Abstract
Clinical outcomes in ovarian cancer are heterogeneous, independent of common features such as stage, response to therapy and grade. This disparity in outcomes warrants further exploration into tumor and host characteristics. One compelling issue is the response of the patient’s immune system to her ovarian cancer. Several studies have confirmed a prominent role for the immune system in modifying disease course. This has led to the identification and evaluation of novel immune-modulating therapeutic approaches such as vaccination and antibody therapy. Antitumor immunity, however, is often negated by immune suppression mechanisms present in the tumor microenvironment. Thus, in the future, research into immunotherapy targeting ovarian cancer will probably become increasingly focused on combination approaches that simultaneously augment immunity while preventing local immune suppression. In this article, we summarize important immunological issues that could influence ovarian cancer outcome, including tumor antigens, endogenous immune responses, immune escape and new and developing immunotherapeutic strategies.
Keywords: antibody therapy, myeloid-derived suppressor cells, regulatory T cells, vaccines
Ovarian cancer has the highest mortality rate of the cancers unique to women. According to the 2010 cancer statistics, there was going to be an estimated 21,880 new cases of ovarian cancer and an estimated 13,850 deaths [1]. The majority (65–75%) of women with ovarian cancer is diagnosed with advanced-stage disease (III and IV) and only approximately 15–20% of these are free of recurrence at 10 years [2–4]. While these statistics are sobering, there are, nevertheless, unique features about ovarian cancer that raise the possibility of increasing the cure rates. First, because it is localized to the peritoneal cavity, ovarian cancer is accessible for initial cytoreductive surgery, which dramatically reduces tumor volume. Second, most ovarian cancers are sensitive to chemotherapy, such that 80% of optimally debulked patients will achieve a complete response to first-line platinum–paclitaxel chemotherapy [3]. Unfortunately, at a median of 16–18 months following chemotherapy, recurrences will manifest, and by 5 years from diagnosis, the majority will have recurred [5,6]. The major determinants of outcome in ovarian cancer are tumor-specific features such as grade, histology, sensitivity to chemotherapy and the extent of the primary surgical debulking. As is evident from high recurrence rates following therapy, minimal residual disease remains in most patients, consisting of cells resistant to initial chemotherapy. It is this minimal residual disease state that can be targeted with innovative therapeutic strategies (e.g., immune-based therapies).
Clinical outcomes in ovarian cancer are quite heterogeneous, with approximately 15% of patients dying of the disease within the first year, and approximately 25% surviving over 5 years from diagnosis [7]. This disparity in outcomes warrants further exploration into tumor and host characteristics. One compelling, and potentially modifiable arena, for study is the response of the patient’s immune system to her ovarian cancer. Thus, research into ovarian cancer has become increasingly focused on the function of the immune system. Despite the observation that ovarian cancer is an immune reactive malignancy, and there are notable regressions in response to immune modulation, there has not been a therapy that has advanced to the point of routine clinical use. Although there are many reasons for this inability to implement effective immune-based treatments, one major reason is that ovarian tumors establish a complex multilayered immune suppression network that effectively neutralizes most attempts at augmenting antitumor immunity (Figure 1). In this article, we present an updated summary of the immunological mechanisms of ovarian cancer including natural immune response, mechanisms of immune escape in the ovarian microenvironment and current immune-based therapies for ovarian cancer being studied in the clinical setting.
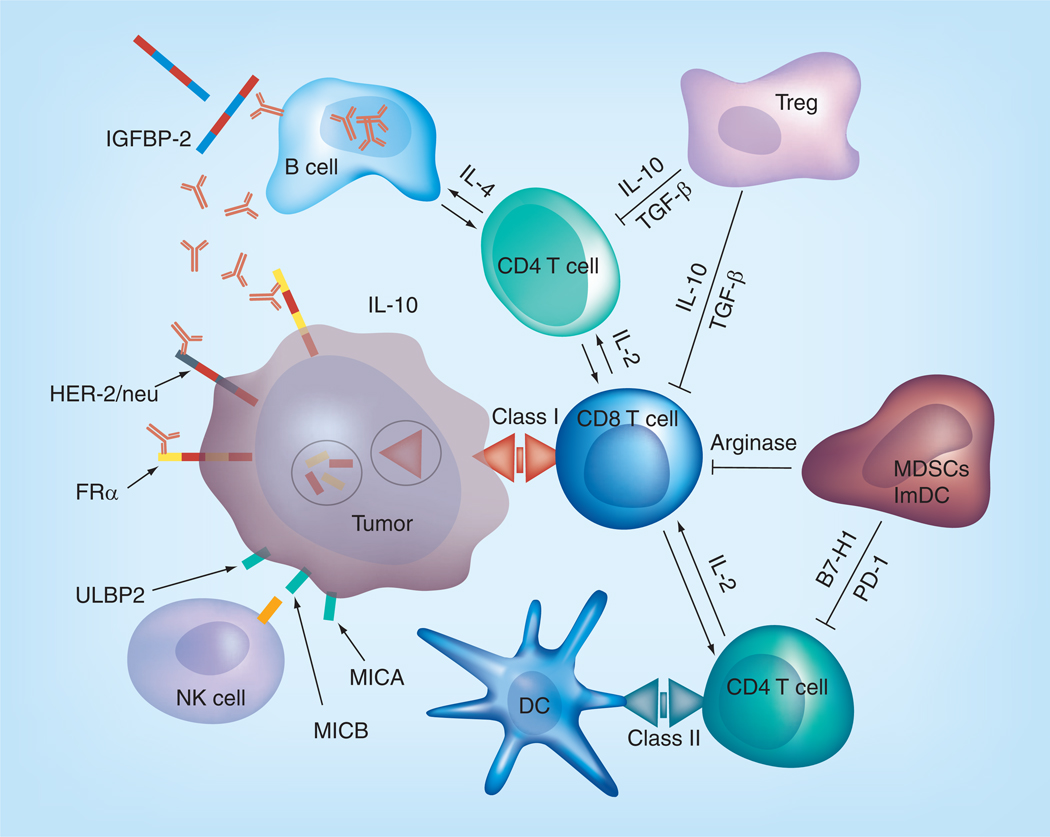
Figure 1. Immune microenvironment in ovarian cancer.
Despite the presence of various immune effector cells (i.e., CD4 T cells, CD8 T cells and NK cells) that can attack the tumor, the presence of a complex immune suppressive network including Tregs, ImDCs and MDSCs along with their mediators (i.e., IL-10, TGF-β, B7-H1, PD-1 and arginase) effectively halts the antitumor immunity.
DC: Dendritic cell; FR: Folate receptor; IGFBP: IGF binding protein; ImDC: Immature DC; MDSC: Myeloid-derived suppressor cell; PD: Programmed death.
Basic principles of immunity
The immune system is constituted by a heterogeneous population of both cellular and molecular effectors that function in an organized and integrated manner to eradicate disease and maintain the overall health of the host, while minimizing off-target activity (e.g., autoimmunity). The immune system is typically divided into two different, but interacting, systems referred to as the innate and the adaptive mechanisms. The innate immune system is the first line of defense that consists of a group of cellular and humoral factors. The cellular component of innate immunity includes NK T cells, mast cells, eosinophils, basophils, macrophages, neutrophils and dendritic cells (DCs), while the humoral factors primarily include cytokines and complement. The major functions of the innate immune system include recruiting immune cells into sites of infection, activation of complement, identification of foreign substances and preparation and activation of the adaptive immune systems. Innate responses are immediate, maximal and antigen independent. In the absence of stimulation, nonactivated innate cells are well known to maintain tolerance and prevent inappropriate immune activation. The adaptive immune response involves both T and B lymphocytes. T lymphocytes produce cytokines or cytolytic molecules, while B cells produce antibodies. Primary adaptive immune responses usually develop after 1 week and are highly antigen-specific. Like the innate immune system, subsets of lymphocytes (e.g., Tregs) are also known to regulate immunity and prevent autoimmunity. Finally, another key feature of the adaptive immune response is generation of immune memory and the resulting rapid and robust responses to previously encountered antigens, a fundamental reason for the widespread clinical success of vaccines.
Natural immune responses to ovarian cancer influence the clinical course of the disease
Observations that date back over two decades have established that there are endogenous immune responses to ovarian tumors; more recently, it has been shown that the quality of the immune responses can have a significant impact on the clinical course of the disease. Although infiltration of T cells and other immune effectors was observed in ovarian cancers as early as 1982 by Haskill and colleagues [8], it would not be until nearly two decades later that the prognostic significance of these cells was appreciated. In their landmark publication in 2003, Zhang and colleagues showed that T-cell infiltration into ovarian tumors was associated with improved survival [9]. For example, among 74 patients with a complete clinical response after debulking and platinum-based therapy, the 5-year survival rate was 73.9% among those patients with CD3+ T cells within their tumor compared with 11.9% among patients without infiltrating T cells [9]. This study also revealed that in tumors with high numbers of tumor-infiltrating T cells the expression of monokines induced by IFN-γ, macrophage-derived chemokines and secondary lymphoid-tissue chemokines were significantly increased as compared with tumors lacking T cells, indicating that these chemokines may be involved in the antitumor response [9]. Although this study did not elucidate the nature of the CD3 T-cell infiltrate with respect to the different subsets of T cells (CD4 T helper [Th] cells, CD8 cytotoxic T cells and CD4 Treg cells), it bolstered the hypothesis that the immune infiltrate has an active role in the clinical course of ovarian cancer.
Whereas helper and cytotoxic T cells, collectively known as effector T cells, exhibit antitumor immune functions, the regulatory T-cell subsets would suppress immunity [10]. Studies in recent years have increasingly focused on whether effector T cells are associated with improved survival, and, if so, what is their phenotype and function. For example, Sato and colleagues studied 117 ovarian cancer cases finding improved survival in patients who had higher numbers of intraepithelial CD8+ T cells compared with patients without intraepithelial CD8 T cells (median survival 55 vs 26 months) [11]. These findings were largely confirmed by Leffers and colleagues in an independent cohort [12]. CD8 cytotoxic T cells are generally thought to be the primary mediators of antitumor immune responses. These cells recognize antigens displayed in the context of MHC (HLA) class I molecules expressed on ovarian cancer cells. Upon recognition of their cognate antigen, CD8 T cells release, among several mediators, perforin and granzyme, which induce apoptosis in target cells [13]. In recent years, however, the identification of regulatory subsets of CD8 T cells has clouded interpretations of tumor-infiltrating CD8 cells [14]. Nonetheless, the interpretation that the vast majority of CD8 T cells are cytotoxic seems reasonable given the recent study by Milne and colleagues who showed a strong positive correlation between levels of CD8 T cells and granzyme B within tumors [15]. Other studies seeking to understand the mechanisms behind lymphocyte recruitment to tumors have used gene expression profiling of serous ovarian tumors with high and low CD8 T-cell infiltration finding two genes differentially expressed in tumors with high versus low CD8 T-cell infiltration: interferon regulatory factor (IRF)-1 and chemokine receptor (CXCR)6 [16,17]. Upregulation of IRF-1 results in the induction of the MHC class I-dependent pathway and activation of transcription factors for MHC class II gene expression. Upregulation of CXCR6, along with other chemokine receptor genes, has been associated with metastasis of several tumors including epithelial cancers. Thus, these studies provide insight into specific genes and pathways associated with recruitment of cytotoxic T cells in ovarian tumors and give possible paths for further immune therapies designed to influence recruitment of helpful T-cell subsets.
In contrast to CD8 cytotoxic T cells, the role of CD4 helper T-cell infiltration is less clear owing to a high prevalence of the CD4 marker on Tregs. Both Sato and Milne observed similar outcomes among patients with or without CD4+ T-cell staining of tumors [11,15]. Kryczek and colleagues found that high levels of IL-17 were associated with greatly improved outcome suggesting that a subset of CD4 Th cells, called Th17 and producing IL-17, may have a direct role in eradicating tumors [18]. Given the abundant expression of IL-17 among innate immune effectors, it is difficult to draw a conclusion as to whether intratumoral Th17 cells might impact the clinical course of the disease [19]; however, the fact that levels of Th17 cells correlate strongly with other prognostic cells (e.g., Tregs) suggests that they might also be predictive of outcome [18].
Other specific subsets of antitumor immune effectors have also been studied but with less than clear results. One example is the subset of NK cells, a group of cytotoxic lymphocytes that have two different mature phenotypes: CD16+CD56dim NK cells, found in the periphery, which have high cytolytic function, and CD16−CD56bright NK cells found in the secondary lymphoid tissue, with inefficient cytotoxicity [20]. These cells have two types of surface receptors: activating (i.e., NKG2) receptors and inhibitory (i.e., KIR) receptors. NK cells have the ability to lyse cells without first having to recognize specific antigens [21]. The balance between inhibitory and activating signals through the different NK receptors is important in the NK cell activation process. NK cells can also be activated in an antigen-dependent manner to mediate antigen-dependent cellular cytotoxicity, which involves binding of cell bound antibodies to Fc receptors on the NK cells. The activating NKG2 receptors bind to stress ligands such as MICA, MICB and ULBP1–3, while KIR receptors bind to MHC class I and class I associated ligands [21,22]. Like other cancers, nearly all ovarian cancers express MICA, MICB and ULBP2 [23]. Furthermore, higher NK cell activity in the peripheral blood of ovarian cancer patients at the time surgery is predictive of improved progression-free survival (PFS) [24]. Despite this, increased numbers of NK cells in peritoneal and pleural effusions of metastatic ovarian carcinoma have been associated with poorer prognosis [25]. The generation of antibody responses to ovarian cancer is a common observation suggesting a role for B cells in disease protection [26–28]. Although antibody-secreting B cells do not need to be at the tumor site to exert antitumor activity, studies evaluating whether B-cell infiltration is associated with improved survival show mixed results [15,25].
Antigen specificity of the ovarian cancer immune response
Since the 1990s, reports have shown heterogeneity of human ovarian cancers with respect to infiltration by immune effectors, particularly effector T cells, and often associated with favorable outcome leading to speculation that ovarian cancer activates an antigen-specific immune response, which kills tumor cells and/or blocks growth. Whether or not immune effectors in the tumor are antigen specific and, importantly, which antigens are tumor rejection antigens remain largely unanswered. Despite this, several ovarian cancer antigens have been identified and in recent years, several studies have demonstrated that patients who have ovarian cancer respond naturally to these antigens, as measured in the peripheral blood or in ascites fluid.
One of the first suggestions that the effector T cells associated with ovarian cancers were specific for antigens overexpressed in the tumor came from Ioannides and colleagues [29]. They found that ascites-derived HLA-A2+ contains CD8 T cells capable of recognizing the human EGF receptor (EGFR)2 (HER-2/neu)-derived peptide, p971–980 [29]. HER-2/neu protein, also known as HER-2, ErbB-2 and c-erbB2, is a transmembrane glycoprotein (185 kDa) that is part of the EGFR family that also includes EGFR-1, HER-3 and HER-4. HER-2/neu comprises a large extracellular domain, a short hydrophobic transmembrane domain and a cytoplasmic intracellular domain containing tyrosine kinase activity [30]. Her2/neu is an attractive immunologic target because of its low-level expression in peripheral tissues and its biologic relevance. HER-2/neu activates signaling pathways that are involved in cellular differentiation, proliferation, migration and apoptosis. Estimates of the percentage of ovarian tumors with HER-2/neu expression have been variable, ranging from 5 to 66% [31–34]. Data in the past decade have been somewhat contradictory with regard to the prognostic relevance of HER-2/neu gene amplification or protein overexpression in ovarian cancer. Some studies show HER-2/neu overexpression and amplification as a poor prognostic factor [32,35], while other studies show no prognostic value associated with this protein [34]. The immune system naturally targets HER-2/neu in ovarian cancer patients, as shown by Karyampudi and colleagues [36]. In that study, peripheral blood T cells from ovarian or breast cancer patients and age-matched women who had not had cancer were tested for immune reactivity against a panel of fifteen epitopes that were predicted to bind to several distinct allelic forms of HLA-DR. Of those epitopes, four were targets of T cells in cancer patients but not healthy donors [36].
Folate receptor (FR)α, previously known as folate-binding protein, is a glycosyl-phosphatidylinositol-linked membrane protein overexpressed in many epithelial cancers [37–39]. Expression of FRα in nonmucinous ovarian tumors is increased approximately 90-fold in comparison with normal epithelial cells [40]. Its expression is also retained on metastatic lesions and recurrent ovarian tumors [41]. Expression of FRα is also relatively limited to a few specific tissues, notably the apical surface of kidney tubule epithelium where it is involved in recovery of folate from the urine [42]. Interest in targeting FRα was initially established by Peoples and colleagues. In their studies, they found that tumor-associated lymphocytes isolated from the malignant ascites of ovarian cancer patients recognized naturally processed and presented HLA-A2 (MHC class I) peptides derived from FRα [43,44]. Using a CD4+ T-cell epitope prediction algorithm in another study, we predicted promiscuous epitopes of FRα and tested for immunity in 30 breast or ovarian cancer patients and 18 healthy donors using ELISPOT [27]. A total of 14 peptides were predicted, and it was found that more than 70% of patients demonstrated immunity to at least one epitope. Patients responded to an average of three epitopes, whereas healthy donors responded to only one. Five of the 14 peptides were recognized by more than 25% of patients, and responses to three peptides were higher in patients than in healthy donors, suggesting that the presence of the tumor augmented immunity. Finally, patients demonstrated elevated levels of FRα antibodies, consistent with a coordinated immune response. Thus, FRα is a promising therapeutic target not only due to its tumor specificity and high-level expression, but also because it is naturally immunogenic.
IGF binding proteins (IGFBPs) are a family of six binding proteins that have 50% homology with each other and have binding and regulatory properties for IGF-1 and −2, therefore having a role in cell proliferation, differentiation and apoptosis. All six family members are secreted and expressed in normal ovarian tissue, although previous studies have shown IGFBP-2 serum levels and expression to be significantly increased in ovarian cancer tissue in comparison with controls [45–48]. We revealed for the first time that IGFBP-2 elicits an in vivo antigen-specific CD4 T-cell immunity in patients with breast and ovarian cancer [49]. In this study, T cells from more than 15% of the patients elicited a response against four HLA-DR-degenerate IGFBP-2 epitopes compared with controls. Moreover, this pool of four IGFBP-2 peptides should cover the majority (~80%) of the patients with tumors with IGFBP-2 overexpression based on the allelic frequencies of the HLA-DR homologs; however, the significant patient response was only 35% [49]. These results implicate IGFBP-2 as a target for vaccine-based therapeutics against ovarian cancer.
Transmembrane mucins are a family of heavily glycosylated proteins with high molecular weights, produced by epithelial tissues, which are involved in coating, lubrication and protection [50]. There are two types of mucins: the extracellular complex mucins found in gastrointestinal and respiratory tracts, and the transmembrane mucins found in glandular and ductal epithelial cells [50]. Of the 11 transmembrane mucins identified to date, three (MUC1, MUC4 and MUC16) have been well characterized and shown to be overexpressed in many types of cancer including ovarian carcinomas [51–54]. MUC16 is the largest membrane-bound mucin protein; it is expressed on the surface of ovarian cancer cells and shed into the bloodstream and peritoneal cavity after proteolytic cleavage. MUC16 contains the CA-125 peptide epitope that is used as a tumor marker for monitoring growth and recurrence of epithelial ovarian cancer [55,56]. MUC1 has been shown to be overexpressed in 50–80% of ovarian tumors and is a prognostic factor for resistance to platinum-based chemotherapy in ovarian cancer [50,53,57]. Although MUC4 is overexpressed in human ovarian cancer, much less is known about its biology. MUC4 is a large glycoprotein that is aberrantly expressed in over 90% of malignant ovarian tumors with very low to an undetectable expression in the normal ovary [53]. Recent studies clearly suggest a pathologic role of MUC4 in ovarian cancer by mediating epithelial to mesenchymal transition, which is involved in metastasis and enhanced tumor aggression [58]. MUC4 also activates HER-2/neu and enhances the motility of ovarian cancer cells [59]. Despite association of MUC4 with pathologic features, there is no association of expression with outcome in ovarian cancer patients [53]. Overexpression and aberrant glycosylation of the mucins are recognized by the immune system. For example, some studies have shown the presence of anti-MUC1 antibodies in healthy individuals as well as in ovarian cancer patients and the levels of anti-MUC1 antibodies inversely correlate with ovarian cancer risk factors [60,61].
Mutation of the p53 gene is one the most common, single genetic alterations in sporadic human epithelial ovarian carcinoma [62]. Either loss of wild-type p53 function, gain of oncogenic function or the ability to activate p53 severely compromises controlled cellular proliferation and growth [63]. As a result of mutation, p53 is overexpressed in nearly 50% of ovarian cancers. In a study of 104 women with ovarian cancer, Goodell and colleagues assessed the levels and clinical impact of anti-p53-specific IgG antibodies. Multivariate analyses showed that the presence of p53 antibodies to be an independent predictor of survival. Specifically, the median survival for antibody positive patients was 51 months compared with 24 months for patients without antibodies [26]. Consistent with the development of IgG antibodies, ovarian cancer patients also develop p53-specific T-cell memory [64].
Epithelial cell adhesion molecule (EpCAM) is a type I membrane glycoprotein constructed by three domains, an extracellular domain, with EGF and thyroglobulin repeat-like domains, a transmembrane domain and an intracellular domain (26-amino acid) termed EpICD [65]. This molecule is expressed in almost all epithelial cell membranes but not on mesodermal or neural cell membranes. EpCAM has a high-level expression on human epithelial cancers with a negative prognostic potential for survival of patients [65]. In a retrospective study carried out by Kobel and colleagues on 500 ovarian cancer patients, EpCAM was highly expressed across all ovarian cancer subtypes [66]. Furthermore, EpCAM-positive tumor cells and ascites-derived exosomes containing EpCAM molecules have also been detected in the majority of ascites samples from ovarian cancer patients [67–69]. Although EPCAM expression in normal epithelial tissue has been detected [67], the expression of this molecule in ascites is highly tumor specific, because normal cells in the peritoneal cavity originate from the mesothelium and do not express EpCAM on their surface. EpCAM is naturally targeted by the immune system but the extent and nature of the immune responses remain undefined [70].
Cancer testis antigens (CTAs) are a group of tumor-associated antigens expressed in normal testis and in some female reproductive organs, trophoblasts and different types of tumors. There are approximately 140 members in 70 families, and the expression of some of these antigens have been studied in different types of tumors and some have been shown to be immunogenic, specifically recognized by cytotoxic T lymphocytes [71]. Some members of the CTA family are expressed in ovarian cancer, including the MAGE, BAGE, LAGE, GAGE, sperm protein 17 (SP17) and the synovial sarcoma X (SSX) genes [72–75]. CTAs are biologically relevant antigens. For example, Zhang and colleagues showed that moderate to high expression of MAGE-1 and MAGE-3 in ovarian cancer tissue (37–57%) positively correlated with tumor differentiation and clinical stage, while GAGE-1/2 and BAGE had relatively low expression, although ovarian cancer patients with ascites had significantly higher BAGE expression [73]. It was found that overexpression of SP17 in ovarian cancer cells results in enhanced migration and chemoresistance [76]. The immune system responds to CTAs in many cancers, including ovarian cancer. Among the CTAs in ovarian cancer, immunity to NY-ESO-1 is best characterized and clinically targeted. Nearly 25% of patients with the disease develop detectable, natural antibody and T-cell immune responses [77,78]. Recent studies show that NY-ESO-1-specific cytotoxic T cells infiltrate into ovarian tumors where they appear to be disabled through the coinduction of programmed death (PD)-1 and LAG-3 by factors in the tumor microenvironment, thus providing a mechanism for tumor growth despite measurable immunity [77]. Collectively, these results show that antitumor immunity is naturally elicited against ovarian cancer and impacts the clinical course of disease. However the antitumor response, as alluded previously with the NY-ESO-1 T cells, is blunted by a hostile immune suppressive microenvironment.
Immune suppression in ovarian cancer
Immune evasion in ovarian tumors involves a complex array of immune suppressive factors and cells that effectively halt the generation and clonal expansion of antitumor immunity. Genetic changes also occur, permitting the tumor cells to be ignored by the immune response. Immune suppression is mediated by factors released from the tumor or by infiltration of the tumors by a variety of either lymphoid or myeloid-derived suppressor cells (MDSCs) or regulatory cells.
The role of CD4 Tregs in the immune evasion of ovarian cancers is well characterized. Tregs are a heterogeneous T-cell subpopulation whose primary function is immune regulation by blocking the function of activated T cells. CD4+ Tregs can be divided into subsets: the thymus-generated naturally occurring Tregs that have a CD4+CD25+Foxp3+ phenotype, and the induced (adaptive) Tr1 Treg and Th3 Tregs that have CD25 variable expression [10,79]. While induced Tregs produce immune- suppressive soluble mediators such as TGF-β and IL-10 to mediate their inhibitory activities and block T-cell proliferation, natural Treg cells use cytokine-dependent, cell contact- dependent or a cytokine/cellular contact-dependent mechanisms to halt T-cell responses [10]. Three other subpopulations of CD4+ Tregs have been proposed that can be differentiated by their level of expression of Foxp3, CD25 and CD45RA [80]. These subpopulations are functionally and phenotypically different: resting Tregs (CD45RA+CD25interFoxp3low) show more active proliferation upon stimulation and increased cytotoxic T lymphocyte antigen-4 expression; activated Tregs (CD45RA− CD25high FOXP3high) die after proliferation but can suppress the proliferation of resting Tregs; and nonregulatory Tregs (CD45−CD25inter Foxp3low) that produced the highest levels of IL-17 when compared with naive non-Treg cells [80]. There are several mechanisms by which tumors, aided by Tregs, can halt the immune response. Tumors can increase the numbers of Tregs in the peripheral blood of cancer patients as reported for several tumor types, including ovarian [81–83]. Tumors can also recruit or induce Treg tumor infiltration as shown by numerous studies that demonstrate intratumoral localization of CD4+CD25+Foxp3+ Tregs in several human cancers. The connection between pathogenesis of Tregs and prognosis in ovarian cancer was first suggested by Curiel and colleagues who showed that accumulation of intratumoral Tregs was associated with poor patient survival [84]. In that study, Tregs, as measured with immunohistochemistry, were associated with a high mortality rate. Two subsequently published manuscripts also demonstrated the importance of Tregs in ovarian cancer pathogenesis and outcome. Wolf and colleagues showed that patients with low levels of intratumoral Foxp3 had substantially improved survival compared with patients with high levels (77 vs 30 months) [85]. Sato and colleagues also determined that the presence of CD4+ Tregs influences the antitumor activity of intratumoral cytotoxic CD8+ T cells [11].
Dendritic cells are classified into two subtypes according to their lineage: plasmacytoid DCs (CD123+, CD45RA+, CD8+, CD11c−, ILT3+, ILT1− and Lin−) and myeloid DCs (MDCs) that express CD11c and CD33, but lack CD45RA and CD123 [86]. Under quiescent conditions, they are present in the body in an immature form and are responsible for detecting danger and sampling of antigens. Upon detection of danger through their pathogen-associated molecular pattern (PAMP) receptors or danger-associated molecular pattern (DAMP) receptors, DCs will mature and migrate to the lymph nodes to activate Th cells and cytotoxic T lymphocytes [86]. Although tumors are able to produce danger signals, they are ineffective in inducing DC maturation and trafficking to lymph nodes, which is thought to be due to tumor-induced alterations in DC differentiation, thus reducing the number of functional cells available for effective T-cell activation [87]. For example, ovarian cancers secrete large amounts of IL-10, which promotes differentiation of DC to CD14+CD1a− macrophage-like cells with reduced T-cell activation properties [87].
Human ovarian cancers are known to contain plasmacytoid and MDCs as well as their precursors. CXCR4 plasmacytoid precursor cells (preDC2s) are attracted into the tumor microenvironment by tumor derived stroma-derived factor (SDF)-1. SDF-1, also known as CXCL12, is a small chemokine that belongs to the intercrine family and CXC subfamily that activate leukocytes and is often induced by proinflammatory stimuli such as lipopolysaccharide, TNF or IL-1 [88]. SDF-1 exists as two variants, SDF-1α and SDF-1β, and has a very specific receptor CXCR4 with unique recognition of SDF-1. This receptor is a G-protein-coupled receptor that is expressed in a wide group of cells including T lymphocytes, B lymphocytes, monocytes and macrophages [88]. Bignotti et al. have identified CXCL12, along with other integrins, among the most highly differentiated genes in metastatic sites of papillary ovarian carcinomas [89]. PreDC2 recruited into the ovarian cancer micro environment induce T cells to release large amounts of IL-10, preventing local T-cell activation [90]. Induction of IL-10 is due, at least in part, to local induction of IL-10+ regulatory CD8 T cells [91]. Although rare in the blood, plasmacytoid DCs and their precursors preferentially accumulate in ovarian cancers. By contrast, MDCs are the dominant DCs in the blood under normal health conditions. Some early studies appear to suggest that there is little accumulation of MDCs in ovarian cancer [90], but more recent studies demonstrate their infiltration. Their role in local immune suppression remains unclear, particularly in the solid tumor mass as opposed to the ascites fluids [92]. MDCs in their immature states are promoters of angiogenesis and vasculogenesis during tumor growth [93–95]. One study suggests that MDCs mediate local immune suppression in ovarian cancer by suppressing the effector function of T cells through the engagement of MDC-expressed B7-H1 [96]. B7-H1, also known as PD-L1 or CD274, is the PD-1 receptor ligand and it is thought that they help in the regulation of T-cell activation [97]. A group showed that DCs expressing B7-H1 inhibit T-cell proliferation directly and also indirectly by promoting the induction of CD25highFoxP3+ Tregs, thus suggesting that B7-H1 is a key player in the tumor micro environment immune suppression [98]. B7-H1 is expressed on the surface of several gynecological cancers, including ovarian, and is associated with poor overall survival in ovarian cancer [99–101]. PD-1 is also shown to be expressed on various adaptive immune effectors, notably CD4 and CD8 T cells, where it negatively regulates cell activation in cancers including ovarian [77,102–104]. The molecular interactions, particularly those associated with PD-1, remain elusive [96]. MDCs can mediate local immune suppression not only by expressing PD-1 and B7-H1 but also by the generation and increased activity of other mediators including arginase, indoleamine 2,3-dioxygenase, nitric oxide (NO) and reactive oxygen species (ROS) [105–107]. Overall, the DC component of ovarian cancers remains an intriguing and underexplored area of ovarian cancer research and could represent a therapeutic target. This concept is borne out by recent murine modeling studies demonstrating improved anti-tumor immunity following specific depletion of DCs [108].
Apart from plasmacytoid DCs and MDCs, there is another subset of myeloid-derived cells, termed MDSCs, which have an increased ability to block local and systemic immune activation [109]. This is a heterogeneous population consisting of macrophages, DCs and granulocytes at early stages of differentiation [95]. MDSCs are classified into two subpopulations, granulocytic and monocytic MDSCs that, in mice, but not humans, are uniquely identified by coexpression of CD11b and Gr1 [110]. MDSCs are known to expand dramatically under various health disturbances such as tumor growth, inflammation and infection [95,111,112], and may have a role in immune suppression of ovarian cancer in murine models; however, their relevance in human ovarian cancers remains uncertain [113].
Neutrophils are potent initiators of non-infectious inflammation, and although recent studies have associated them with tumor progression and metastasis, the mechanism is still not well understood but may be due to blockade in adaptive immune responses. A study carried out by Klink and colleagues evaluated the interactions between neutrophils and ovarian cancer cells [114]. They showed that direct contact between ovarian cancer cells and neutrophils elicited enhanced ROS production, increased adhesion ability and upregulation of CD11b/CD18 expression in neutrophils from ovarian cancer patients compared with control neutrophils [114]. The neutrophil-to-lymphocyte ratio (NLR) serves as an indirect measurement of inflammatory status. Many recent studies showed that elevated NLR is an independent prognostic factor associated with an increase in disease recurrence in several cancers [115–119]. A recent study by Cho and colleagues evaluated the prognostic significance of NLR in patients with ovarian cancer compared with patients with benign gynecological tumors and healthy controls [116]. They showed that patients with advanced ovarian cancer and high preoperative NLR had decreased overall survival compared to patients with low NLR [116]. Overall, these results showed that neutrophils have a potential immune deregulating role in ovarian cancer and, in the future, they may become targets for immune-based therapies for advanced and metastatic ovarian cancer.
Aside from the recruitment of suppressive cells into the tumor microenvironment, tumor cells themselves express a variety of molecules that directly block immune responses. Studies show that MUC16 (the protein source of CA-125) facilitates peritoneal metastasis of ovarian tumors and adds to the immune suppressive tumor microenvironment by inhibiting the activity of NK cells [120–123]. A recent study by Gubbels and colleagues sheds more light onto the role and importance of MUC16 on cancer cells [122]. For example, they showed that MUC16 acts as an inhibitor of the NK–tumor conjugation of ovarian cancer cells. NK cells killed cells expressing low levels of MUC16 more effectively, with approximately 20% more NK-cell lysis and a two- to three-fold increase in NK leukemia cell lysis when compared with lysis of cells with high MUC16 expression [122]. Furthermore, a recent study by Krockenberger and colleagues showed that macrophage migration inhibitory factor (MIF; inducer of inflammation and promoter of tissue repair) inhibits the antitumor immune response against ovarian cancer cells by downregulating NKG2D receptor in NK cells [124].
Recent immunological approaches for treatment of ovarian cancer
Passive antibody therapies
HER-2/neu antibodies, namely trastuzumab and pertuzumab, have been extensively studied as therapeutics for the small subset of patients with tumors that overexpress HER-2/neu (Table 1). Trastuzumab (Herceptin® [Genentech, CA, USA]) is a humanized monoclonal antibody that targets the HER-2/neu extracellular domain and inhibits HER-2/neu-positive tumor cell proliferation. This antibody is the standard of care for patients with HER-2/neu+ breast cancer [125]. Few clinical trials have been reported on the use of trastuzumab in ovarian cancer. One study performed on 41 patients with advanced epithelial ovarian cancer showed that trastuzumab administered as a single agent therapy yielded a low response rate (~7%) and a median progression-free interval of only 2 months in patients with recurrent or advanced ovarian cancer [31]. A Phase II clinical study looked at the efficacy and tolerance of trastuzumab when combined with paclitaxel and carboplatin in seven patients with taxane/carboplatin- resistant ovarian cancer and HER-2/neu overexpression [126]. In this study the combination treatment elicited a clinical response in three patients with a median PFS of 2.9 months and overall survival of 12.3 months. Another clinical study performed on 33 patients with mucinous ovarian carcinoma showed approximately 18% HER-2 expression, and of the three patients treated with chemotherapy and trastuzumab, one had complete effective response and another had partial response, suggesting that trastuzumab may be effective for this subset of individuals [127].
Table 1.
Immune-based clinical trials for ovarian cancer.
| Agent | Antigen targeted | Patient population | Patients (n) |
Clinical description | Ref. |
|---|---|---|---|---|---|
| Passive antibody therapies | |||||
| Trastuzumab | HER-2/neu | Recurrent or persistent ovarian or primary peritoneal cancer, 2+ or 3+ Her-2/neu overexpression |
41 | Single agent, group wide, Phase II; overall objective RR: 7.3%; SD: 39%; PFS: 2 months |
[31] |
| Trastuzumab + TC | HER-2/neu | Recurrent TC-resistant ovarian cancer + Her-2/neu overexpression |
7 | Open-label, noncomparative, prospective, Phase II; CR: 43%; PFS: 2.9 months; OS: 12.3 months |
[126] |
| Pertuzumab | HER-2/neu | Refractory or recurrent platinum- resistant ovarian cancer |
117 | Open-label, single agent, Phase II; overall RR: 4.3%; overall SD: 6.8%; overall PFS: 6.6 weeks; OS: 52.7 weeks; cohort 1 PFS: 7.6 weeks; OS: 46.7 weeks; cohort 2 PFS: 6.1 weeks; OS not yet reached |
[129] |
| Pertuzumab + gemcitabine | HER-2/neu | Platinum-resistant ovarian, fallopian tube or primary peritoneal cancer |
130 | Randomized, double-blind, Phase II; gemcitabine + placebo: PFS: 2.6 months; PR: 4.6%; OS: 13.1 months; gemcitabine + pertuzumab PFS: 2.9 months; PR: 13.8%; OS: 13 months |
[131] |
| Pertuzumab + carboplatin | HER-2/neu | Platinum-sensitive ovarian cancer | 149 | Ongoing Phase II, carboplatin + paclitaxel or gemcitabine: RR: 52%; PFS: 31.3 weeks; pertuzumab + carboplatin: RR: 64%; PFS: 34.1 weeks |
[132,133] |
| Farletuzumab | FRα | Platinum-resistant or refractory ovarian, fallopian tube or primary peritoneal cancer |
25 | Open-label, dose-escalation, Phase I, well tolerated at 12.5–400 mg/m2; SD: 35%; three patients with SD had extended treatment with 3–17% decrease in tumor size from baseline |
[137] |
| Farletuzumab + taxane/carboplatin | FRα | Platinum-sensitive ovarian cancer patients with first recurrence |
44 | Open-label, Phase II, farletuzumab alone: frequent SD but no objective RR; farletuzumab + taxane/ carboplatin: 89% normalized CA-125, RR: 64% in first progression-free interval <12 months, RR: 71% in first progression-free interval >12 months |
[138–140] |
| Catumaxomab | EpCAM | Recurrent ascites from pretreated refractory ovarian cancer |
23 | Open-label, dose-escalation, Phase I/II, safe and well tolerated at 10–200-µg doses, 100% decrease in ascites and EpCAM+ tumor cells |
[142] |
| Catumaxomab + paracentesis | EpCAM | Symptomatic malignant ascites with epithelial ovarian and nonovarian cancers |
258† | Open-label, randomized, Phase ll/lll, paracentesis alone: puncture-free survival: 11 days; TP: 11 days; OS: 81 days; catumaxomab + paracentesis: puncture- free survival 52 days; TP: 71 days; OS: 110 days |
[143] |
| Adoptive T-cell therapies | |||||
| Autologous T-cell specific for FRα | FRα | Recurrent or residual epithelial FRα+ ovarian cancer with metastasis |
14 | Phase I, cohort 1 = T cells + IL-2, cohort 2 = T cells + allogeneic PBMCs; well tolerated in both groups, no clinical response observed |
[146] |
| Autologous MUC1-stimulated T cells | MUC-1 | Residual or recurrent epithelial ovarian cancer |
7 | Phase I, four patients completed three cycles: one patient is disease free + maintained low CA-125, one patient had 16-month survival + decrease in CA-125 and two patients had 3–5-month survival + increase CA-125 |
[147] |
| Vaccine therapies | |||||
| Multipeptide vaccine | HER-2/neu, FRα, MAGE-A1 |
Primary peritoneal, fallopian tube or ovarian cancer with HLA-A1+ HLA-A2+ or HLA-A3+ |
9 | Phase I, well tolerated, 89% had progression of disease, one patient disease-free after 19 months; CD8+ T-cell response in all nine patients |
[148] |
| NY-ESO-1b peptide vaccine | NY-ESO-1b | High-risk epithelial ovarian cancer in first clinical remission |
9 | Phase I, well tolerated, after in vitro presensitation 75% had CD8+ T-cell response, 67% patients recurred, PFS: 13 months, three patients remain disease-free |
[149] |
| p53 synthetic long peptide vaccine | p53 | Recurrent epithelial ovarian cancer | 20 | Phase I, well tolerated, p53-specific CD4+ T-cell proliferation in 82.4% patients, SD: 10% |
[150] |
Ovarian cancer group = 129; non-ovarian cancer group = 129.
CR: Complete response; EpCAM: Epithelial cell adhesion molecule; FR: Folate receptor; MUC: Mucin; OS: Median overall survival; PBMC: Peripheral blood mononuclear cell; PFS: Median progression-free survival; PR: Partial response; RR: Response rate; SD: Stable disease; TC: Paclitaxel/carboplatin; TP: Median time to next therapeutic paracentesis.
A recent preclinical study by Wilken and colleagues explored the mechanism behind the trastuzumab resistance in four HER-2+ ovarian cancer cell lines. They showed that ovarian cancer cells treated with trastuzumab for 12 weeks and later treated with a HER-1-targeted drug, such as cetuximab or gefitinib, were effectively growth-inhibited [128]. This study suggests that trastuzumab may increase its effect when combined with a second HER-2/neu-targeted drug. In this case, it appears that trastuzumab induces the cells to rely on other growth factor receptors such as the EGFR and, after inhibiting this second pathway, the cancer cell’s growth can be effectively halted.
Pertuzumab (rhuMAb 2C4, Omnitarg [Genentech, CA, USA]) is a HER-2/neu targeted monoclonal antibody that binds to a different epitope than trastuzumab, inhibiting the formation of HER-2/neu heterodimers with other HER-family receptor and halting the proliferation of ovarian cancer cells [129,130]. Pertuzumab is the HER-2 inhibitor that has been mostly studied in clinical trials for ovarian cancer (Table 1). The first clinical study was carried out by Gordon and colleagues and showed that pertuzumab as a monotherapy elicited a 4.3% partial response, 6.8% stable disease for 6 months and 14.5% of total clinical activity, with a median PFS of 6.6 weeks in patients with recurrent ovarian carcinoma [129]. A study carried out in platinum-resistant patients showed that the combination therapy of pertuzumab with gemcitabine resulted in a response rate of 13.8% with 2.9 months PFS as compared with a 4.6% response rate and PFS of 1.5 months in the gemcitabine and placebo group [131]. A recent ongoing study of 152 platinum- sensitive patients evaluated the activity of pertuzumab and carboplatin-based therapy compared with carboplatin therapy alone [132]. Preliminary results demonstrated that pertuzumab with carboplatin is well tolerated, has a 64% response rate and PFS of 34.1 weeks in comparison with 52% response rate and PFS of 31.3 weeks in the single-agent carboplatin group [132,133].
Several antihuman FRα antibodies have been previously studied in ovarian cancer. Farletuzumab (MORAb-003) is a humanized monoclonal antibody, optimized from the original murine LK26 antibody, which has high affinity for FRα [134]. Preclinical in vivo and in vitro studies have shown that farletuzumab binds specifically to ovarian cancer cells, has high efficacy in decreasing tumor growth with very low to no toxicity, and is a useful antibody for radioimmunoscintigraphy of ovarian cancer cells expressing FRα [135,136]. A clinical study performed by Konner and colleagues reported that farletuzumab as a single therapy in platinum-resistant ovarian cancer patients was safe and well tolerated at doses between 12.5 and 400 mg/m2 [137]. Moreover, 35% of the patients treated with one cycle (four weekly doses) had stable disease and three of these patients received extended therapy showing 3–17% mean decreases in tumor size. Another recent clinical study showed the results of farletuzumab as single-agent therapy or in combination with platinum and taxane in platinum-sensitive ovarian cancer patients with first recurrence of disease [138,139]. In this study, the group treated with farletuzumab combination therapy showed a 7% complete response, 63% partial response and 89% of the patients achieved normal CA-125. Moreover, four patients still remain in remission with median extension of the secondary remission being 11 months [140]. Ongoing and future Phase III studies include adjuvant use of farletuzumab as a monotherapy and in combination with chemotherapy in patients with recurrent ovarian cancer.
Catumaxomab is a trifunctional antibody with bispecificity for EpCAM in tumor cells and CD3 antigen in T cells. These new types of antibodies induce a tri-cell complex of tumor cells, T cells and accessory immune cells (e.g., macrophages, DCs and NK cells) owing to their unique Fc-composition of mouse IgG2a and rat IgG2b[141]. This drug has been approved in Europe since 2009 for the treatment of malignant ascites with EpCAM-positive carcinomas where first-hand therapy is not available or no longer viable. This drug has also been tested in the clinical setting as a new therapeutic strategy for ovarian cancer patients with symptomatic malignant ascites. A clinical study from Burges and colleagues showed good tolerance of catumaxomab in patients with recurrent ascites due to ovarian cancer [142]. They demonstrated a significant decrease in production of ascites in all 23 patients (only one patient required paracentesis at day 28) with a decrease in the number of intraperitoneal EpCAM+ tumor cells from 540,000 per 106 analyzed cells prior to treatment to 39 per 106 following the last infusion. Another recent clinical trial using catumaxomab in patients with malignant ascites due to epithelial cancers (129 ovarian and 129 nonovarian) assessed the efficacy and safety of catumaxomab and paracentesis compared with paracentesis alone [143]. This study showed that catumaxomab reduced the ascites volume and the time between paracentesis in both cancer groups, but most significantly in the ovarian cancer group along with an overall survival of 110 days compared with 81 days in the control group. Moreover, the catumaxomab-treated group elicited decreased EpCAM+ tumor cell count and increased CD45+ leukocytes in the ascites at the end of treatment when compared with the paracentesis alone.
Adoptive T-cell therapies
Adoptive T-cell therapy involves ex vivo activation, expansion and reinfusion of antigen-specific T cells [144]. The approach has been used successfully for the treatment of malignant melanoma and is envisioned to be useful for ovarian cancer based on the importance of T cells in relapse [145]. From a technical and infrastructure standpoint, adoptive T-cell therapy remains a challenge. Thus far, only two clinical trials of adoptive T-cell therapy in ovarian cancer have been reported. The first clinical trial by Kershaw and colleagues tested the safety and feasibility of infusion of T cells genetically modified to express a FRα-receptor antibody coupled to the signaling motif of the Fc receptor γ-chain [146]. The study was performed in patients with recurrent disease. While the infusions were shown to be safe, no clinical activity was observed, probably due to two notable problems. First, the infused T cells did not persist for long periods of time in large numbers, as required for tumor eradication in melanoma [145]. Second, an inhibitory factor developed in half of the patients, which significantly reduced the ability of the gene-modified T cells to respond against the tumor. The second clinical trial involved the infusion of nonmodified MUC1-specific Th1 effector cells [147]. MUC1-specific T cells were derived by repeated stimulation and expansion of leukapheresis-derived cells with the 20-mer MUC1 peptide, GSTAPPAHGVTSAPATAPAP. Three doses of between 108 and 109 cells (largely CD4 T cells) were administered intraperitoneally into seven patients. Three discontinued treatment due to local inflammation or obstruction at the intraperitoneal port; in the four remaining patients, treatment was shown to increase systemic MUC1 immune responses, which could have contributed to the long-term survival observed in two of the patients.
Vaccine therapies
Peptide vaccines are a very attractive type of active immunotherapy that offer several advantages including specificity, stability and the ability to be manufactured to increase their immunogenicity. To date, a few clinical studies have been published using peptide vaccines for ovarian cancer focusing on the safety and immunogenicity of the vaccines (Table 1). One study of a multipeptide vaccine constructed from three ovarian cancer-associated antigens, HER-2/neu (amino acids 369–377 and 754–762), MAGE-A1 (amino acids 161–169 and 96–104) and FRα (amino acids 191–199), for the treatment of advanced ovarian cancer was reported to be safe and well tolerated [148]. This study demonstrated that this vaccine was immunogenic with detection of CD8+ T-cell response in all treated patients, one detected ex vivo and eight after in vitro stimulation. Despite the CD8+ T-cell response, 89% of the patients had progression of their disease. In a Phase I trial by Diefenbach and colleagues, NY-ESO-1b peptide (amino acids 157–165) vaccine was studied as an adjuvant therapy in nine patients with high-risk ovarian cancer [149]. Results showed no ex vivo CD8+ T-cell responses; however, after in vitro presensitization with either NY-ESO-1b peptide or recombinant adenovirus, 75% of the treated patients showed CD8+ T-cell immunity and remained present 3 weeks after vaccination. In another study, a p53 synthetic long-peptide (SLP) vaccine in patients with recurrent ovarian cancer showed that this vaccine is safe with only grade 1 or 2 adverse events observed in all patients after four immunizations [150]. This vaccine was capable of inducing proliferation of p53-specific CD4+ T cells, predominantly of the Th2 phenotype, in 82.4% of the patients, although only 10% of the patients had stable disease and the rest showed disease progression.
While most of these vaccines being tested target the generation of T cells, the fact that antibody immunity correlates with disease outcomes in patients makes antibody- inducing vaccines a logical option. To that end, Kaumaya determined the activity of a HER-2/neu antibody- inducing chimeric peptide (amino acids 628–647 and 316–339) vaccine with a promiscuous T-cell epitope (amino acids 280–302) from the measles virus fusion protein in patients with metastatic solid tumors including patients with advanced ovarian cancer [151]. This study demonstrated that this vaccine is moderately well tolerated (20% severe adverse events) and elicits IgG antibodies at all doses. Moreover, of six patients that showed clinical benefit, two were patients with ovarian cancer who had a high antibody response to the vaccine [151]. Overall, the few vaccine trials that have been conducted reveal that patients can mount immunity safely to tumor antigens paving the way for advanced clinical trials designed to test clinical efficacy. Optimism remains that vaccines can eradicate residual tumor to prevent recurrence or disease progression. Indeed, Hernando and colleagues recently published a case report demonstrating vaccine potency in a patient with recurrent metastatic ovarian cancer [152]. The 61-year-old patient has been previously treated twice with surgical debulking and platinum based therapy. At third recurrence 4 months following the last therapy, the patient presented with a bulky axillary lymph node metastasis and a para-aortic mass. At that time, a vaccination regimen with autologous DCs engineered to express FRα was initiated, and ten immunizations were given at 4-week intervals. CT scans before and 3 months after the last treatment showed a marked, albeit partial, shrinkage in tumor. CA-125 levels, which were rising just prior to vaccine, immediately declined to baseline following the first two vaccinations. A follow-up CT scan at 16 months following the start of vaccination showed greater than 50% regression. Immune monitoring revealed that the patient developed strong FRα-specific T-cell immunity to the vaccine. Remarkably, at 11 months following the last vaccine, CA-125 levels remained at baseline showing that vaccines can induce long-term remission.
Future perspective
There is some agreement in the field of cancer immunotherapy that combination approaches could lead to synergism and greater efficacy. A number of strategies have emerged, some of which are being tested clinically. For example, the efficacy of trastuzumab therapy may be improved by combining with agents that boost T-cell immunity. In one study, zum Buschenfelde found that pretreatment of HER-2/neu+ ovarian tumor cells with trastuzumab enhanced the cytolytic activity of HER-2/neu-specific T cells against the HER-2/neu-overexpressing tumors in vitro [153]. Although the mechanism is unclear, it is possible that trastuzumab promotes the internalization and degradation of HER-2/neu, resulting in increased presentation of HER-2/neu MHC class I epitopes, which may lead to greater activation and expansion of HER-2/neu- specific T cells. A clinical trial of combination trastuzumab and HER-2/neu vaccination in breast cancer was recently reported by Disis and colleagues [154]. A total of 22 patients with stage 4 HER-2/neu-overexpressing breast cancer receiving trastuzumab therapy were vaccinated with an HER-2/ neu T-cell peptide-based vaccine. The patients had either no evidence of disease or stable masses at the time of therapy. The combination was well tolerated with 15% of patients experiencing an asymptomatic decline in left ventricular ejection fraction below the normal range during combination therapy. While many patients had pre-existing immunity to HER-2/neu when treated with trastuzumab alone, that immunity could be significantly boosted and maintained with vaccination. Importantly, at a median follow-up of 36 months from the first vaccine, the median overall survival in the study population has not been reached.
In summary, circulating or tumor-associated Tregs may block the immune response; subsequently, there is interest in preconditioning patients to inhibit Tregs in order to augment immunity. Agents such as cyclophosphamide, which augment immune-based therapies in both human and mouse studies are thought to involve Treg depletion [155]. Other strategies that are being explored to inhibit the function of Tregs include anticytotoxic T lymphocyte antigen-4 monoclonal antibodies and CD25-targeted agents such as denileukin diftitox [156]. Alternatively, targeted therapeutics, such as small molecule inhibitors, could also work in concert with vaccines. An example is inhibitors of TGF-β, a growth factor with multiple roles in cancer [157,158]. TGF-β promotes tumor progression, invasion and metastasis by inducing epithelial-to-mesenchymal transition, migration and release of VEGF [157]. TGF-β also directly inhibits cytotoxic actions of tumor-infiltrating CD8 T cells [159]. Thus, an agent that simultaneously blocks immunosuppression and tumor progression may be more effective by providing sufficient time for the immune system to expand and destroy residual tumor burden. Many combinatorial strategies are currently being tested and time will tell whether such mixing will be therapeutically effective.
Executive summary.
Basic principles of immunity
-
▪
The innate immune system is the first line of defense that consists of:
-
–
A cellular component that includes NKT cells, mast cells, eosinophils, basophils, macrophages, neutrophils and dendritic cells.
-
–
Humoral factors that include mainly cytokines and complement.
-
–
-
▪
The adaptive immune system involves both T and B lymphocytes.
-
▪
Adaptive immune responses usually develop after 1 week, are highly antigen specific and capable of generating immunologic memory.
Natural immune response to ovarian cancer
-
▪
There is a natural immune response associated with ovarian cancers shown to have a significant impact on the clinical course of the disease.
-
▪
CD8+ T cells tumor infiltration is shown to improve survival in ovarian cancer patients.
Ovarian cancer antigens
-
▪
Several ovarian cancer antigens have been identified and studied (i.e., folate receptor α, HER2/neu, IGF binding protein 2, p53, mucins, epithelial cell adhesion molecule and NY-ESO-1) demonstrating that ovarian cancer patients respond naturally to these antigens.
Immune suppression in ovarian cancer
-
▪
Despite the fact that antitumor immunity is naturally elicited against ovarian cancers, the antitumor response is blunted by a complex immune suppressive microenvironment.
-
▪
Immune suppression is mediated by factors released from the tumor or by infiltration of the tumors by a variety of either lymphoid or myeloid-derived suppressor or regulatory cells.
Immunological treatments
-
▪
Immunological approaches for ovarian cancer have been studied in recent clinical trials that include antibody therapies (i.e., farletuzumab, pertuzumab and catumaxomab), adoptive T-cell therapies and vaccines with promising results.
Conclusion
-
▪
Ovarian cancer is an immune reactive malignancy with a complex immune microenvironment.
-
▪
Future direction of this field should focus on a synergistic combination of therapies that can generate immunity and target immune suppression, hopefully with greater results.
Acknowledgments
Work on ovarian cancers in the authors’ laboratories is supported by the Minnesota Ovarian Cancer Alliance (Keith L Knutson), the Fred C and Katherine B Andersen Foundation (Keith L Knutson and Kimberly R Kalli), R01-CA122443 (to Ellen L Goode) and the Mayo Clinic Ovarian Cancer SPORE (P50-CA136393 to Lynn C Hartmann, Keith L Knutson and Ellen L Goode).
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
- 1.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J. Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Aletti GD, Gallenberg MM, Cliby WA, et al. Current management strategies for ovarian cancer. Mayo Clin. Proc. 2007;82(6):751–770. doi: 10.4065/82.6.751. [DOI] [PubMed] [Google Scholar]
- 3.Cannistra SA. Cancer of the ovary. N. Engl. J. Med. 2004;351(24):2519–2529. doi: 10.1056/NEJMra041842. [DOI] [PubMed] [Google Scholar]
- 4.Ozols RF, Bundy BN, Greer BE, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J. Clin. Oncol. 2003;21(17):3194–3200. doi: 10.1200/JCO.2003.02.153. [DOI] [PubMed] [Google Scholar]
- 5.Winter WE, 3rd, Maxwell GL, Tian C, et al. Prognostic factors for stage III epithelial ovarian cancer: a Gynecologic Oncology Group study. J. Clin. Oncol. 2007;25(24):3621–3627. doi: 10.1200/JCO.2006.10.2517. [DOI] [PubMed] [Google Scholar]
- 6.Hoskins P, Vergote I, Cervantes A, et al. Advanced ovarian cancer: Phase III randomized study of sequential cisplatin-topotecan and carboplatin-paclitaxel vs carboplatin-paclitaxel. J. Natl Cancer Inst. 2010;102(20):1547–1556. doi: 10.1093/jnci/djq362. [DOI] [PubMed] [Google Scholar]
- 7.Tanner B, Hasenclever D, Stern K, et al. ErbB-3 predicts survival in ovarian cancer. J. Clin. Oncol. 2006;24(26):4317–4323. doi: 10.1200/JCO.2005.04.8397. [DOI] [PubMed] [Google Scholar]
- 8.Haskill S, Becker S, Fowler W, et al. Mononuclear-cell infiltration in ovarian cancer. I. Inflammatory-cell infiltrates from tumour and ascites material. Br. J. Cancer. 1982;45(5):728–736. doi: 10.1038/bjc.1982.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang L, Conejo-Garcia JR, Katsaros D, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N. Engl. J. Med. 2003;348(3):203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 10.Knutson KL, Disis ML, Salazar LG. CD4 regulatory T cells in human cancer pathogenesis. Cancer Immunol. Immunother. 2007;56(3):271–285. doi: 10.1007/s00262-006-0194-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sato E, Olson SH, Ahn J, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc. Natl Acad. Sci. USA. 2005;102(51):18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leffers N, Gooden MJ, de Jong RA, et al. Prognostic significance of tumor-infiltrating T-lymphocytes in primary and metastatic lesions of advanced stage ovarian cancer. Cancer Immunol. Immunother. 2009;58(3):449–459. doi: 10.1007/s00262-008-0583-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ashton-Rickardt PG. The granule pathway of programmed cell death. Crit. Rev. Immunol. 2005;25(3):161–182. doi: 10.1615/critrevimmunol.v25.i3.10. [DOI] [PubMed] [Google Scholar]
- 14.Filaci G, Fenoglio D, Fravega M, et al. CD8+ CD28- T regulatory lymphocytes inhibiting T cell proliferative and cytotoxic functions infiltrate human cancers. J. Immunol. 2007;179(7):4323–4334. doi: 10.4049/jimmunol.179.7.4323. [DOI] [PubMed] [Google Scholar]
- 15.Milne K, Kobel M, Kalloger SE, et al. Systematic analysis of immune infiltrates in high-grade serous ovarian cancer reveals CD20, FoxP3 and TIA-1 as positive prognostic factors. PLoS One. 2009;4(7):E6412. doi: 10.1371/journal.pone.0006412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Callahan MJ, Nagymanyoki Z, Bonome T, et al. Increased HLA-DMB expression in the tumor epithelium is associated with increased CTL infiltration and improved prognosis in advanced-stage serous ovarian cancer. Clin. Cancer Res. 2008;14(23):7667–7673. doi: 10.1158/1078-0432.CCR-08-0479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leffers N, Fehrmann RS, Gooden MJ, et al. Identification of genes and pathways associated with cytotoxic T lymphocyte infiltration of serous ovarian cancer. Br. J. Cancer. 2010;103(5):685–692. doi: 10.1038/sj.bjc.6605820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kryczek I, Banerjee M, Cheng P, et al. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood. 2009;114(6):1141–1149. doi: 10.1182/blood-2009-03-208249. ▪ Demonstrates that increasing IL-17 levels in ascites are associated with improved patient outcomes in ovarian cancer.
- 19.Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat. Rev. Immunol. 2010;10(7):479–489. doi: 10.1038/nri2800. [DOI] [PubMed] [Google Scholar]
- 20.Perussia B, Chen Y, Loza MJ. Peripheral NK cell phenotypes: multiple changing of faces of an adapting, developing cell. Mol. Immunol. 2005;42(4):385–395. doi: 10.1016/j.molimm.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez S, Lopez-Soto A, Suarez-Alvarez B, et al. NKG2D ligands: key targets of the immune response. Trends Immunol. 2008;29(8):397–403. doi: 10.1016/j.it.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 22.Lopez-Larrea C, Suarez-Alvarez B, Lopez-Soto A, et al. The NKG2D receptor: sensing stressed cells. Trends Mol. Med. 2008;14(4):179–189. doi: 10.1016/j.molmed.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 23.Li K, Mandai M, Hamanishi J, et al. Clinical significance of the NKG2D ligands, MICA/B and ULBP2 in ovarian cancer: high expression of ULBP2 is an indicator of poor prognosis. Cancer Immunol. Immunother. 2009;58(5):641–652. doi: 10.1007/s00262-008-0585-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garzetti GG, Cignitti M, Ciavattini A, et al. Natural killer cell activity and progression-free survival in ovarian cancer. Gynecol. Obstet. Invest. 1993;35(2):118–120. doi: 10.1159/000292678. [DOI] [PubMed] [Google Scholar]
- 25.Dong HP, Elstrand MB, Holth A, et al. NK- and B-cell infiltration correlates with worse outcome in metastatic ovarian carcinoma. Am. J. Clin. Pathol. 2006;125(3):451–458. [PubMed] [Google Scholar]
- 26. Goodell V, Salazar LG, Urban N, et al. Antibody immunity to the p53 oncogenic protein is a prognostic indicator in ovarian cancer. J. Clin. Oncol. 2006;24(5):762–768. doi: 10.1200/JCO.2005.03.2813. ▪ First study that shows that antigen-specific immune response predict improved survival in late stage ovarian cancer.
- 27.Knutson KL, Krco CJ, Erskine CL, et al. T-cell immunity to the folate receptor a is prevalent in women with breast or ovarian cancer. J. Clin. Oncol. 2006;24(26):4254–4261. doi: 10.1200/JCO.2006.05.9311. [DOI] [PubMed] [Google Scholar]
- 28.Tchabo NE, Mhawech-Fauceglia P, Caballero OL, et al. Expression and serum immunoreactivity of developmentally restricted differentiation antigens in epithelial ovarian cancer. Cancer Immun. 2009;9:6. [PMC free article] [PubMed] [Google Scholar]
- 29.Ioannides CG, Fisk B, Fan D, et al. Cytotoxic T cells isolated from ovarian malignant ascites recognize a peptide derived from the HER-2/neu proto-oncogene. Cell Immunol. 1993;151(1):225–234. doi: 10.1006/cimm.1993.1233. [DOI] [PubMed] [Google Scholar]
- 30.Doherty JK, Bond C, Jardim A, et al. The HER-2/neu receptor tyrosine kinase gene encodes a secreted autoinhibitor. Proc. Natl Acad. Sci. USA. 1999;96(19):10869–10874. doi: 10.1073/pnas.96.19.10869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bookman MA, Darcy KM, Clarke-Pearson D, et al. Evaluation of monoclonal humanized anti-HER2 antibody, trastuzumab, in patients with recurrent or refractory ovarian or primary peritoneal carcinoma with overexpression of HER2: a Phase II trial of the Gynecologic Oncology Group. J. Clin. Oncol. 2003;21(2):283–290. doi: 10.1200/JCO.2003.10.104. [DOI] [PubMed] [Google Scholar]
- 32.Camilleri-Broet S, Hardy-Bessard AC, Le Tourneau A, et al. HER-2 overexpression is an independent marker of poor prognosis of advanced primary ovarian carcinoma: a multicenter study of the GINECO group. Ann. Oncol. 2004;15(1):104–112. doi: 10.1093/annonc/mdh021. [DOI] [PubMed] [Google Scholar]
- 33.Karaferic A, Jovanovic D, Jelic S. Expression of HER2/neu, estrogen and progesterone receptors, CA 125 and CA19-9 on cancer cell membrane in patients with serous and mucinous carcinoma of the ovary. J. BUON. 2009;14(4):635–639. [PubMed] [Google Scholar]
- 34.Tuefferd M, Couturier J, Penault-Llorca F, et al. HER2 status in ovarian carcinomas: a multicenter GINECO study of 320 patients. PLoS One. 2007;2(11):E1138. doi: 10.1371/journal.pone.0001138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hogdall EV, Christensen L, Kjaer SK, et al. Distribution of HER-2 overexpression in ovarian carcinoma tissue and its prognostic value in patients with ovarian carcinoma: from the Danish MALOVA Ovarian Cancer Study. Cancer. 2003;98(1):66–73. doi: 10.1002/cncr.11476. [DOI] [PubMed] [Google Scholar]
- 36.Karyampudi L, Formicola C, Erskine CL, et al. A degenerate HLA-DR epitope pool of HER-2/ neu reveals a novel in vivo immunodominant epitope, HER-2/neu88–102. Clin. Cancer Res. 2010;16(3):825–834. doi: 10.1158/1078-0432.CCR-09-2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown Jones M, Neuper C, Clayton A, et al. Rationale for folate receptor a targeted therapy in ‘high risk’ endometrial carcinomas. Int. J. Cancer. 2008;123(7):1699–1703. doi: 10.1002/ijc.23686. [DOI] [PubMed] [Google Scholar]
- 38.Dainty LA, Risinger JI, Morrison C, et al. Overexpression of folate binding protein and mesothelin are associated with uterine serous carcinoma. Gynecol. Oncol. 2007;105(3):563–570. doi: 10.1016/j.ygyno.2006.10.063. [DOI] [PubMed] [Google Scholar]
- 39.Elnakat H, Ratnam M. Role of folate receptor genes in reproduction and related cancers. Front. Biosci. 2006;11:506–519. doi: 10.2741/1815. [DOI] [PubMed] [Google Scholar]
- 40.Parker N, Turk MJ, Westrick E, et al. Folate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assay. Anal. Biochem. 2005;338(2):284–293. doi: 10.1016/j.ab.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 41.Kalli KR, Oberg AL, Keeney GL, et al. Folate receptor a as a tumor target in epithelial ovarian cancer. Gynecol. Oncol. 2008;108(3):619–626. doi: 10.1016/j.ygyno.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kelemen LE. The role of folate receptor a in cancer development, progression and treatment: cause, consequence or innocent bystander? Int. J. Cancer. 2006;119(2):243–250. doi: 10.1002/ijc.21712. [DOI] [PubMed] [Google Scholar]
- 43.Peoples GE, Anderson BW, Fisk B, et al. Ovarian cancer-associated lymphocyte recognition of folate binding protein peptides. Ann. Surg. Oncol. 1998;5(8):743–750. doi: 10.1007/BF02303486. [DOI] [PubMed] [Google Scholar]
- 44.Peoples GE, Anderson BW, Lee TV, et al. Vaccine implications of folate binding protein, a novel cytotoxic T lymphocyte-recognized antigen system in epithelial cancers. Clin. Cancer Res. 1999;5(12):4214–4223. [PubMed] [Google Scholar]
- 45.Baron-Hay S, Boyle F, Ferrier A, et al. Elevated serum insulin-like growth factor binding protein-2 as a prognostic marker in patients with ovarian cancer. Clin. Cancer Res. 2004;10(5):1796–1806. doi: 10.1158/1078-0432.ccr-0672-2. [DOI] [PubMed] [Google Scholar]
- 46.Lancaster JM, Sayer RA, Blanchette C, et al. High expression of insulin-like growth factor binding protein-2 messenger RNA in epithelial ovarian cancers produces elevated preoperative serum levels. Int. J. Gynecol. Cancer. 2006;16(4):1529–1535. doi: 10.1111/j.1525-1438.2006.00623.x. [DOI] [PubMed] [Google Scholar]
- 47.Wang H, Rosen DG, Wang H, et al. Insulin-like growth factor-binding protein 2 and 5 are differentially regulated in ovarian cancer of different histologic types. Mod. Pathol. 2006;19(9):1149–1156. doi: 10.1038/modpathol.3800637. [DOI] [PubMed] [Google Scholar]
- 48.Yan XJ, Tian Y, Wang C, et al. [The expressions and clinical significance of IGFBP-2, −3 in both serum and tumor tissues in patients with epithelial ovarian cancer] Sichuan Da Xue Xue Bao Yi Xue Ban. 2009;40(4):639–643. [PubMed] [Google Scholar]
- 49.Kalli KR, Krco CJ, Hartmann LC, et al. An HLA-DR-degenerate epitope pool detects insulin-like growth factor binding protein 2-specific immunity in patients with cancer. Cancer Res. 2008;68(12):4893–4901. doi: 10.1158/0008-5472.CAN-07-6726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bafna S, Kaur S, Batra SK. Membrane-bound mucins: the mechanistic basis for alterations in the growth and survival of cancer cells. Oncogene. 2010;29(20):2893–2904. doi: 10.1038/onc.2010.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bafna S, Singh AP, Moniaux N, et al. MUC4, a multifunctional transmembrane glycoprotein, induces oncogenic transformation of NIH3T3 mouse fibroblast cells. Cancer Res. 2008;68(22):9231–9238. doi: 10.1158/0008-5472.CAN-08-3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Senapati S, Sharma P, Bafna S, et al. The MUC gene family: their role in the diagnosis and prognosis of gastric cancer. Histol. Histopathol. 2008;23(12):1541–1552. doi: 10.14670/HH-23.1541. [DOI] [PubMed] [Google Scholar]
- 53.Chauhan SC, Singh AP, Ruiz F, et al. Aberrant expression of MUC4 in ovarian carcinoma: diagnostic significance alone and in combination with MUC1 and MUC16 (CA125) Mod. Pathol. 2006;19(10):1386–1394. doi: 10.1038/modpathol.3800646. [DOI] [PubMed] [Google Scholar]
- 54.Singh AP, Chauhan SC, Bafna S, et al. Aberrant expression of transmembrane mucins, MUC1 and MUC4, in human prostate carcinomas. Prostate. 2006;66(4):421–429. doi: 10.1002/pros.20372. [DOI] [PubMed] [Google Scholar]
- 55.Bast RC, Jr, Xu FJ, Yu YH, et al. CA 125: the past and the future. Int. J. Biol. Markers. 1998;13(4):179–187. doi: 10.1177/172460089801300402. [DOI] [PubMed] [Google Scholar]
- 56.Yin BW, Lloyd KO. Molecular cloning of the CA125 ovarian cancer antigen: identification as a new mucin, MUC16. J. Biol. Chem. 2001;276(29):27371–27375. doi: 10.1074/jbc.M103554200. [DOI] [PubMed] [Google Scholar]
- 57.Wang L, Ma J, Liu F, et al. Expression of MUC1 in primary and metastatic human epithelial ovarian cancer and its therapeutic significance. Gynecol. Oncol. 2007;105(3):695–702. doi: 10.1016/j.ygyno.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 58.Ponnusamy MP, Lakshmanan I, Jain M, et al. MUC4 mucin-induced epithelial to mesenchymal transition: a novel mechanism for metastasis of human ovarian cancer cells. Oncogene. 2010;29(42):5741–5754. doi: 10.1038/onc.2010.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ponnusamy MP, Singh AP, Jain M, et al. MUC4 activates HER2 signalling and enhances the motility of human ovarian cancer cells. Br. J. Cancer. 2008;99(3):520–526. doi: 10.1038/sj.bjc.6604517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Terry KL, Titus-Ernstoff L, McKolanis JR, et al. Incessant ovulation, mucin 1 immunity, and risk for ovarian cancer. Cancer Epidemiol. Biomarkers Prev. 2007;16(1):30–35. doi: 10.1158/1055-9965.EPI-06-0688. [DOI] [PubMed] [Google Scholar]
- 61.Oei AL, Moreno M, Verheijen RH, et al. Induction of IgG antibodies to MUC1 and survival in patients with epithelial ovarian cancer. Int. J. Cancer. 2008;123(8):1848–1853. doi: 10.1002/ijc.23725. [DOI] [PubMed] [Google Scholar]
- 62.Plisiecka-Halasa J, Dansonka-Mieszkowska A, Kraszewska E, et al. Loss of heterozygosity, microsatellite instability and TP53 gene status in ovarian carcinomas. Anticancer Res. 2008;28(2A):989–996. [PubMed] [Google Scholar]
- 63.Corney DC, Flesken-Nikitin A, Choi J, et al. Role of p53 and Rb in ovarian cancer. Adv. Exp. Med. Biol. 2008;622:99–117. doi: 10.1007/978-0-387-68969-2_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lambeck A, Leffers N, Hoogeboom BN, et al. P53-specific T cell responses in patients with malignant and benign ovarian tumors: implications for p53 based immunotherapy. Int. J. Cancer. 2007;121(3):606–614. doi: 10.1002/ijc.22710. [DOI] [PubMed] [Google Scholar]
- 65.Munz M, Baeuerle PA, Gires O. The emerging role of EpCAM in cancer and stem cell signaling. Cancer Res. 2009;69(14):5627–5629. doi: 10.1158/0008-5472.CAN-09-0654. [DOI] [PubMed] [Google Scholar]
- 66.Kobel M, Kalloger SE, Boyd N, et al. Ovarian carcinoma subtypes are different diseases: implications for biomarker studies. PLoS Med. 2008;5(12):E232. doi: 10.1371/journal.pmed.0050232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Balzar M, Winter MJ, de Boer CJ, et al. The biology of the 17-1A antigen (Ep-CAM) J. Mol. Med. 1999;77(10):699–712. doi: 10.1007/s001099900038. [DOI] [PubMed] [Google Scholar]
- 68.Runz S, Keller S, Rupp C, et al. Malignant ascites-derived exosomes of ovarian carcinoma patients contain CD24 and EpCAM. Gynecol. Oncol. 2007;107(3):563–571. doi: 10.1016/j.ygyno.2007.08.064. [DOI] [PubMed] [Google Scholar]
- 69.Diaz-Arias AA, Loy TS, Bickel JT, et al. Utility of BER-EP4 in the diagnosis of adenocarcinoma in effusions: an immunocytochemical study of 232 cases. Diagn. Cytopathol. 1993;9(5):516–521. doi: 10.1002/dc.2840090509. [DOI] [PubMed] [Google Scholar]
- 70.Kim JH, Herlyn D, Wong KK, et al. Identification of epithelial cell adhesion molecule autoantibody in patients with ovarian cancer. Clin. Cancer Res. 2003;9(13):4782–4791. [PubMed] [Google Scholar]
- 71.Stevenson BJ, Iseli C, Panji S, et al. Rapid evolution of cancer/testis genes on the X chromosome. BMC Genomics. 2007;8:129. doi: 10.1186/1471-2164-8-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Valmori D, Qian F, Ayyoub M, et al. Expression of synovial sarcoma X (SSX) antigens in epithelial ovarian cancer and identification of SSX-4 epitopes recognized by CD4+ T cells. Clin. Cancer Res. 2006;12(2):398–404. doi: 10.1158/1078-0432.CCR-05-1902. [DOI] [PubMed] [Google Scholar]
- 73.Zhang S, Zhou X, Yu H, et al. Expression of tumor-specific antigen MAGE, GAGE and BAGE in ovarian cancer tissues and cell lines. BMC Cancer. 2010;10:163. doi: 10.1186/1471-2407-10-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hofmann M, Ruschenburg I. mRNA detection of tumor-rejection genes BAGE, GAGE, and MAGE in peritoneal fluid from patients with ovarian carcinoma as a potential diagnostic tool. Cancer. 2002;96(3):187–193. doi: 10.1002/cncr.10622. [DOI] [PubMed] [Google Scholar]
- 75.Straughn JM, Jr, Shaw DR, Guerrero A, et al. Expression of sperm protein 17 (Sp17) in ovarian cancer. Int. J. Cancer. 2004;108(6):805–811. doi: 10.1002/ijc.11617. [DOI] [PubMed] [Google Scholar]
- 76.Li FQ, Han YL, Liu Q, et al. Overexpression of human sperm protein 17 increases migration and decreases the chemosensitivity of human epithelial ovarian cancer cells. BMC Cancer. 2009;9:323. doi: 10.1186/1471-2407-9-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Matsuzaki J, Gnjatic S, Mhawech-Fauceglia P, et al. Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc. Natl Acad. Sci. USA. 2010;107(17):7875–7880. doi: 10.1073/pnas.1003345107. ▪ Interesting study demonstrating that PD-1 is associated with negative regulation of cell activation in ovarian cancer tumor-infiltrating lymphocytes.
- 78.Odunsi K, Jungbluth AA, Stockert E, et al. NY-ESO-1 and LAGE-1 cancer-testis antigens are potential targets for immunotherapy in epithelial ovarian cancer. Cancer Res. 2003;63(18):6076–6083. [PubMed] [Google Scholar]
- 79.Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat. Rev. Immunol. 2003;3(3):253–257. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- 80.Miyara M, Yoshioka Y, Kitoh A, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30(6):899–911. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 81.Meloni F, Morosini M, Solari N, et al. Foxp3 expressing CD4+ CD25+ and CD8+CD28-T regulatory cells in the peripheral blood of patients with lung cancer and pleural mesothelioma. Hum. Immunol. 2006;67(1–2):1–12. doi: 10.1016/j.humimm.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 82.Audia S, Nicolas A, Cathelin D, et al. Increase of CD4+ CD25+ regulatory T cells in the peripheral blood of patients with metastatic carcinoma: a Phase I clinical trial using cyclophosphamide and immunotherapy to eliminate CD4+ CD25+ T lymphocytes. Clin. Exp. Immunol. 2007;150(3):523–530. doi: 10.1111/j.1365-2249.2007.03521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li X, Ye DF, Xie X, et al. Proportion of CD4+CD25+ regulatory T cell is increased in the patients with ovarian carcinoma. Cancer Invest. 2005;23(5):399–403. [PubMed] [Google Scholar]
- 84. Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat. Med. 2004;10(9):942–949. doi: 10.1038/nm1093. ▪ Important study demonstrating an association between tumor-infiltrating Tregs and poor patient survival.
- 85.Wolf D, Wolf AM, Rumpold H, et al. The expression of the regulatory T cell-specific forkhead box transcription factor FoxP3 is associated with poor prognosis in ovarian cancer. Clin. Cancer Res. 2005;11(23):8326–8331. doi: 10.1158/1078-0432.CCR-05-1244. [DOI] [PubMed] [Google Scholar]
- 86.Fricke I, Gabrilovich DI. Dendritic cells and tumor microenvironment: a dangerous liaison. Immunol. Invest. 2006;35(3–4):459–483. doi: 10.1080/08820130600803429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen F, Hou M, Ye F, et al. Ovarian cancer cells induce peripheral mature dendritic cells to differentiate into macrophagelike cells in vitro. Int. J. Gynecol. Cancer. 2009;19(9):1487–1493. doi: 10.1111/IGC.0b013e3181bb70c6. [DOI] [PubMed] [Google Scholar]
- 88.Teicher BA, Fricker SP. CXCL12 (SDF-1)/CXCR4 pathway in cancer. Clin. Cancer Res. 2010;16(11):2927–2931. doi: 10.1158/1078-0432.CCR-09-2329. [DOI] [PubMed] [Google Scholar]
- 89.Bignotti E, Tassi RA, Calza S, et al. Gene expression profile of ovarian serous papillary carcinomas: identification of metastasis-associated genes. Am. J. Obstet. Gynecol. 2007;196(3):245, E1–E11. doi: 10.1016/j.ajog.2006.10.874. [DOI] [PubMed] [Google Scholar]
- 90.Zou W, Machelon V, Coulomb-L’Hermin A, et al. Stromal-derived factor-1 in human tumors recruits and alters the function of plasmacytoid precursor dendritic cells. Nat. Med. 2001;7(12):1339–1346. doi: 10.1038/nm1201-1339. [DOI] [PubMed] [Google Scholar]
- 91.Wei S, Kryczek I, Zou L, et al. Plasmacytoid dendritic cells induce CD8+ regulatory T cells in human ovarian carcinoma. Cancer Res. 2005;65(12):5020–5026. doi: 10.1158/0008-5472.CAN-04-4043. [DOI] [PubMed] [Google Scholar]
- 92.Wertel I, Polak G, Bednarek W, et al. Dendritic cell subsets in the peritoneal fluid and peripheral blood of women suffering from ovarian cancer. Cytometry B Clin. Cytom. 2008;74(4):251–258. doi: 10.1002/cyto.b.20410. [DOI] [PubMed] [Google Scholar]
- 93.Kerbel RS. Tumor angiogenesis. N. Engl. J. Med. 2008;358(19):2039–2049. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schmid MC, Varner JA. Myeloid cells in the tumor microenvironment: modulation of tumor angiogenesis and tumor inflammation. J. Oncol. 2010 doi: 10.1155/2010/201026. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ostrand-Rosenberg S. Myeloid-derived suppressor cells: more mechanisms for inhibiting antitumor immunity. Cancer Immunol. Immunother. 2010;59(10):1593–1600. doi: 10.1007/s00262-010-0855-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Curiel TJ, Wei S, Dong H, et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat. Med. 2003;9(5):562–567. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- 97.Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol. Rev. 2010;236:219–242. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wilcox RA, Feldman AL, Wada DA, et al. B7-H1 (PD-L1, CD274) suppresses host immunity in T-cell lymphoproliferative disorders. Blood. 2009;114(10):2149–2158. doi: 10.1182/blood-2009-04-216671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hamanishi J, Mandai M, Iwasaki M, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc. Natl Acad. Sci. USA. 2007;104(9):3360–3365. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ghebeh H, Mohammed S, Al-Omair A, et al. The B7-H1 (PD-L1) T lymphocyte-inhibitory molecule is expressed in breast cancer patients with infiltrating ductal carcinoma: correlation with important high-risk prognostic factors. Neoplasia. 2006;8(3):190–198. doi: 10.1593/neo.05733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Karim R, Jordanova ES, Piersma SJ, et al. Tumor-expressed B7-H1 and B7-DC in relation to PD-1+ T-cell infiltration and survival of patients with cervical carcinoma. Clin. Cancer Res. 2009;15(20):6341–6347. doi: 10.1158/1078-0432.CCR-09-1652. [DOI] [PubMed] [Google Scholar]
- 102.Keir ME, Butte MJ, Freeman GJ, et al. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Keir ME, Francisco LM, Sharpe AH. PD-1 and its ligands in T-cell immunity. Curr. Opin. Immunol. 2007;19(3):309–314. doi: 10.1016/j.coi.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 104.Keir ME, Liang SC, Guleria I, et al. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J. Exp. Med. 2006;203(4):883–895. doi: 10.1084/jem.20051776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Scarlett UK, Cubillos-Ruiz JR, Nesbeth YC, et al. In situ stimulation of CD40 and Toll-like receptor 3 transforms ovarian cancer-infiltrating dendritic cells from immunosuppressive to immunostimulatory cells. Cancer Res. 2009;69(18):7329–7337. doi: 10.1158/0008-5472.CAN-09-0835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cubillos-Ruiz JR, Rutkowski M, Conejo-Garcia JR. Blocking ovarian cancer progression by targeting tumor microenvironmental leukocytes. Cell Cycle. 2010;9(2):260–268. doi: 10.4161/cc.9.2.10430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sperner-Unterweger B, Neurauter G, Klieber M, et al. Enhanced tryptophan degradation in patients with ovarian carcinoma correlates with several serum soluble immune activation markers. Immunobiology. 2011;216(3):296–301. doi: 10.1016/j.imbio.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 108.Huarte E, Cubillos-Ruiz JR, Nesbeth YC, et al. Depletion of dendritic cells delays ovarian cancer progression by boosting antitumor immunity. Cancer Res. 2008;68(18):7684–7691. doi: 10.1158/0008-5472.CAN-08-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009;9(3):162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Movahedi K, Guilliams M, Van den Bossche J, et al. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood. 2008;111(8):4233–4244. doi: 10.1182/blood-2007-07-099226. [DOI] [PubMed] [Google Scholar]
- 111.Youn JI, Nagaraj S, Collazo M, et al. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J. Immunol. 2008;181(8):5791–5802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Serafini P, Borrello I, Bronte V. Myeloid suppressor cells in cancer: recruitment, phenotype, properties, and mechanisms of immune suppression. Semin. Cancer Biol. 2006;16(1):53–65. doi: 10.1016/j.semcancer.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 113.Yang R, Cai Z, Zhang Y, et al. CD80 in immune suppression by mouse ovarian carcinoma-associated Gr-1+CD11b+ myeloid cells. Cancer Res. 2006;66(13):6807–6815. doi: 10.1158/0008-5472.CAN-05-3755. [DOI] [PubMed] [Google Scholar]
- 114.Klink M, Jastrzembska K, Nowak M, et al. Ovarian cancer cells modulate human blood neutrophils response to activation in vitro. Scand. J. Immunol. 2008;68(3):328–336. doi: 10.1111/j.1365-3083.2008.02139.x. [DOI] [PubMed] [Google Scholar]
- 115.An X, Ding PR, Li YH, et al. Elevated neutrophil to lymphocyte ratio predicts survival in advanced pancreatic cancer. Biomarkers. 2010;15(6):516–522. doi: 10.3109/1354750X.2010.491557. [DOI] [PubMed] [Google Scholar]
- 116.Cho H, Hur HW, Kim SW, et al. Pre-treatment neutrophil to lymphocyte ratio is elevated in epithelial ovarian cancer and predicts survival after treatment. Cancer Immunol. Immunother. 2009;58(1):15–23. doi: 10.1007/s00262-008-0516-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ding PR, An X, Zhang RX, et al. Elevated preoperative neutrophil to lymphocyte ratio predicts risk of recurrence following curative resection for stage IIA colon cancer. Int. J. Colorectal Dis. 2010;25(12):1427–1433. doi: 10.1007/s00384-010-1052-0. [DOI] [PubMed] [Google Scholar]
- 118.Kim HS, Han KH, Chung HH, et al. Neutrophil to lymphocyte ratio for preoperative diagnosis of uterine sarcomas: a case-matched comparison. Eur. J. Surg. Oncol. 2010;36(7):691–698. doi: 10.1016/j.ejso.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 119.Shimada H, Takiguchi N, Kainuma O, et al. High preoperative neutrophil-lymphocyte ratio predicts poor survival in patients with gastric cancer. Gastric Cancer. 2010;13(3):170–176. doi: 10.1007/s10120-010-0554-3. [DOI] [PubMed] [Google Scholar]
- 120.Gubbels JA, Belisle J, Onda M, et al. Mesothelin-MUC16 binding is a high affinity, N-glycan dependent interaction that facilitates peritoneal metastasis of ovarian tumors. Mol. Cancer. 2006;5(1):50. doi: 10.1186/1476-4598-5-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Patankar MS, Jing Y, Morrison JC, et al. Potent suppression of natural killer cell response mediated by the ovarian tumor marker CA125. Gynecol. Oncol. 2005;99(3):704–713. doi: 10.1016/j.ygyno.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 122.Gubbels JA, Felder M, Horibata S, et al. MUC16 provides immune protection by inhibiting synapse formation between NK and ovarian tumor cells. Mol. Cancer. 2010;9:11. doi: 10.1186/1476-4598-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Belisle JA, Horibata S, Jennifer GA, et al. Identification of Siglec-9 as the receptor for MUC16 on human NK cells, B cells, and monocytes. Mol. Cancer. 2010;9:118. doi: 10.1186/1476-4598-9-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Krockenberger M, Dombrowski Y, Weidler C, et al. Macrophage migration inhibitory factor contributes to the immune escape of ovarian cancer by down-regulating NKG2D. J. Immunol. 2008;180(11):7338–7348. doi: 10.4049/jimmunol.180.11.7338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hudis CA. Trastuzumab – mechanism of action and use in clinical practice. N. Engl. J. Med. 2007;357(1):39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- 126.Ray-Coquard I, Guastalla JP, Allouache D, et al. HER2 overexpression/amplification and trastuzumab treatment in advanced ovarian cancer: a GINECO Phase II study. Clin. Ovarian Cancer. 2008;1(1):54–59. [Google Scholar]
- 127.McAlpine JN, Wiegand KC, Vang R, et al. HER2 overexpression and amplification is present in a subset of ovarian mucinous carcinomas and can be targeted with trastuzumab therapy. BMC Cancer. 2009;9:433. doi: 10.1186/1471-2407-9-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wilken JA, Webster KT, Maihle NJ. Trastuzumab sensitizes ovarian cancer cells to EGFR-targeted Therapeutics. J. Ovarian Res. 2010;3:7. doi: 10.1186/1757-2215-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gordon MS, Matei D, Aghajanian C, et al. Clinical activity of pertuzumab (rhuMAb 2C4), a HER dimerization inhibitor, in advanced ovarian cancer: potential predictive relationship with tumor HER2 activation status. J. Clin. Oncol. 2006;24(26):4324–4332. doi: 10.1200/JCO.2005.05.4221. [DOI] [PubMed] [Google Scholar]
- 130.Takai N, Jain A, Kawamata N, et al. 2C4, a monoclonal antibody against HER2, disrupts the HER kinase signaling pathway and inhibits ovarian carcinoma cell growth. Cancer. 2005;104(12):2701–2708. doi: 10.1002/cncr.21533. [DOI] [PubMed] [Google Scholar]
- 131.Makhija S, Amler LC, Glenn D, et al. Clinical activity of gemcitabine plus pertuzumab in platinum-resistant ovarian cancer, fallopian tube cancer, or primary peritoneal cancer. J. Clin. Oncol. 2010;28(7):1215–1223. doi: 10.1200/JCO.2009.22.3354. [DOI] [PubMed] [Google Scholar]
- 132.Kaye SB, Poole CJ, Bidzinksi M, et al. A randomised Phase II study evaluating the combination of carboplatin-based chemotherapy with pertuzumab (P) versus carboplatin-based therapy alone in patients with relapsed, platinum sensitive ovarian cancer. J. Clin. Oncol. 2008;26 Suppl doi: 10.1093/annonc/mds282. Abstract 5520. [DOI] [PubMed] [Google Scholar]
- 133.Langdon SP, Faratian D, Nagumo Y, et al. Pertuzumab for the treatment of ovarian cancer. Expert Opin. Biol. Ther. 2010;10(7):1113–1120. doi: 10.1517/14712598.2010.487062. [DOI] [PubMed] [Google Scholar]
- 134.Kalli KR. MORAb-003, a fully humanized monoclonal antibody against the folate receptor a, for the potential treatment of epithelial ovarian cancer. Curr. Opin. Investig. Drugs. 2007;8(12):1067–1073. [PubMed] [Google Scholar]
- 135.Ebel W, Routhier EL, Foley B, et al. Preclinical evaluation of MORAb-003, a humanized monoclonal antibody antagonizing folate receptor-α. Cancer Immun. 2007;7:6. [PMC free article] [PubMed] [Google Scholar]
- 136.Smith-Jones PM, Pandit-Taskar N, Cao W, et al. Preclinical radioimmunotargeting of folate receptor α using the monoclonal antibody conjugate DOTA-MORAb-003. Nucl. Med. Biol. 2008;35(3):343–351. doi: 10.1016/j.nucmedbio.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Konner JA, Bell-McGuinn KM, Sabbatini P, et al. Farletuzumab, a humanized monoclonal antibody against folate receptor a, in epithelial ovarian cancer: a Phase I study. Clin. Cancer Res. 2010;16(21):5288–5295. doi: 10.1158/1078-0432.CCR-10-0700. [DOI] [PubMed] [Google Scholar]
- 138.Armstrong DK, Coleman R, White AJ, et al. Efficacy and safety of farletuzumab, a humanized monoclonal antibody to folate receptor a, in platinum-sensitive relapsed ovarian cancer subjects: preliminary data from a Phase-2 study. Eur. J. Cancer. 2009;7 Suppl. 2 Abstract 450. [Google Scholar]
- 139.White AJ, Coleman R, Armstrong DK, et al. Efficacy and safety of farletuzumab, a humanized monoclonal antibody to folate receptor a, in platinum-sensitive relapsed ovarian cancer subjects: final data from a multicenter Phase II study. J. Clin. Oncol. 2010;28 Suppl. 15 Abstract 5001. [Google Scholar]
- 140.Spannuth WA, Sood AK, Coleman RL. Farletuzumab in epithelial ovarian carcinoma. Expert Opin. Biol. Ther. 2010;10(3):431–437. doi: 10.1517/14712591003592069. [DOI] [PubMed] [Google Scholar]
- 141.Ruf P, Gires O, Jager M, et al. Characterisation of the new EpCAM-specific antibody HO-3: implications for trifunctional antibody immunotherapy of cancer. Br. J. Cancer. 2007;97(3):315–321. doi: 10.1038/sj.bjc.6603881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Burges A, Wimberger P, Kumper C, et al. Effective relief of malignant ascites in patients with advanced ovarian cancer by a trifunctional anti-EpCAM x anti-CD3 antibody: a Phase I/II study. Clin. Cancer Res. 2007;13(13):3899–3905. doi: 10.1158/1078-0432.CCR-06-2769. [DOI] [PubMed] [Google Scholar]
- 143.Heiss MM, Murawa P, Koralewski P, et al. The trifunctional antibody catumaxomab for the treatment of malignant ascites due to epithelial cancer: results of a prospective randomized Phase II/III trial. Int. J. Cancer. 2010;127(9):2209–2221. doi: 10.1002/ijc.25423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Knutson KL, Wagner W, Disis ML. Adoptive T cell therapy of solid cancers. Cancer Immunol. Immunother. 2006;55(1):96–103. doi: 10.1007/s00262-005-0706-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rosenberg SA, Dudley ME. Adoptive cell therapy for the treatment of patients with metastatic melanoma. Curr. Opin. Immunol. 2009;21(2):233–240. doi: 10.1016/j.coi.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kershaw MH, Westwood JA, Parker LL, et al. A Phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin. Cancer Res. 2006;12(20 Pt 1):6106–6115. doi: 10.1158/1078-0432.CCR-06-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Dobrzanski MJ, Rewers-Felkins KA, Quinlin IS, et al. Autologous MUC1-specific Th1 effector cell immunotherapy induces differential levels of systemic Treg cell subpopulations that result in increased ovarian cancer patient survival. Clin. Immunol. 2009;133(3):333–352. doi: 10.1016/j.clim.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chianese-Bullock KA, Irvin WP, Jr, Petroni GR, et al. A multipeptide vaccine is safe and elicits T-cell responses in participants with advanced stage ovarian cancer. J. Immunother. 2008;31(4):420–430. doi: 10.1097/CJI.0b013e31816dad10. [DOI] [PubMed] [Google Scholar]
- 149.Diefenbach CS, Gnjatic S, Sabbatini P, et al. Safety and immunogenicity study of NY-ESO-1b peptide and montanide ISA-51 vaccination of patients with epithelial ovarian cancer in high-risk first remission. Clin. Cancer Res. 2008;14(9):2740–2748. doi: 10.1158/1078-0432.CCR-07-4619. [DOI] [PubMed] [Google Scholar]
- 150.Leffers N, Lambeck AJ, Gooden MJ, et al. Immunization with a P53 synthetic long peptide vaccine induces P53-specific immune responses in ovarian cancer patients, a Phase II trial. Int. J. Cancer. 2009;125(9):2104–2113. doi: 10.1002/ijc.24597. [DOI] [PubMed] [Google Scholar]
- 151.Kaumaya PT, Foy KC, Garrett J, et al. Phase I active immunotherapy with combination of two chimeric, human epidermal growth factor receptor 2, B-cell epitopes fused to a promiscuous T-cell epitope in patients with metastatic and/or recurrent solid tumors. J. Clin. Oncol. 2009;27(31):5270–5277. doi: 10.1200/JCO.2009.22.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hernando JJ, Park TW, Fischer HP, et al. Vaccination with dendritic cells transfected with mRNA-encoded folate-receptor-a for relapsed metastatic ovarian cancer. Lancet Oncol. 2007;8(5):451–454. doi: 10.1016/S1470-2045(07)70142-0. [DOI] [PubMed] [Google Scholar]
- 153.zum Buschenfelde CM, Hermann C, Schmidt B, et al. Antihuman epidermal growth factor receptor 2 (HER2) monoclonal antibody trastuzumab enhances cytolytic activity of class I-restricted HER2-specific T lymphocytes against HER2-overexpressing tumor cells. Cancer Res. 2002;62(8):2244–2247. [PubMed] [Google Scholar]
- 154.Disis ML, Wallace DR, Gooley TA, et al. Concurrent trastuzumab and HER2/ neu-specific vaccination in patients with metastatic breast cancer. J. Clin. Oncol. 2009;27(28):4685–4692. doi: 10.1200/JCO.2008.20.6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ercolini AM, Machiels JP, Chen YC, et al. Identification and characterization of the immunodominant rat HER-2/neu MHC class I epitope presented by spontaneous mammary tumors from HER-2/neu-transgenic mice. J. Immunol. 2003;170(8):4273–4280. doi: 10.4049/jimmunol.170.8.4273. [DOI] [PubMed] [Google Scholar]
- 156.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat. Rev. Cancer. 2005;5(4):263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 157.Muraoka-Cook RS, Dumont N, Arteaga CL. Dual role of transforming growth factor β in mammary tumorigenesis and metastatic progression. Clin. Cancer Res. 2005;11(2 Pt 2):S937–S943. [PubMed] [Google Scholar]
- 158.Halder SK, Beauchamp RD, Datta PK. A specific inhibitor of TGF-β receptor kinase, SB-431542, as a potent antitumor agent for human cancers. Neoplasia. 2005;7(5):509–521. doi: 10.1593/neo.04640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.von Boehmer H. Mechanisms of suppression by suppressor T cells. Nat. Immunol. 2005;6(4):338–344. doi: 10.1038/ni1180. [DOI] [PubMed] [Google Scholar]