Abstract
To date, the major role of HPV16E6 in cancer has been considered to be its ability to inhibit the p53 tumor-suppressor protein, thereby thwarting p53-mediated cytotoxic responses to cellular stress signals. Here, we show that HPV16E6-dependent c-fos oncogenic protein expression contributes to AP-1 complex formation under oxidative stress in SiHa cells (HPV16-positive squamous cell carcinoma of the cervix). In addition, we examined the role of HPV16E6 in TGF-α-induced c-fos expression and found that the c-fos protein expression induced by TGF-α is HPV16E6 dependent. Thus, our results provide the first evidence that HPV16E6 contributes to AP-1 complex formation after both ligand-dependent and independent EGFR activation, suggesting a new therapeutic approach to the treatment of HPV-associated tumors.
1. Introduction
HPV (human papillomavirus) infection is a hallmark of uterine cervical cancer and is thought to be important, if not causal, for some types of tumorigenesis [1–3]. Two HPV-encoded proteins, E6 and E7, inactivate and inhibit the expression of the p53 and RB tumor suppressor proteins, respectively. Nevertheless, HPV infection is not sufficient for tumorigenesis, because HPV infection is also present in healthy individuals [4], and the overexpression of E6/E7 alone is capable of immortalizing primary human epithelial cells but does not induce tumor cell transformation [5]. Thus, understanding the conditions that stimulate HPV-infected cells is crucial for the development of effective treatments for HPV-associated tumors.
The transcription factor AP-1, which is composed of heterodimers of members of the c-Jun and c-Fos families, regulates various cellular processes such as enhanced proliferation, apoptosis, and tumor metastasis [6]. In particular, among the various members of the AP-1 family, c-fos acts as a tumor promoter, and c-fos upregulation causes cellular transformation that is characterized by colony formation in soft-agar and tumor formation in nude mice [7, 8]. In addition, when c-fos binds to c-jun, it increases the gene expression of cyclinD1 and contributes to the potentiation of malignancy [9–11].
In cervical cancer, the AP-1 complex formed during tumor development consists of a c-fos/c-jun heterodimer [9, 12–14], and c-fos/c-jun formation is also implicated in HPV-induced esophageal tumor development [15]. In fact, various c-fos target genes are reported to be expressed at higher levels in cervical cancer cells with comparison to normal cervical epithelial cells [16]. While c-fos showed very low expression in normal samples and moderate expression in cervical premalignant lesions, the tumor tissues showed very strong expression [13]. It is, therefore, reasonable to assume that c-fos regulation plays a fundamental role in HPV-induced tumor development. However, the extracellular conditions in which c-fos expression and AP-1 complex formation are induced in HPV-infected cells are unknown.
A shift in the composition of AP-1 from fra-1/c-jun to c-fos/c-jun heterodimers occurs during HPV-infected tumor development [7, 14]. The same shift in AP-1 composition also takes place under oxidative stress, such as after UV-B exposure [10]. As transient transfection experiments showed that HPV16E6 induces the transcription of the c-fos promoter [17], we hypothesized that under oxidative stress HPV16E6 function might contribute to c-fos expression and c-fos/c-jun heterodimer formation.
There are at least 15 cancer-associated HPV types, and of these, HPV16 is found in more than 50% of HPV-positive cancer tissues [1, 2]. Here, we used SiHa cells (HPV16-positive squamous cell carcinoma of the cervix) and showed that H2O2 and TGF-α, which are promoters of the multistep carcinogenic process [18, 19], induced c-fos/c-jun heterodimer formation in SiHa cells, whereas o-phenanthroline inhibited H2O2-induced c-fos upregulation. H2O2 and TGF-α-induced c-fos upregulation was impaired in SiHa cells transfected with siRNA HPV16E6. This result further implicates H2O2 and TGF-α signaling in the pathogenesis of HPV-induced tumor development.
2. Material and Methods
2.1. Reagents
H2O2 was purchased from WAKO (Tokyo, Japan), and o-phenanthroline was obtained from Sigma-Aldrich (S.t. Louis, Mo, USA).
2.2. Cell Culture and Treatment
The SiHa and Caski cell line used in our study was obtained from the American Type Culture Collection. SiHa cells were routinely cultured in DMEM (Invitrogen) supplemented with 5% fetal bovine serum (Sigma) at 37°C under 5% CO2. SiHa cells were serum-deprived for at least 16 hours before being stimulated with H2O2 (1 mM). To observe the effect of an Fe2+ chelating agent on the activation of c-fos, o-phenanthroline (0.2 mM) was added to the culture medium 1 hour before stimulation with H2O2.
2.3. SDS–PAGE and Western Blotting
The cell cultures (80–100% confluent) were washed twice with ice-cold phosphate buffered saline and lysed in 1 × Laemmli sample buffer supplemented with a protease inhibitor cocktail (Roche) and a phosphatase inhibitor cocktail (Roche). The cells were scraped and homogenized at 4°C with a syringe and a thin needle, and the cell lysates were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE, 10% resolving gel). The resultant proteins were electrophoretically transferred onto nitrocellulose membranes (Pall Corporation), before being incubated for 2 h at room temperature with blocking buffer containing 4% nonfat dried milk in TBS (20 mM Tris (pH 7.6) and 150 mM NaCl). They were then washed with TBS-T (0.1% Tween-20) and incubated overnight at 4°C in TBS buffer containing 4% BSA (Wako) and appropriate antibodies. The antibodies used in the experiment were anti-c-fos (Santa Cruz, sc-52, 1 : 500) and anti-c-jun (Cell Signaling, L70B11, 1 : 500), and the secondary antibodies were Donkey Antirabbit IgG/HRP (Amersham NA934, 1 : 5000) and Goat Antimouse IgG/HRP (Chemicon, AP124P 1 : 5000).
The protein bands were visualized using an enhanced chemiluminescence (ECL) detection system (GE Healthcare Bio-Sciences). The signals from the membrane were detected and imaged using a LAS-4000 imager (Fujifilm Corporation). The membranes were then stripped (30 min at 56°C) in stripping buffer containing 100 mM 2-mercaptoethanol, 2% SDS, and 62.5 mM Tris–HCl (pH 6.7) and reprobed with β-actin or tubulin as a loading control. All Western blots were performed at least three times for each experiment. To quantify the Western blot data, densitometric analysis of ECL-exposed blots was performed using the NIH ImageJ software (version 1.45f).
2.4. CoIP Assay
The antibodies used for the Western blot analysis were c-Fos, c-Jun, and CBP/β. For the c-Fos coIP assay, SiHa cells were lysed in a modified RIPA buffer (50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1 mM EDTA, and 1% NP-40) and a protease inhibitor mixture (Roche). CoIP was performed using 2 μg of the c-Fos antibody or the respective isotype control.
2.5. Preparation of Small Interfering RNA and Transfection
Synthesized siRNA duplexes were obtained from Invitrogen. The siRNA sequences targeting HPV16E6 corresponded to nucleotides 5′-ACCGUUGUGUGAUUUGUUATT-3′ (siRNA1-HPV16E6) and 5′-UAACAAAUCACACAACGGUTT-3′ (siRNA2-HPV16E6) of the coding region [20]. The sequence of the negative control (siRNA-scrambled) was as follows: 5′-CCAUUCCGAUCCUGAUCCGTT-3′ and 5′-CGGAUCAGGAUCGGAAUGGTT-3′. Cells in the exponential growth phase were plated in a 35 mm dish containing antibiotic-free medium at 30% confluence and then transfected with siRNA (siRNA-HPV16E6 or siRNA-scrambled) using Oligofectamine (Invitrogen) and Opti-MEM I (Invitrogen), according to the manufacturer's protocol. Silencing was examined 24 h after transfection.
2.6. RT-PCR
Total RNA were extracted from the harvested cells using Isogen (Nippon Gene). After the removal of genomic DNA, approximately 1 μg of RNA was used to generate cDNA using Reverse Transcriptase M-MLV (RNase H-) (TaKaRa) and random hexamers (Fermentas). The primer sequences used were as follows: 5′-CGGAATTCATGCACCAAAAGCGAAC-3′ (sense) and 5′-CCCAAGCTTACAGCTGGGTTTCTCT-3′ (antisense) for HPV16E6 [21] and 5′-CAGGGCTGCTTTTAACTCTG-3′ (sense) and 5′-GATGATCTTGAGGCTGTTGTC-3′ (anti-sense) for GAPDH. The PCR reactions for HPV16E6 were run for 30 cycles. GAPDH amplification products were used as a loading control. All amplification products were separated in 2% agarose gels. All RT-PCR were performed at least three times for each experiment.
2.7. Statistical Analysis
Data are expressed as the mean ± s.e.m. The paired-sample t-test was performed for statistical comparisons. For all tests, a P value of less than 0.05 was considered to be significant.
3. Results
3.1. o-phenanthroline Inhibits H2O2-Induced c-Fos Expression
The upregulation of c-fos has been observed after H2O2 treatment in both epithelial and endothelial cells [19, 22], which raises the possibility that SiHa cells might respond to H2O2 in a similar manner. To test this, we analyzed c-fos protein expression after H2O2 treatment in SiHa cells. Western blotting analysis revealed aberrantly high levels of c-fos protein expression after H2O2 treatment (Figure 1(a)). In the H2O2-treated SiHa cells, the expression of the AP-1 family member c-jun was also upregulated (Figure 1(a)). o-phenanthroline is an iron chelator [23] that protects cells from cancer progression by inhibiting the Fenton reaction, and it has been reported that in a mouse model of gastric cancer the intraperitoneal injection of o-phenanthroline significantly reduced the incidence of gastric cancers [24] by inhibiting the Fenton reaction. The effect of o-phenanthroline on c-fos expression in SiHa cells is unclear; therefore, we tested the effect of o-phenanthroline on H2O2-induced c-fos expression in SiHa cells by pre-treating SiHa cells with o-phenanthroline before H2O2 treatment. As a result, we found that o-phenanthroline markedly inhibited H2O2-induced c-fos expression (Figure 1(b)).
Figure 1.
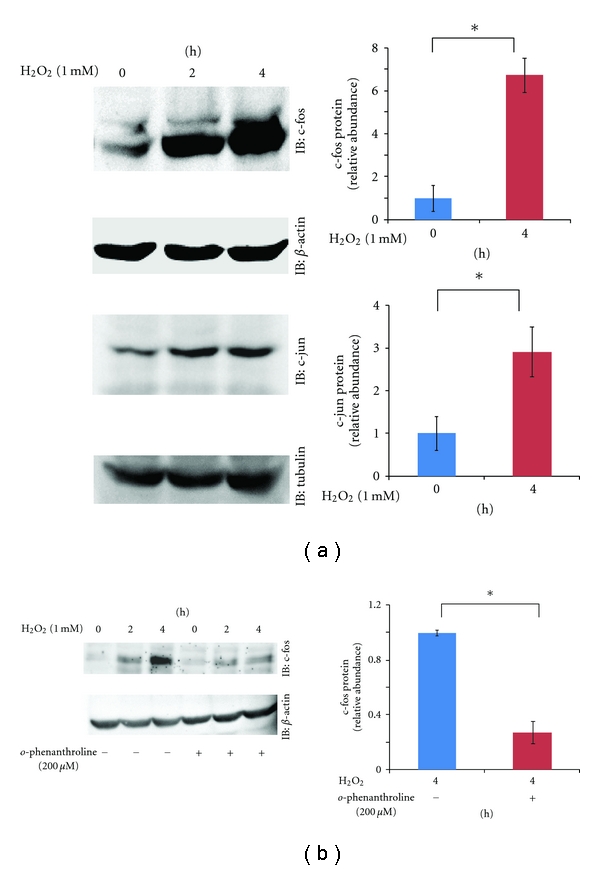
(a) Upregulation of AP-1 gene expression in SiHa cells at the indicated time points after H2O2 (1 mM) exposure. Representative blots are shown and include β-actin or tubulin as a loading control, along with the results of densitometric analysis (0 h, 4 h; normalized to β-actin or tubulin). *P < 0.05, n = 3. (b) Inhibitory effect of o-phenanthroline on H2O2-induced c-fos upregulation. Cells were pretreated for 1 hour with or without o-phenanthroline (200 μM) before H2O2 (1 mM) exposure. Representative blots are shown and include β-actin as a loading control, along with the results of densitometric analysis (4 h; normalized to β-actin). *P < 0.05, n = 4.
3.2. Characterization of c-Fos/c-jun Heterodimers after Ligand-Dependent and Independent EGFR Activation
The upregulation of c-fos observed in epithelial cells after H2O2 treatment is mainly mediated through EGFR activation [18]. We, therefore, examined c-fos protein expression in SiHa cells that had been treated with the EGFR ligand TGF-α. Western blotting analysis revealed aberrantly high levels of c-fos protein expression after TGF-α treatment (Figure 2(a)). We then investigated c-fos/c-jun heterodimer formation in SiHa cells. We stimulated SiHa cells with H2O2 or TGF-α for 4 hours, immunoprecipitated c-fos, and determined its partner AP-1 family member by Western blotting (Figure 2(b)). Interactions between c-fos and c-jun were observed after both H2O2 and TGF-α stimulation (Figure 2(b): the IP with isotype control did not show any specific band).
Figure 2.
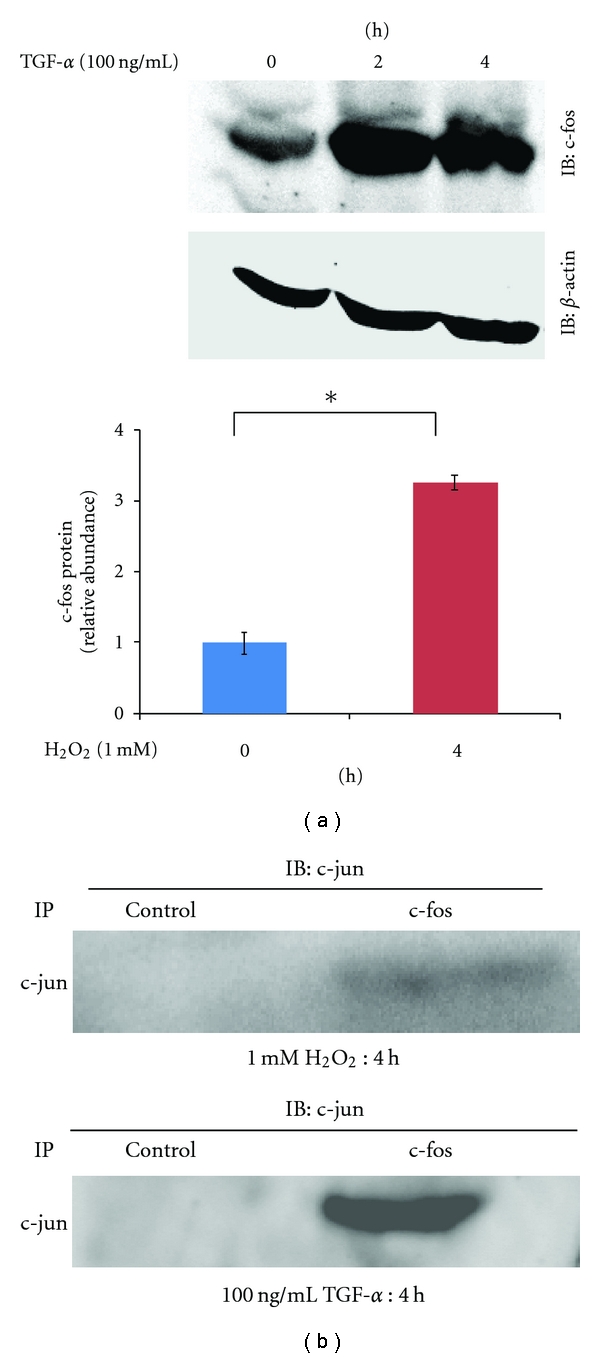
(a) c-fos upregulation in SiHa cells after TGF-α (100 ng/mL) treatment. Cells were harvested at the indicated time points and used for Western blotting analysis. Representative blots are shown and include β-actin as a loading control, along with the results of densitometric analysis (0 h, 4 h; normalized to β-actin). *P < 0.01, n = 3. (b) IP of c-fos was performed using whole-cell extracts. Coprecipitated proteins were detected by Western blotting analysis using antibodies specific to c-jun.
3.3. HPV16E6 Mediates H2O2 and TGF-α-Induced c-Fos Expression
We next tested whether HPV16E6 is necessary for the c-fos expression induced by H2O2 or TGF-α treatment. To directly address the role of HPV16E6 protein in c-fos expression, we transfected SiHa cells with siRNA HPV16E6 (Figure 3(a)). A scrambled nonspecific siRNA duplex was used as a negative control. We observed siRNA HPV16E6-dependent reductions in H2O2 and TGF-α induced c-fos expression (Figures 3(b) and 3(c)). Similar results that siRNA HPV16E6-dependent reduction in c-fos expression induced by H2O2 treatment were obtained in another cervical cancer cell line, Caski cell (Figure 3(d)). These data suggest that HPV16E6 is necessary for H2O2 and TGF-α induced-c-fos expression.
Figure 3.
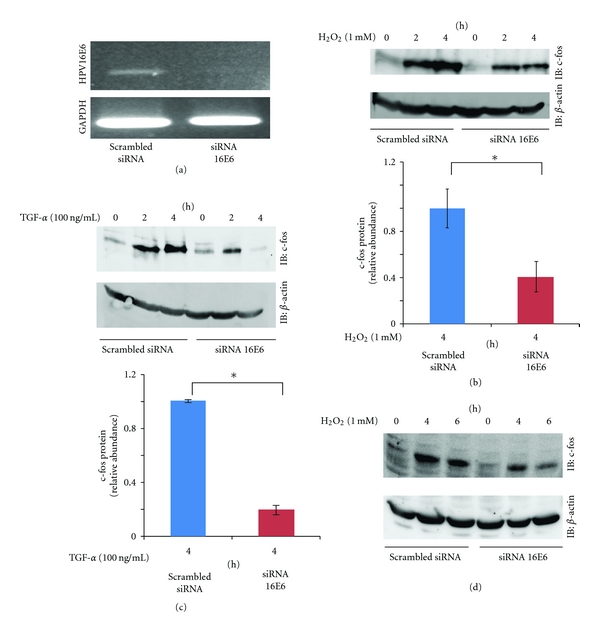
HPV16E6-mediated upregulation of c-fos in SiHa cells. (a) The mRNA levels of HPV16E6 were detected by RT-PCR after HPV16E6 siRNA transfection into SiHa cells. Cells were transfected with scrambled siRNA or HPV16E6 siRNA for 24 hours. (b) HPV16E6-mediated upregulation of c-fos expression in SiHa cells after H2O2 exposure. The transfected cells were exposed to H2O2 (1 mM) and harvested at the indicated time points, before being subjected to Western blotting analysis. Representative blots are shown and include β-actin as a loading control, along with the results of densitometric analysis (4 h; normalized to β-actin). *P < 0.05, n = 4. (c) HPV16E6-mediated upregulation of c-fos expression in SiHa cells after TGF-α exposure. Transfected cells were exposed to TGF-α (100 ng/mL) and harvested at the indicated time points, before being subjected to Western blotting analysis. Representative blots are shown and include β-actin as a loading control, along with the results of densitometric analysis (4 h; normalized to β-actin). *P < 0.05, n = 4. (d) HPV16E6-mediated upregulation of c-fos expression in Caski cells after H2O2 exposure. The transfected cells were exposed to H2O2 (1 mM) and harvested at the indicated time points, before being subjected to Western blotting analysis.
4. Discussion
Our findings provide new insights into the mechanism of c-fos/c-jun heterodimer formation during the development of cervical cancer. First, our findings show that c-fos/c-jun heterodimer formation results from both H2O2 and TGF-α stimulation. The current view is that in cervical cancer c-fos/c-jun heterodimer formation results from the inefficient expression of the ternary complex factor Net [25], but the evidence we have uncovered for HPV16E6 dependent c-fos expression after H2O2 or TGF-α stimulation broadens the scope of the mechanisms of c-fos/c-jun heterodimer formation in extracellular conditions such as oxidative stress. For example, the observed shift in AP-1 composition from fra-1/c-jun to c-fos/c-jun heterodimers in HaCaT keratinocytes after UV-B exposure [10] suggests that after being induced by extracellular and environmental stimuli, c-fos expression and c-fos/c-jun heterodimer formation contribute to the pathological process of HPV positive skin cancer. Therefore, studies of c-fos expression, HPV infection, and skin tumor development are necessary.
Second, our data indicate that HPV16E6 is necessary and sufficient for c-fos/c-jun heterodimer formation to occur in SiHa cells under both H2O2 and TGF-α stimulation. In the presence of transition metals such as iron and copper, H2O2 can be oxidized into extremely reactive and toxic HO• via the Fenton reaction. The hydroxyl radical (HO•) then activates EGFR [26]. Our data show that H2O2 and TGF-α are stimulators of c-fos/c-jun heterodimer formation and confirm that the iron chelator is an inhibitor of c-fos expression that acts by inhibiting the Fenton reaction. The negative effect of o-phenanthroline on HPV16E6 mediated c-fos expression might explain why iron chelation inhibits growth and induces the apoptosis of HPV-positive carcinoma cells [27]. Tumorigenic cervical cells express higher levels of intracellular iron [28], which creates an environment that favors HPV16E6-mediated c-fos/c-jun heterodimer formation in HPV-infected cells. This implies that iron is necessary for c-fos/c-jun heterodimer formation. The precise triggers of H2O2 expression around cervical epithelial cells are unknown, but it has been proposed that chronic inflammation triggers the accumulation of macrophages in the vicinity of the cervix, and that these cells subsequently produce the antimicrobial molecule H2O2.
Finally, our data indicate that cervical cancer, the hallmark of which is HPV infection, is an EGFR-sensitive disease regardless of ligand-dependent or ligand-independent stimulation. EGFR mAb therapies are effective for treating squamous cell carcinoma of the head and neck and are also being evaluated in other HPV-associated tumors [29]. One of the best-known mechanisms of EGFR mAb therapy resistance is ligand-independent EGFR activation [30]. This was observed in studies of squamous carcinoma cells (T-Hep3) that naturally overexpress the uPA receptor, one of which found that EGFR was activated by the uPA receptor in a ligand-independent fashion, resulting in resistance to EGFR mAb [31]. Therapies such as the use of o-phenanthroline, or alternative approaches such as the use of small molecule EGFR tyrosine kinase inhibitors that block the intracellular tyrosine kinase domain, could hold great potential for the treatment of head and neck cancer.
References
- 1.Zelkowitz R. Cancer: HPV casts a wider shadow. Science. 2009;323(5914):580–581. doi: 10.1126/science.323.5914.580. [DOI] [PubMed] [Google Scholar]
- 2.Wang X, Tian X, Liu F, et al. Detection of HPV DNA in esophageal cancer specimens from different regions and ethnic groups: a descriptive study. BMC Cancer. 2010;10, article no. 19 doi: 10.1186/1471-2407-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akgül B, Cooke JC, Storey A. HPV-associated skin disease. Journal of Pathology. 2006;208(2):165–175. doi: 10.1002/path.1893. [DOI] [PubMed] [Google Scholar]
- 4.do Sacramento PR, Babeto E, Colombo J, et al. The prevalence of human papillomavirus in the oropharynx in healthy individuals in a Brazilian population. Journal of Medical Virology. 2006;78(5):614–618. doi: 10.1002/jmv.20583. [DOI] [PubMed] [Google Scholar]
- 5.Al Moustafa AE, Foulkes WD, Wong A, et al. Cyclin D1 is essential for neoplastic transformation induced by both E6/E7 and E6/E7/ErbB-2 cooperation in normal cells. Oncogene. 2004;23(30):5252–5256. doi: 10.1038/sj.onc.1207679. [DOI] [PubMed] [Google Scholar]
- 6.Eferl R, Wagner EF. AP-1: a double-edged sword in tumorigenesis. Nature Reviews Cancer. 2003;3(11):859–868. doi: 10.1038/nrc1209. [DOI] [PubMed] [Google Scholar]
- 7.Soto U, Das BC, Lengert M, Finzer P, Zur Hausen H, Rösl F. Conversion of HPV 18 positive non-tumorigenic HeLa-fibroblast hybrids to invasive growth involves loss of TNF-α mediated repression of viral transcription and modification of the AP-1 transcription complex. Oncogene. 1999;18(21):3187–3198. doi: 10.1038/sj.onc.1202765. [DOI] [PubMed] [Google Scholar]
- 8.Joo A, Aburatani H, Morii E, Iba H, Yoshimura A. STAT3 and MITF cooperatively induce cellular transformation through upregulation of c-fos expression. Oncogene. 2004;23(3):726–734. doi: 10.1038/sj.onc.1207174. [DOI] [PubMed] [Google Scholar]
- 9.Turatti E, da Costa Neves A, de Magalhães MH, de Sousa SO. Assessment of c-Jun, c-Fos and cyclin D1 in premalignant and malignant oral lesions. Journal of oral science. 2005;47(2):71–76. doi: 10.2334/josnusd.47.71. [DOI] [PubMed] [Google Scholar]
- 10.Catani MV, Savini I, Rossi A, Melino G, Avigliano L. Biological role of vitamin C in keratinocytes. Nutrition Reviews. 2005;63(3):81–90. doi: 10.1111/j.1753-4887.2005.tb00125.x. [DOI] [PubMed] [Google Scholar]
- 11.Cooper SJ, MacGowan J, Ranger-Moore J, Young MR, Colburn NH, Bowden GT. Expression of dominant negative c-jun inhibits ultraviolet B-induced squamous cell carcinoma number and size in an SKH-1 hairless mouse model. Molecular Cancer Research. 2003;1(11):848–854. [PubMed] [Google Scholar]
- 12.De Wilde J, De-Castro Arce J, Snijders PJF, Meijer CJLM, Rösl F, Steenbergen RDM. Alterations in AP-1 and AP-1 regulatory genes during HPV-induced carcinogenesis. Cellular Oncology. 2008;30(1):77–87. doi: 10.1155/2008/279656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prusty BK, Das BC. Constitutive activation of transcription factor AP-1 in cervical cancer and suppression of human papillomavirus (HPV) transcription and AP-1 activity in HeLa cells by curcumin. International Journal of Cancer. 2005;113(6):951–960. doi: 10.1002/ijc.20668. [DOI] [PubMed] [Google Scholar]
- 14.Soto U, Denk C, Finzer P, Hutter KJ, Zur Hausen H, Rösl F. Genetic complementation to non-tumorigenicity in cervical-carcinoma cells correlates with alterations in AP-1 composition. International Journal of Cancer. 2000;86(6):811–817. doi: 10.1002/(sici)1097-0215(20000615)86:6<811::aid-ijc9>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 15.Hussain S, Bharti AC, Salam I, et al. Transcription factor AP-1 in esophageal squamous cell carcinoma: alterations in activity and expression during human papillomavirus infection. BMC Cancer. 2009;9, article no. 329 doi: 10.1186/1471-2407-9-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rajkumar T, Sabitha K, Vijayalakshmi N, et al. Identification and validation of genes involved in cervical tumourigenesis. BMC Cancer. 2011;11, article 80 doi: 10.1186/1471-2407-11-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morosov A, Phelps WC, Raychaudhuri P. Activation of the c-fos gene by the HPV16 oncoproteins depends upon the cAMP-response element at -60. Journal of Biological Chemistry. 1994;269(28):18434–18440. [PubMed] [Google Scholar]
- 18.Kina S, Nakasone T, Takemoto H, et al. Regulation of chemokine production via oxidative pathway in HeLa cells. Mediators of Inflammation. 2009;2009:5 pages. doi: 10.1155/2009/183760. Article ID 183760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang RP, Peng A, Golard A, et al. Hydrogen peroxide promotes transformation of rat liver non-neoplastic epithelial cells through activation of epidermal growth factor receptor. Molecular Carcinogenesis. 2001;30(4):209–217. doi: 10.1002/mc.1030. [DOI] [PubMed] [Google Scholar]
- 20.An J, Mo D, Liu H, et al. Inactivation of the CYLD deubiquitinase by HPV E6 mediates hypoxia-induced NF-κB activation. Cancer Cell. 2008;14(5):394–407. doi: 10.1016/j.ccr.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Au Yeung CL, Tsang TY, Yau PL, Kwok TT. Human papillomavirus type 16 E6 induces cervical cancer cell migration through the p53/microRNA-23b/urokinase-type plasminogen activator pathway. Oncogene. 2011;30(21):2401–2410. doi: 10.1038/onc.2010.613. [DOI] [PubMed] [Google Scholar]
- 22.Ichiki T, Tokunou T, Fukuyama K, Iino N, Masuda S, Takeshita A. Cyclic AMP response element-binding protein mediates reactive oxygen species-induced c-fos expression. Hypertension. 2003;42(2):177–183. doi: 10.1161/01.HYP.0000079791.26014.04. [DOI] [PubMed] [Google Scholar]
- 23.Guyton KZ, Liu Y, Gorospe M, Xu Q, Holbrook NJ. Activation of mitogen-activated protein kinase by H2O2: role in cell survival following oxidant injury. Journal of Biological Chemistry. 1996;271(8):4138–4142. doi: 10.1074/jbc.271.8.4138. [DOI] [PubMed] [Google Scholar]
- 24.Tatsuta M, Iishi H, Baba M, et al. Suppression by iron chelator phenanthroline of sodium chloride-enhanced gastric carcinogenesis induced by N-methyl-N′-nitro-N-nitrosoguanidine in Wistar rats. Cancer Letters. 2003;191(1):9–16. doi: 10.1016/s0304-3835(01)00797-2. [DOI] [PubMed] [Google Scholar]
- 25.Van Riggelen J, Buchwalter G, Soto U, et al. Loss of net as repressor leads to constitutive increased c-fos transcription in cervical cancer cells. Journal of Biological Chemistry. 2005;280(5):3286–3294. doi: 10.1074/jbc.M409915200. [DOI] [PubMed] [Google Scholar]
- 26.Helfrich YR, Sachs DL, Voorhees JJ. The biology of skin aging. US Dermatology. 2009;4(1):48–51. [Google Scholar]
- 27.Simonart T, Boelaert JR, Mosselmans R, et al. Antiproliferative and apoptotic effects of iron chelators on human cervical carcinoma cells. Gynecologic Oncology. 2002;85(1):95–102. doi: 10.1006/gyno.2001.6570. [DOI] [PubMed] [Google Scholar]
- 28.Disbrow GL, Baege AC, Kierpiec KA, et al. Dihydroartemisinin is cytotoxic to papillomavirus-expressing epithelial cells in vitro and in vivo. Cancer Research. 2005;65(23):10854–10861. doi: 10.1158/0008-5472.CAN-05-1216. [DOI] [PubMed] [Google Scholar]
- 29.Vermorken JB, Mesia R, Rivera F, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. New England Journal of Medicine. 2008;359(11):1116–1127. doi: 10.1056/NEJMoa0802656. [DOI] [PubMed] [Google Scholar]
- 30.Camp ER, Summy J, Bauer TW, Liu W, Gallick GE, Ellis LM. Molecular mechanisms of resistance to therapies targeting the epidermal growth factor receptor. Clinical Cancer Research. 2005;11(1):397–405. [PubMed] [Google Scholar]
- 31.Liu D, Aguirre Ghiso JA, Estrada Y, Ossowski L. EGFR is a transducer of the urokinase receptor initiated signal that is required for in vivo growth of a human carcinoma. Cancer Cell. 2002;1(5):445–457. doi: 10.1016/s1535-6108(02)00072-7. [DOI] [PubMed] [Google Scholar]


