. chabaudi and P. berghei after oral administration and possessed promising pharmacokinetic properties. It was selected for further preclinical development. To overcome the development of resistance against CQ, a class of hybrid molecules, which we term “reversed chloroquines” (RCQs), 23 was designed by Burgess et al.[27] A prototype molecule, N’-(7-chloroquinolin-4-yl)-N-[3-(10,11-dihydrodibenzo[b,f]azepin-5-yl)propyl]-N-methylpropane-1,3-diamine 24 was found to be effective at low nanomolar concentrations against both CQS and CQR strains of P. falciparum.
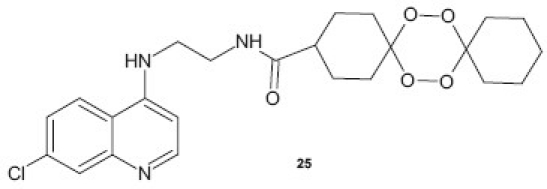
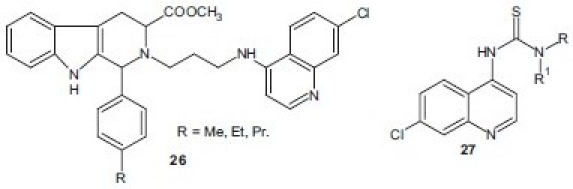
Combination of two pharmacophores in a single matrix with synergistic effect is one of the approaches in drug design for the development of potent bioactive molecules. 1,2,4-trioxane as an active motif of artemisinin, a naturally occurring potent antimalarial, has resulted in the development of newer class of synthetic peroxide-containing drugs as antimalarials. Opsenica et al.[28] have synthesized chimeric molecules consisting of two pharmacophores, tetraoxane and 7-chloro-4-aminoquinoline, and showed relatively potent in vitro antimalarial activities, with IC90 values for the P. falciparum strain W2 in the range of 2.26-12.44 nM. Compound 25 exhibited potent in vivo activity in cured mice. Likewise, a series of hybrid molecules 2-[3-(7-chloro-quinolin-4-ylamino)-alkyl]-1-(substitutedphenyl)-2,3,4,9-tetrahydro-1H-β-carbolines 26 have been synthesized by Gupta et al.[29] and screened for their in vitro antimalarial activity. Some of derivatives showed minimum inhibitory concentration MIC in the range of 0.05–0.11 μM and were several folds more active than CQ in vitro. A series of new 7-chloroquinolinyl thiourea 27 derivatives derived from the corresponding 4,7-dichloroquinoline isothiocyanate were prepared and evaluated for in vitro antimalarial and anticancer activity. The most active compound from the series displayed an inhibitory IC50 value of 1.2 μM against the D10 strain of P. falciparum.[30]
Piperazine Incorporated Chloroquine Analogs
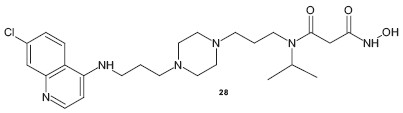
A variety of piperazine incorporated chloroquinie analogs have been designed and synthesized to improve its efficacy and its effectiveness against CQR strains. Flipo et al.[31] reported a new class of piperazine incorporated CQ analog as an inhibitor of PfA-M1, a neutral zinc aminopeptidase of P. falciparum, a new potential target for the discovery of new antimalarial agents, and compound 28 showed an IC50 of 854 nM. Likewise, Ryckebusch et al. reported a series of N1 -(7-chloro-4-quinolyl)-1,4-bis(3-aminopropyl)piperazine derivatives 29 and evaluated their antimalarial activity against a CQR strain of P. falciparum.[32]
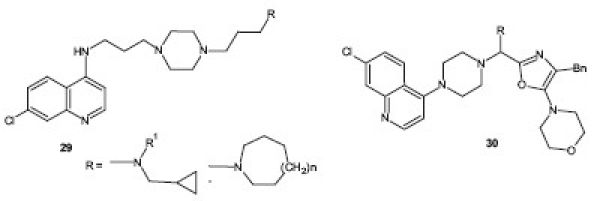
A series of 4-aminoquinoline-containing 2,4,5-trisubstituted aminoxazoles 30 and piperazine/morpholine were synthesized and screened in vitro against two strains of the P. falciparum parasite by Musonda et al. A number of compounds exhibited significantly more potent activity than the standard drug CQ.[33]
Amodiaquine Analog
AQ is a 4-aminoquinoline, structurally related to CQ in which N,N-diethyl-pentane-1,4-diamine chain is replaced by 2-[(diethylamino)methyl]phenol and was found to be more effective against CQR parasites. But its use is compromised by its hepatotoxicity and its ability to cause agranulocytosis. Pharmacologic studies have revealed that a regioisomer of AQ, in which the positions of the groups on the hydroxyl aniline ring have been swapped, cannot be oxidized to the quinone imine and was comparatively less hepatotoxic. Further optimization led to the discovery of N-t-butyl-isoquine 31 , which is now being transitioned toward antimalarial clinical trials.[34,35]
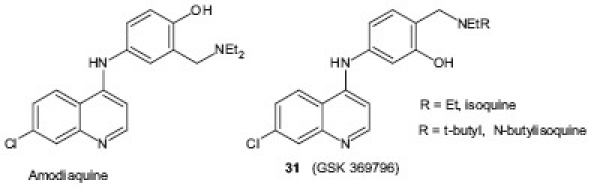
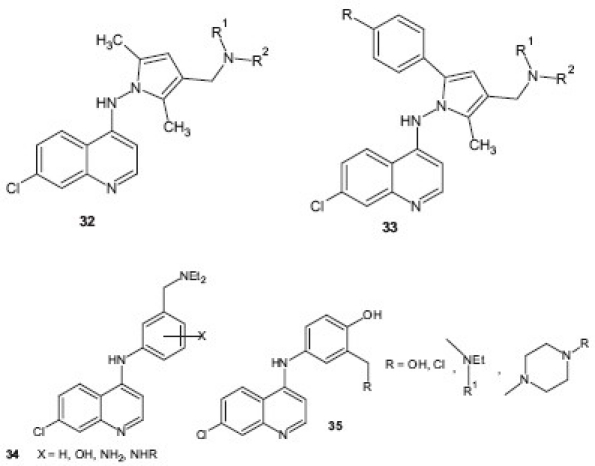
Casagrande et al.[36] designed a new class of antimalarial agents by replacing the phenolic ring of AQ, tebuquine, and isoquine with other aromatic nuclei and reported that several compounds containing pyrrole analogs 32 , 33 displayed high activity against both D10 (CQS) and W-2 (CQR) strains of P. falciparum. Similarly, Sandrine et al.[37] studied the significance of the 4′-phenolic group in the antimalarial activity and/or cytotoxicity of AQ and synthesized newer analogs of AQ in which the phenolic group was either shifted or modified. Among the compounds tested, new amino derivative 34 displayed the greatest selectivity index toward the most CQR strain and was active in mice infected by P. berghei. A series of new AQ derivatives bearing modified lateral basic chains as new agents 35 with both antimalarial and antileishmanial activities were reported by Guglielmo et al. Most of the compounds displayed antileishmanial activity in low micromolar range and were cytotoxic with a narrow therapeutic window.[38]
8-Aminoquinoline Analogs
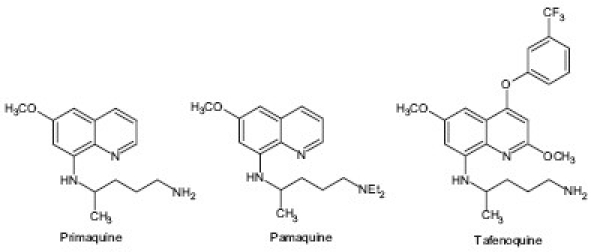
8-aminoquinoline is another class of quinoline-based antimalarial drug that contains three members, primaquine, tafenoquine and pamaquine, which are used in the treatment of malaria. They may be used to eradicate malarial hypnozoites from the liver and have been used for malarial prophylaxis. Pamaquine is no longer available, but primaquine is still used routinely worldwide as a part of the treatment of P. vivax and P. ovale malaria. Tafenoquine is currently in Phase III clinical trials and is still not available for prescription.
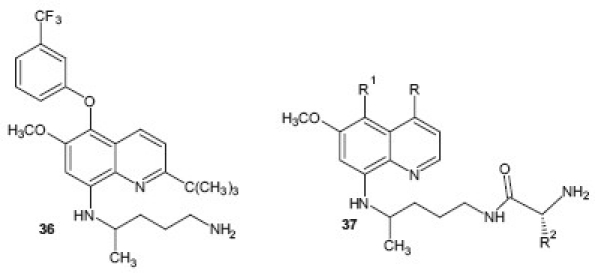
The structural exploitation of this class is still going on and numerous reports are available on recent developments based on this phrmacophore. Jain et al.[39] reported a series of 8-quinolinamines related to 2-tert-butylprimaquine and evaluated them for their in vitro antimalarial activity. Among the compound tested, analog 36 was found to exhibit curative antimalarial activity at a dose of 25 mg/kg/day × 4 in a P. berghei infected mice model, and produced suppressive activity at a lower dose of 10 mg/kg/day × 4. The research group further studied the antimalarial activity of amino acid, e.g., alanine, lysine, ornithine and valine conjugated to primaquine and other 8-quinolinamine 37. The results of this study represent a development of highly potent 8-quinolinamine derivatives for antimalarial activity. Compound N1-[4-(5-butoxy-4-ethyl-6-methoxy-8-quinolylamino)pentyl]-(2S)-2,6-diaminohexanamide that showed curative activity at 5 mg/kg in the P. berghei test emerged as the most effective compound and compound N1-[4-(4-ethyl-5-hexoxy-6-methoxy-8-quinolylamino)pentyl]-(2S)-2,6-diaminohexanamide exhibited curative activity at 50 mg/kg against P. yoelii nigeriensis in mice and was found to be most potent analog against multidrug resistant strain.[40]
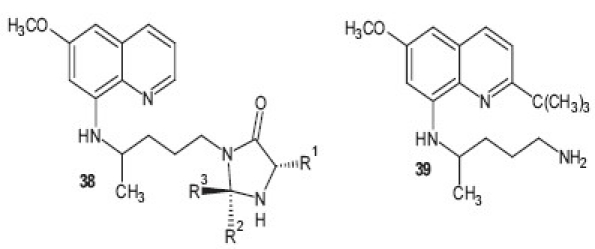
Imidazolidin-4-one derivatives of primaquine were synthesized as potential double prodrugs of the parent drug by Araújo et al.[41] and the derivatives were found to inhibit the development of the sporogonic cycle of P. berghei, affecting the appearance of oocysts in the midguts of the mosquitoes. These imidazolidin-4-one derivatives 38 of primaquine represent novel transmission-blocking antimalarials. To avoid the unwanted metabolic pathway of the quinoline ring of primaquine has results in development of new stearically hindered derivative of primaquine where C-2 position of drug is blocked by metabolically stable bulky alkyl group [R = C(CH3)3]. This compound 39 showed excellent antimalarial efficacy against P. berghei in vivo and was highly potent against multidrug resistant P. yoelii nigeriensis strain. This study describes the discovery of a highly potent blood-schizontocidal antimalarial analog 2 , completely devoid of MetHb toxicity.[42]
Acridine and Quinoline Fused Analog
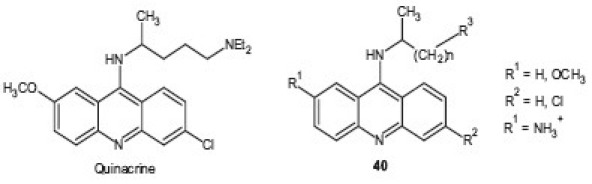
Quinacrine is an acridine-based antiprotozoal which has been used as an antimalarial agent. It is also used in other protozoal infections which include giardiasis, where quinacrine is indicated as a primary agent for patients with metronidazole-resistant giardiasis and for patients who should not receive or cannot tolerate metronidazole. Many useful structural modifications of acridine and its derivatives are in progress to generate potential antimalarial drugs based on this nucleus. Guetzoyan et al.[43] reported a series of acridine derivatives 40, 41 and their in vitro antimalarial activity evaluation against one CQ-susceptible strain (3D7) and three CQR strains (W2, Bre1 and FCR3) of P. falciparum. Structure activity relationship SAR of compounds showed that two positives charges as well as 6-chloro and 2-methoxy substituents on the acridine ring were required to exert a good antimalarial activity and compounds possessing these features inhibited the growth of the CQ-susceptible strain with an IC50 ≤ 0.07 μM, close to that of CQ, and better than CQ against CQR strains with IC50 ≤ 0.3 μM. Among them, compound 9-(6-ammonioethylamino)-6-chloro-2-methoxyacridinium dichloride displayed a promising antimalarial activity in vitro with a quite good selectivity index
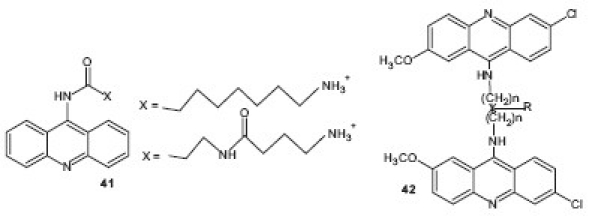
versus mammalian cell on the CQ-susceptible strain and promising selectivity on other strains. A series of bis(9-amino-6-chloro-2-methoxyacridine) derivatives 42 in which acridine moieties were joined by alkanediamines, polyamines, or polyamines substituted by a side chain, were synthesized and tested for their antimalarial activity by Girault et al.[44] The results of the study revealed the importance of the nature of the linker and its side chain for antiparasitic activity, cytotoxicity, and cellular localization. Some of the compounds displayed IC50 values ranging from 8 to 18 nM against different P. falciparum strains while three others totally inhibited Trypanosoma brucei at 1.56 μM.
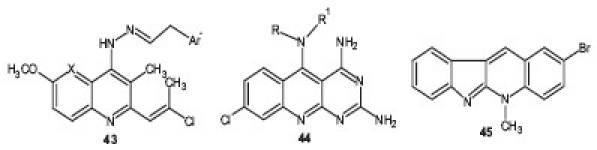
In another study, a series N2-acrydinylhydrazones 43 were synthesized and tested for their antimalarial properties. These compounds showed remarkable in vitro anti-plasmodial activity, especially against CQR strains.[45] Joshi et al. designed and synthesized a series of novel 5-substituted amino-2,4-diamino-8-chloropyrimido-[4,5-b]quinolines 44 based on the pharmacophore developed for potent antimalarial activity, using Chem-X and MOE softwares. The compounds were evaluated by Rane's test for blood schizonticidal activity in mice infected by P. berghei. Based on the Mean Survival Time (MST) data of the nine compounds evaluated, three were found to have curative potential when compared with CQ.[46] On the basis of the original lead neocryptolepine or 5-methyl-5H-indolo[2,3-b]quinoline, an alkaloid from Cryptolepis sanguinolenta, derivatives were prepared using a biradical cyclization methodology and screened for their antimalarial activity and cytotoxicity on human MRC-5 cells. 2-Bromoneocryptolepine 45 was the most selective compound with an IC50 value against CQR P. falciparum of 4.0 μM. 2-bromoneocryptolepine showed a low affinity for DNA and no inhibition of human topoisomerase II, in contrast to cryptolepine.[47]
Organometallic Ferrocene Analog of Quinoline
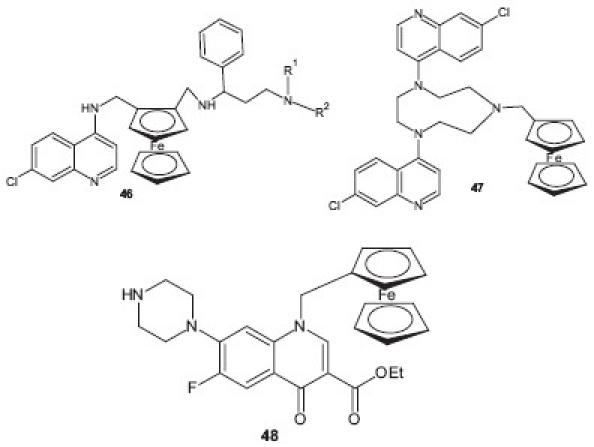
This is one of the recent approaches evolved for the design and development of newer potent derivatives for malarial chemotherapy. In these molecules, which are generally analogs of CQ, a ferrocene nucleus (dicyclopentadienyl iron) is localized at different sites of molecule and then evaluated for antimalarial activity.[48] Based on this approach, a new series of organic and organometallic dual drugs 46 were designed as potential antimalarial agents by Wenzel et al.[49] Several compounds not only showed a marked antimalarial activity with IC50 and IC90 values in the low nanomolar range, but also showed a high cytotoxicity against mammalian cells. The newly designed compounds revealed high DNA binding properties, especially for the GC-rich domains. Altogether, these dual drugs seem to be more appropriate to be developed as antiproliferative agents against mammalian cancer cells than Plasmodium parasites. Similarly, easily accessible ferrocenic quinoline derivatives were synthesized by Biot et al.[50] from triazacyclononane and were evaluated for their antiplasmodial properties against CQS (HB3) and CQR (Dd2) P. falciparum. Compound 7-chloro-4-[4-(7-chloro-4-quinolyl)-7-ferrocenylmethyl-1,4,7-triazacyclononan-1-yl]quinoline 47 showed potent antimalarial activity in vitro against the CQR strain Dd2. Dubar et al.[51] carried out the derivatization of the fluoroquinolone, ciprofloxacin, with a ferrocene nucleus (dicyclopentadienyl iron) resulting in new achiral compounds 48 which were found to be 10- to 100-fold more active than ciprofloxacin against P. falciparum CQ-susceptible and CQR strains. These achiral derivatives killed parasites more rapidly than ciprofloxacin. These derivatives appeared to be promising new leads and creating a new family of antimalarial agents.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Technical Report Series. Geneva: World Health Organization; 2000. WHO Expert Committee on Malaria. 20th Report. [PubMed] [Google Scholar]
- 2.Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature. 2005;434:214–7. doi: 10.1038/nature03342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bathurst I, Hentschel C. Medicines for Malaria Venture: Sustaining antimalarial drug development. Trends Parasitology. 2006;22:301–7. doi: 10.1016/j.pt.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 4.Gelb MG. Drug discovery for malaria: A very challenging and timely endeavor. Curr Opin Chem Biol. 2007;11:440–5. doi: 10.1016/j.cbpa.2007.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenthal PJ. Antimalarial drug discovery: Old and new approaches. J Exp Biol. 2003;206:3735–44. doi: 10.1242/jeb.00589. [DOI] [PubMed] [Google Scholar]
- 6.Robert A, Benoit-Vical F, Dechy-Cabaret O, Meunier B. From classical antimalarial drugs to new compounds based on the mechanism of action of artemisinin. Pure Appl Chem. 2001;73:1173–88. [Google Scholar]
- 7.Coslédan F, Fraisse L, Pellet A, Guillou F, Mordmüller B, Kremsner PG, et al. Selection of a trioxaquine as an antimalarial drug candidate. Proc Natl Acad Sci U S A. 2008;105:17579–84. doi: 10.1073/pnas.0804338105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen M, Theander TG, Christensen BS, Hviid L, Zhai L, Kharazmi A. Licochalcone A, a new antimalarial agent, inhibits in vitro growth of the human malaria parasite Plasmodium falciparum and protects mice from P.yoelii infection. Antimicrob Agents Chemothe. 1994;38:1470–5. doi: 10.1128/aac.38.7.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charris JE, Domínguez JN, Gamboa N, Rodrigues JR, Angel JE. Synthesis and antimalarial activity of E-2-quinolinylbenzocycloalcanones. Eur J Med Chem. 2005;40:875–81. doi: 10.1016/j.ejmech.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 10.Domínguez JN, Charris JE, Lobo G, Gamboa-Domínguez N, Moreno MM, Riggione F, et al. Synthesis of quinolinyl chalcones and evaluation of their antimalarial activity. Eur J Med Chem. 2001;36:555–60. doi: 10.1016/s0223-5234(01)01245-4. [DOI] [PubMed] [Google Scholar]
- 11.Charris JE, Lobo GM, Camacho J, Ferrer R, Barazarte A, Domínguez JN, et al. Synthesis and Antimalarial Activity of (E) 2-(2’-Chloro-3’- Quinolinylmethyl idene)-5,7-Dimethoxyindanones. Lett Drug Des Discovery. 2007;4:49–54. [Google Scholar]
- 12.Liu M, Wilairat P, Go M-L. Antimalarial Alkoxylated and Hydroxylated Chalones: Structure—Activity Relationship Analysis. J Med Chem. 2001;44:4443–52. doi: 10.1021/jm0101747. [DOI] [PubMed] [Google Scholar]
- 13.Davis TM, Hung TY, Sim IK, Karunajeewa HA, Ilett KF. Piperaquine: A Resurgent Antimalarial Drug. Drugs. 2005;65:75–87. doi: 10.2165/00003495-200565010-00004. [DOI] [PubMed] [Google Scholar]
- 14.Ayad F, Tilley L, Deady LW. Synthesis, antimalarial activity and inhibition of haem detoxification of novel bisquinolines. Bioorg Med Chem. 2001;11:2075–7. doi: 10.1016/s0960-894x(01)00383-3. [DOI] [PubMed] [Google Scholar]
- 15.Srivastava S, Tewari S, Chauhan PM, Puri SK, Bhaduri AP, Pandey VC. Synthesis of bisquinolines and their in vitro ability to produce methemoglobin in canine hemolysate. Bioorg Med Chem Lett. 1999;09:653–8. doi: 10.1016/s0960-894x(99)00058-x. [DOI] [PubMed] [Google Scholar]
- 16.Vennerstrom JL, Ager AL, Jr, Dorn A, Andersen SL, Gerena L, Ridley RG, et al. Bisquinolines. 2. Antimalarial N,N-Bis(7-chloroquinolin-4-yl) heteroalkanediamines. J Med Chem. 1998;41:4360–4. doi: 10.1021/jm9803828. [DOI] [PubMed] [Google Scholar]
- 17.Faruk Khan MO, Levi MS, Tekwani BL, Wilson NH, Borne RG. Synthesis of isoquinuclidine analogues of chloroquinie: Antimalarial and antileishmanial activity. Bioorg Med Chem. 2007;15:3919–25. doi: 10.1016/j.bmc.2006.11.024. [DOI] [PubMed] [Google Scholar]
- 18.Solomon VR, Puri SK, Srivastava K, Katti SB. Design and synthesis of new antimalarial agents from 4-aminoquinoline. Bioorg Med Chem. 2005;13:2157–65. doi: 10.1016/j.bmc.2004.12.051. [DOI] [PubMed] [Google Scholar]
- 19.Solomon VR, Haq W, Srivastava K, Puri SK, Katti SB. Synthesis and Antimalarial Activity of Side Chain Modified 4-Aminoquinoline Derivatives. J Med Chem. 2007;50:394–8. doi: 10.1021/jm061002i. [DOI] [PubMed] [Google Scholar]
- 20.Cunico W, Cechinel CA, Bonacorso HG, Martins MAP, Zanatta N, De Souza MV, et al. Antimalarial activity of 4-(5-trifluoromethyl-1H-pyrazol-1-yl)-chloroquine analogues. Bioorg Med Chem Lett. 2006;16:649–53. doi: 10.1016/j.bmcl.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 21.Sparatore A, Basilico N, Casagrande M, Parapini S, Taramelli D, Brun R, et al. Antimalarial activity of novel pyrrolizidinyl derivatives of 4-aminoquinoline. Bioorg Med Chem Lett. 2008;18:3737–40. doi: 10.1016/j.bmcl.2008.05.042. [DOI] [PubMed] [Google Scholar]
- 22.Navarro M, Vásquez S, Sánchez-Delgado RA, Pérez H, Sinou V, Schrével J. Toward a Novel Metal-Based Chemotherapy against Tropical Diseases.7. Synthesis and in vitro Antimalarial Activity of New Gold—Chloroquine Complexes. J Med Chem. 2004;47:5204–9. doi: 10.1021/jm049792o. [DOI] [PubMed] [Google Scholar]
- 23.Ray S, Madrid PB, Catz P, LeValley SE, Furniss MJ, Rausch LL, et al. Development of a New Generation of 4-Aminoquinoline Antimalarial Compounds Using Predictive Pharmacokinetic and Toxicology Models. J Med Chem. 2010;53:3685–95. doi: 10.1021/jm100057h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Madrid PB, Liou AP, DeRisi JL, Guy KR. Incorporation of an Intramolecular Hydrogen-Bonding Motif in the Side Chain of 4-Aminoquinolines Enhances Activity against Drug-Resistant P.falciparum. J Med Chem. 2006;49:4535–43. doi: 10.1021/jm0600951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gemma S, Campiani G, Butini S, Joshi BP, Kukreja G, Coccone SS, et al. Combining 4-Aminoquinoline- and Clotrimazole-Based Pharmacophores toward Innovative and Potent Hybrid Antimalarials. J Med Chem. 2009;52:502–13. doi: 10.1021/jm801352s. [DOI] [PubMed] [Google Scholar]
- 26.Gemma S, Campiani G, Butini S, Joshi BP, Kukreja G, Coccone SS, et al. Clotrimazole Scaffold as an Innovative Pharmacophore Towards Potent Antimalarial Agents: Design, Synthesis, and Biological and Structure-Activity Relationship Studies. J Med Chem. 2008;51:1278–94. doi: 10.1021/jm701247k. [DOI] [PubMed] [Google Scholar]
- 27.Burgess SJ, Selzer A, Kelly JX, Smilkstein MJ, Riscoe MK, Peyton DH. A Chloroquine-like Molecule Designed to Reverse Resistance in Plasmodium falciparum. J Med Chem. 2006;49:5623–5. doi: 10.1021/jm060399n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Opsenica I, Opsenica D, Lanteri CA, Anova L, Milhous WK, Smith KS, et al. New Chimeric Antimalarials with 4-Aminoquinoline Moiety Linked to a Tetraoxane Skeleton. J Med Chem. 2008;51:6216–9. doi: 10.1021/jm8006905. [DOI] [PubMed] [Google Scholar]
- 29.Gupta L, Srivastava K, Singh S, Puri SK, Chauhan PMS. Synthesis of 2-[3-(7-Chloro-quinolin-4-ylamino)-alkyl]-1-(substituted phenyl)-2,3,4,9-tetrahydro-1H-β-carbolines as a new class of antimalarial agents. Bioorg. Med. Chem. Lett. 2008;18:3306–3309. doi: 10.1016/j.bmcl.2008.04.030. [DOI] [PubMed] [Google Scholar]
- 30.Mahajan A, Yeh S, Nell M, Van Rensburg CE, Chibale K. Synthesis of new 7-chloroquinolinyl thiourea and their biological investigation as potential antimalarial and anticancer agents. Bioorg Med Chem Lett. 2007;17:5683–5. doi: 10.1016/j.bmcl.2007.07.049. [DOI] [PubMed] [Google Scholar]
- 31.Flipo M, Florent I, Grellier P, Sergheraert C, Deprez-Poulain R. Design, synthesis and antimalarial activity of novel, quinoline-based, zinc metallo-aminopeptidase inhibitor. Bioorg Med Chem Lett. 2003;13:2659–62. doi: 10.1016/s0960-894x(03)00550-x. [DOI] [PubMed] [Google Scholar]
- 32.Ryckebusch A, Debreu-Fontaine M-A, Mouray E, Grellier P, Sergheraert C, Melnyk P. Synthesis and antimalarial evaluation of N1-(7-chloro-4-quinolyl)-1,4-bis(3-aminopropyl) Piperazine derivatives. Bioorg Med Chem Lett. 2005;15:297–302. doi: 10.1016/j.bmcl.2004.10.080. [DOI] [PubMed] [Google Scholar]
- 33.Musonda CC, Little S, Yardley V, Chibale K. Application of multicomponant reaction to antimalarial drug discovery.Part 3: Discovery of aminoxazole 4-aminoquinolines with potent antiplasmodial activity in vitro. Bioorg Med Chem Lett. 2007;17:4733–6. doi: 10.1016/j.bmcl.2007.06.070. [DOI] [PubMed] [Google Scholar]
- 34.O’Neill PM, Park BK, Shone AE, Maggs JL, Roberts P, Stocks PA, et al. Candidate Selection and Preclinical Evaluation of N-tert-Butyl Isoquine (GSK369796), An Affordable and Effective 4-Aminoquinoline Antimalarial for the 21st Century. J Med Chem. 2009;52:1408–15. doi: 10.1021/jm8012618. [DOI] [PubMed] [Google Scholar]
- 35.O’Neill PM, Mukhtar A, Stocks PA, Randle LE, Hindley S, Ward SA, et al. Isoquine and Related Amodiaquine Analogues: A New Generation of Improved 4-Aminoquinoline Antimalarials. J Med Chem. 2003;46:4933–45. doi: 10.1021/jm030796n. [DOI] [PubMed] [Google Scholar]
- 36.Casagrande M, Basilico N, Parapini S, Romeo S, Taramelli D, Sparatore A. Novel amodiaquine congeners as potent antimalarial agent. Bioorg Med Chem. 2008;16:6813–23. doi: 10.1016/j.bmc.2008.05.068. [DOI] [PubMed] [Google Scholar]
- 37.Sandrine D-C, Paunescu E, Maes L, Mouray E, Sergheraert C, Grellier p, et al. Synthesis and antimalarial activity of new analogues of amodiaquine. Eur J Med Chem. 2008;43:252–60. doi: 10.1016/j.ejmech.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 38.Guglielmo S, Bertinaria M, Rolando B, Crosetti M, Fruttero R, Yardley V, et al. A new series of amodiaquine analogues modified in the basic side chain with in vitro antileishmanial and antiplasmodial activity. Eur J Med Chem. 2009;44:5071–9. doi: 10.1016/j.ejmech.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 39.Jain M, Khan SI, Tekwani BL, Jacob MR, Singh S, Jain R, et al. Synthesis, antimalarial, antileishmanial and antimicrobial activities of some 8-quinolinamine analogues. Bioorg Med Chem. 2005;13:4458–66. doi: 10.1016/j.bmc.2005.04.034. [DOI] [PubMed] [Google Scholar]
- 40.Vangapandu S, Sachdeva S, Jain M, Singh S, Singh PP, Jain R, et al. Quinolinamine conjugated with amino acids are exhibiting potent blood-schizontocidal antimalarial activities. Bioorg Med Chem. 2004;12:239–47. doi: 10.1016/j.bmc.2003.10.028. [DOI] [PubMed] [Google Scholar]
- 41.Araújo MJ, Bom J, Capela R, Casimiro C, Chambel P, Gomes P, et al. Imidazolidin-4-one Derivatives of Primaquine as Novel Transmission-Blocking Antimalarials. J Med Chem. 2005;48:888–92. doi: 10.1021/jm0494624. [DOI] [PubMed] [Google Scholar]
- 42.Jain M, Vangapandu S, Sachdeva S, Singh S, Singh PP, Jena GB, et al. Discovery of a Bulky 2-tert-Butyl Group Containing Primaquine Analogue That Exhibits Potent Blood-Schizontocidal Antimalarial Activities and Complete Elimination of Methemoglobin Toxicity. J Med Chem. 2004;47:285–7. doi: 10.1021/jm0304562. [DOI] [PubMed] [Google Scholar]
- 43.Guetzoyan L, Yu X-M, Ramiandrasoa F, Pethe S, Rogier C, Pradines B, et al. Antimalarial acridines: Synthesis, in vitro activity against P. falciparum and interaction with hematin. Bioorg Med Chem. 2009;17:8032–9. doi: 10.1016/j.bmc.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 44.Girault S, Grellier P, Berecibar A, Maes L, Mouray E, Lemière P, et al. Antimalarial, Antitrypanosomal, and Antileishmanial Activities and Cytotoxicity of Bis(9-amino-6-chloro-2-methoxyacridines): Influence of the Linker. J Med Chem. 2000;43:2646–54. doi: 10.1021/jm990946n. [DOI] [PubMed] [Google Scholar]
- 45.Gemma S, Kukreja G, Fattorusso C, Persico M, Romano MP, Altarelli M, et al. Synthesis of N1-arylidene-N2-quinolyl- and N2-acridinylhydrazones as potent antimalarial agents active against CQ-resistant P. falciparum strains. Bioorg Med Chem Lett. 2006;16:5384–8. doi: 10.1016/j.bmcl.2006.07.060. [DOI] [PubMed] [Google Scholar]
- 46.Joshi AA, Narkhede SS, Viswanathan CL. Design, synthesis and evaluation of 5-substituted amino-2,4-diamino-8-chloropyrimido-[4,5-b]quinolines as novel antimalarials. Bioorg Med Chem Lett. 2005;15:73–6. doi: 10.1016/j.bmcl.2004.10.037. [DOI] [PubMed] [Google Scholar]
- 47.Jonckers TH, Miert S, Cimanga K, Bailly C, Colson P, De Pauw-Gillet M-C, et al. Synthesis, Cytotoxicity, and Antiplasmodial and Antitrypanosomal Activity of New Neocryptolepine Derivatives. J Med Chem. 2002;45:3497–508. doi: 10.1021/jm011102i. [DOI] [PubMed] [Google Scholar]
- 48.Domarle O, Blampain G, Agnaniet H, Nzadiyabi T, Lebibi J, Brocard J, et al. In vitro Antimalarial Activity of a New Organometallic Analog, Ferrocene-Chloroquine. Antimicrob Agents Chemother. 1998;42:540–4. doi: 10.1128/aac.42.3.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wenzel NI, Chavain N, Wang Y, Friebolin W, Maes L, Pradines B, et al. Antimalarial versus Cytotoxic Properties of Dual Drugs Derived From 4-Aminoquinolines and Mannich Bases: Interaction with DNA. J Med Chem. 2010;53:3214–26. doi: 10.1021/jm9018383. [DOI] [PubMed] [Google Scholar]
- 50.Biot C, Dessolin J, Ricard I, Dive D. Easily synthesized antimalarial ferrocene triazacyclononane quinoline conjugates. J Organomet Chem. 2004;689:4678–82. [Google Scholar]
- 51.Dubar F, Anquetin G, Pradines B, Dive D, Khalife J, Biot C. Enhancement of the Antimalarial Activity of Ciprofloxacin Using a Double Prodrug/Bioorganometallic Approach. J Med Chem. 2009;52:7954–7. doi: 10.1021/jm901357n. [DOI] [PubMed] [Google Scholar]


