Abstract
Rats given extended access to high-fat high-sugar food show behavioral and physiological changes that are similar to those caused by drugs of abuse. However, parallels between drug and food “addiction” should be drawn with caution.
After half a century of research on the neurobiology of food and drug reward, Princeton professor Bartley Hoebel proposed that sugar can be addictive1. But can eating, even in an unhealthy, seemingly compulsive way, be legitimately labeled an addiction? A study in this issue by Johnson and Kenny2, using rat models, supports Hoebel’s controversial view that it can. Before we consider the implications of this, and suggest some caveats, let’s examine what Johnson and Kenny found.
Johnson and Kenny2 examined rats using behavioral models borrowed from drug-addiction research, but, instead of being given access to cocaine or heroin, the rats were given access to a cafeteria-style diet of energy-dense (high fat and/or high carbohydrate) food, including bacon, sausage, cheesecake, pound cake, frosting and chocolate. The diet had two behavioral effects that were similar to those of exposure to addictive drugs.
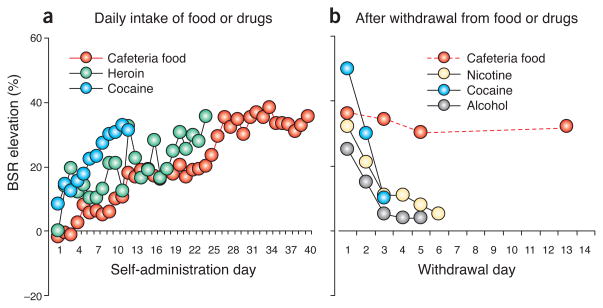
The first effect was disruption of sensitivity to brain-stimulation reward (BSR). Before the rat ‘cafeteria’ opened for business, the rats had spent 10–14 d learning to turn a wheel for electrical stimulation of the lateral hypothalamus. They were then divided into three groups, with one receiving a diet of standard laboratory rat chow, one receiving the standard diet along with restricted access (1 h per d) to the cafeteria food and the third receiving the standard diet and extended access (18–23 h per d) to the cafeteria food. All of the rats weighed 300–350 g when the exposure started. Over the next 40 d, the first two groups gained 80–100 g, which is developmentally typical, whereas the extended-access group gained almost twice as much. The BSR threshold, the minimal level of electrical current required to keep the rats turning the wheel, remained stable in the chow-fed and restricted-access rats but increased in the extended-access rats, reflecting a disruption in brain reward function. Similar disruptions occur after self-administration of addictive drugs (Fig. 1a)3,4. Notably, the reward disruption associated with the cafeteria diet persisted at least 14 d after access ended, which is substantially longer than disruptions observed after withdrawal from nicotine, cocaine or alcohol (Fig. 1b)5–7.
Figure 1.
Prolonged access to cafeteria food causes persistent elevations in threshold for BSR: comparison with drugs of abuse. (a) BSR threshold during daily intake of cafeteria food or drugs. (b) BSR threshold after loss of access to cafeteria food or drugs. Data were redrawn from Johnson and Kenny2 and refs. 3–7. In these studies, rats performed an operant response to obtain rewarding electrical brain stimulation into the median forebrain bundle at the level of the lateral hypothalamus. BSR threshold is defined as the minimum intensity of electrical stimulation that maintains operant responding. Increased BSR threshold is hypothesized to reflect decreased sensitivity of the brain reward system. Extended access to cafeteria food causes progressive disruption of the brain reward system that persists for long periods after loss of access to the food. In contrast, although extended access to abused drugs also causes progressive disruption of the brain reward system, this disruption dissipates in the first few days after withdrawal from the drugs.
The second behavioral effect involved a hallmark of addiction in humans: insensitivity to adverse consequences of drug self-administration. This has been successfully modeled in animal models of drug addiction. Another three groups of rats were given different types of food access for more than 40 d as described above, then some of the rats from each group were exposed to a fear-conditioning procedure in which an electric shock was paired with a light cue. On a subsequent test day, the rats were given access to the cafeteria food in the presence of the now fear-inducing light. The light suppressed cafeteria-food intake in the rats that had only received chow and the rats that had been given limited access to cafeteria food, but not in the rats that had extended access to the cafeteria food. Thus, as with addictive drugs, extended access to cafeteria food led to reward-seeking that was seemingly compulsive in that it was insensitive to a cue that warned of impending punishment.
In addition to these behavioral parallels between cafeteria-food intake and drug self-administration, Johnson and Kenny2 found a neurophysiological parallel between the two. Drawing on prior findings that human drug addiction and obesity are each associated with decreased expression of D2 dopamine receptors in the striatum8, the authors examined D2 receptor expression in the dorsal striatum of their rats after more than 40 d of exposure to cafeteria food and found that it was inversely related to weight gain. To determine whether reduced D2 receptor expression was actually causing addiction-like behaviors, the authors used a viral vector to knock down receptor expression in the dorsal striatum of rats that were exposed to cafeteria food for just 14 d, a period that is normally not long enough to induce changes in BSR threshold or fear-cue-induced suppression of feeding. When D2 receptor expression was knocked down, these addiction-like behavioral changes were seen within 14 d. This is an interesting, although anatomically imperfect, parallel with prior findings; escalation of voluntary cocaine intake in rats is associated with low D2 receptor expression in ventral, not dorsal, striatum9.
Johnson and Kenny’s work2 extends previous results from rat studies that had suggested addiction-like properties of prolonged access to palatable food. For example, earlier work has shown that intermittent sugar intake leads to physiological and behavioral symptoms on discontinuation that are similar to those seen during opiate withdrawal1 and a binge-like intake of sugar that to some degree resembled the behavior of rats given unlimited access to psychostimulants1. Rats that are given a choice between a sweet saccharin solution and cocaine strongly prefer saccharin10. Moreover, increased anxiety and other withdrawal-like symptoms after loss of access to high-fat food are mediated by the neuropeptide corticotropin-releasing factor, which also mediates symptoms of drug withdrawal11. Finally, studies using the reinstatement procedure (an animal model of drug relapse) have found overlaps between the neuronal mechanisms through which stressors or cues can cause rats to resume seeking of drugs or palatable food after loss of access12.
Given all of this, how far shall we go in drawing parallels between drug addiction and food addiction? Unlike drugs, food is essential for survival, but frequent consumption of bacon, sausage and cheesecake (the rats’ cafeteria diet) is not. The availability of such foods in most developed societies has increased so quickly that, similar to addictive drugs, they may stimulate brain reward systems more powerfully than we have evolved to handle, signaling a false fitness benefit and thereby reinforcing unhealthy patterns of consumption. In that respect, a parallel is defensible. But if we accept that parallel, there are at least two major caveats.
The first caveat is that food addiction is not identical to public health’s cause célèbre, obesity. If diagnostic criteria for food addiction were written to parallel the current diagnostic criteria for drug addiction, focusing on patterns of consumption that are maladaptive or problematic in any way, one could even argue that food addiction is neither necessary nor sufficient for obesity. The current draft of the Diagnostic and Statistical Manual includes criteria for a food addiction–like syndrome known as binge-eating disorder (BED), which is characterized by distress-inducing, subjectively hard-to-control episodes in which one eats “an amount of food that is definitely larger than most people would eat in a similar period of time under similar circumstances.” The cumulative lifetime risk of BED in the US is only 3.9%; even when combined with subthreshold BED and “any binge eating,” this only rises to 11%, about one-third of the current prevalence of adult obesity (body mass index ≥ 30), 34%. Among adults with BED, the point prevalence of obesity is 42%, which is only about 8% higher than that seen in the general population13. BED is also distinct from obesity in terms of prognosis (BED is associated with a lower quality of life than obesity) and treatment response (BED responds to antidepressants, but obesity generally does not).
Of course, food addiction could be defined more broadly as frequent heavy consumption of energy-dense foods without frank bingeing. In that case, its overlap with obesity is surely much greater (although there are probably no reliable statistics to quantify the extent of the overlap), but there are still reasons to avoid drawing an easy equivalence. For example, it has been argued that the effects of behavior on weight could be subverted by metabolic defense of a ‘set point’14. A high set point could result from overeating, but could also be established pre/perinatally and could be influenced by environmental factors that do not even involve food15. There is vigorous debate about the interactions of genetic, environmental and behavioral causes of obesity, but it is best to be leery of any account that overwhelmingly attributes obesity to the behavior of the obese.
The second caveat is that, in the realm of behavioral causes of obesity, if we invoke the concept of addiction, we need to remember what we have learned from the study of other addictions: addiction does not obliterate the capacity for choice. Even addiction to intravenous heroin and crack cocaine can be highly responsive to consequences when the consequences (for example, money) are sufficiently large and predictable. Despite Johnson and Kenny’s findings2 of changes in BSR sensitivity, human addicts are not always hyporesponsive to alternative rewards, even in studies that have been interpreted as evidence that they are. This caveat is important because it underlies behaviorally based treatments for addiction. And if the kinds of alternative-reinforcer treatments that are effective in drug addiction can reduce regular overindulgence in energy-dense food (with or without frank bingeing), health benefits are likely to accrue regardless of whether appreciable weight loss occurs.
To restate the two caveats, whatever entity we call food addiction should not be seen as an excuse for unhealthy eating and the unhealthy eating associated with food addiction should not be equated with obesity. Johnson and Kenny’s rat data2 suggest something interesting but not something that reduces to an enticing headline or sound bite. We would be mistrustful of any summary simpler than this: given enough access to cheesecake and bacon, rats display patterns of eating that resemble those that account to some unknown degree for human obesity and these patterns seem behaviorally similar to, and share some neurophysiological substrates with, patterns of drug self-administration and withdrawal symptoms that resemble those seen in drug addiction.
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Avena NM, Rada P, Hoebel BG. Neurosci Biobehav Rev. 2008;32:20–39. doi: 10.1016/j.neubiorev.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson PM, Kenny PJ. Nat Neurosci. 2010;13:635–641. doi: 10.1038/nn.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmed SH, Kenny PJ, Koob GF, Markou A. Nat Neurosci. 2002;5:625–626. doi: 10.1038/nn872. [DOI] [PubMed] [Google Scholar]
- 4.Kenny PJ, Chen SA, Kitamura O, Markou A, Koob GF. J Neurosci. 2006;26:5894–5900. doi: 10.1523/JNEUROSCI.0740-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Epping-Jordan MP, Watkins SS, Koob GF, Markou A. Nature. 1998;393:76–79. doi: 10.1038/30001. [DOI] [PubMed] [Google Scholar]
- 6.Markou A, Koob GF. Neuropsychopharmacology. 1991;4:17–26. [PubMed] [Google Scholar]
- 7.Schulteis G, Markou A, Cole M, Koob GF. Proc Natl Acad Sci USA. 1995;92:5880–5884. doi: 10.1073/pnas.92.13.5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Volkow ND, Wise RA. Nat Neurosci. 2005;8:555–560. doi: 10.1038/nn1452. [DOI] [PubMed] [Google Scholar]
- 9.Dalley JW, et al. Science. 2007;315:1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lenoir M, Serre F, Cantin L, Ahmed SH. PLoS One. 2007;2:e698. doi: 10.1371/journal.pone.0000698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cottone P, et al. Proc. Natl. Acad. Sci. USA; 2009. pp. 20016–20020. [Google Scholar]
- 12.Nair SG, Adams-Deutsch T, Epstein DH, Shaham Y. Prog Neurobiol. 2009;89:18–45. doi: 10.1016/j.pneurobio.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wonderlich SA, Gordon KH, Mitchell JE, Crosby RD, Engel SG. Int J Eat Disord. 2009;42:687–705. doi: 10.1002/eat.20719. [DOI] [PubMed] [Google Scholar]
- 14.Major GC, Doucet E, Trayhurn P, Astrup A, Tremblay A. Int J Obes (Lond) 2007;31:204–212. doi: 10.1038/sj.ijo.0803523. [DOI] [PubMed] [Google Scholar]
- 15.Heindel JJ, vom Saal FS. Mol Cell Endocrinol. 2009;304:90–96. doi: 10.1016/j.mce.2009.02.025. [DOI] [PubMed] [Google Scholar]