Abstract
Objectives
Tachycardia-induced relaxation abnormalities were evaluated in myocardium from patients with normal ejection fraction (EF).
Background
Diastolic dysfunction and left ventricular (LV) hypertrophy are closely linked. Tachycardia can induce heart failure symptoms in otherwise asymptomatic patients. To study the effects of tachycardia on myocardial contractility and relaxation we evaluated the effects of increasing pacing rates in myocardial biopsies obtained from patients with normal EF.
Methods
LV biopsies were obtained during coronary bypass surgery. Myocardial strip preparations were electrically paced at rates from 60/min to180/min. Diastolic resting tone was assessed by crossbridge deactivation. Calcium transporting systems were functionally examined and myofilament calcium sensitivity was studied.
Results
Seven preparations developed incomplete relaxation with increased diastolic tension development at increasing pacing rates. This was absent in the remaining seven preparations. Incomplete relaxation was found to be associated with increased LV mass and left atrial volume. Crossbridge deactivation showed that these preparations also had a significant resting tone. Additional functional analyses suggest that incomplete relaxation is associated with disproportionately elevated cellular calcium loads due to a reduced sarcolemmal calcium extrusion reserve.
Conclusions
Tachycardia-induced incomplete relaxation was associated with increased LV mass and left atrial volumes. We also found a disproportionately increased calcium load at high rates and a substantial resting tone due to diastolic crossbridge cycling. These observations may play a role in reduced exercise tolerance and tachycardia induced diastolic dysfunction.
Keywords: diastole, hypertrophy, tachycardia, calcium
INTRODUCTION
Increased LV wall thickness resulting in cardiac hypertrophy is a frequent cause of diastolic dysfunction in patients with normal ejection fraction (1,2). While the majority of patients are asymptomatic at rest, they often develop symptoms with exertion and tachycardia. Moreover, these patients are at risk to develop acute onset heart failure symptoms that are frequently associated with tachycardia and hypertension. These clinical observations suggest an important dynamic component of diastolic dysfunction that may be different from abnormalities present at rest. Previous studies have shown increased LV end-diastolic chamber stiffness as well as increased resting tension in demembranated cardiomyocytes from symptomatic patients with diastolic dysfunction (3,4). These findings have generally been ascribed to structural changes in the myocardium such as altered extra-cellular matrix collagen content and changes in the expression or post-translational modification of structural cardiomyocyte proteins such as titin (5).
Based on the above clinical observations we speculated that myocardium from patients with normal ejection fractions and increased LV mass may display dynamic relaxation abnormalities in vitro when subjected to rates that are typical for tachycardia. These abnormalities should manifest as incomplete relaxation with continued active force generation in diastole. To test this hypothesis we studied myocardial strip preparations from patients with normal ejection fractions. After the preparations underwent a pacing protocol that encompassed rates from 60/min to 180/min they were categorized into two groups. One group displayed a normal relaxation behavior up to the highest rates, whereas the other group developed incomplete relaxation as the rate was increased. Thereafter, echocardiographic and other clinical data were disclosed to determine whether LV mass and other parameters of diastolic dysfunction are associated with incomplete relaxation. To further investigate the underlying cellular mechanisms we also performed a series of physiological and pharmacological experiments in the same preparations. This allowed us to test for the presence of active force generation in resting preparations and assess calcium handling and myofilament calcium sensitivity.
METHODS
Patient Population
LV myocardial biopsies were obtained from 17 patients at the time of aortocoronary bypass grafting (CBG). All patients had LVEF ≥50% and normal regional wall motion based on pre-operative echocardiography. Patients with objective evidence of myocardial ischemia in the week prior to CBG were excluded. Three patients underwent aortic valve replacement (AVR) for aortic stenosis in addition to CBG. Patients with a history of myocardial infarction, diabetes mellitus, chronic renal dysfunction (creatinine >2.0) and any other significant valve disorders were excluded. Consent forms, tissue biopsy techniques, and experimental protocols were approved by the Institutional Review Board. Three biopsies could not be sculpted into excitable preparations, leaving a total of 14 experiments.
Clinical Data
Age, sex, pre-operative blood pressures, and pertinent details of the medical history were tabulated. LVEF was calculated by volumetric assessment using the apical four and two-chamber views. LV chamber dimensions, wall thickness, mass and regional wall motion were assessed in accordance with published guidelines (6). Pulsed Doppler mitral inflow and lateral mitral annular tissue Doppler signals were analyzed for the following parameters of diastolic function: E wave peak velocities and lateral E’ wave tissue velocity to calculate E/E’. Left atrial volume was calculated using the area-length method. LV mass and LA volume were normalized to body surface area. Concentric LV hypertrophy criteria were >115g/m2 in men and >95g/m2 in women with a normal LV enddiastolic diameter (<59mm in men, <53mm in women). LV end-diastolic pressure (LVEDP) at pre-operative catheterization was also tabulated.
Muscle Preparation and Mechanical Parameters
The biopsies were obtained from the anterior LV wall and microdissected into strip preparations (cross-sectional area 0.7±0.3mm2) as previously described (7,8). The preparations were then transferred to the muscle chamber, containing oxygenated Tyrode solution at 37°C, and attached to a force transducer and stimulating electrodes. Isometric force was evoked at a stimulation rate of 60/min. Preload was applied until the developed tension reached a maximum. Core hypoxia was excluded by perfusion arrest (8). Force parameters were recorded using an IonOptix recording system (Milton, MA). A reservoir above the muscle chamber allowed for precise delivery of rapid cooling solution. Contraction and relaxation parameters were defined as previously described (7,8). An exponential time constant, tau, was calculated from non-linear least squares fitting of a single exponential equation to the force data after half maximal relaxation.
Experimental Protocol
The experimental sequence for each preparation was as follows:
a. Pacing Protocol
Relaxation was evaluated after increasing the stimulation rate in 30/min increments over a range from 60/min to 180/min, to encompass normal human resting and tachycardia rates. Measurements were taken after steady state conditions were reached. The onset-rate of incomplete relaxation was defined as the stimulation rate at which diastolic tension started to rise. If diastolic tension did not increase the preparations were assigned to the complete (normal) relaxation group. The magnitude of the rise of diastolic tension due to incomplete relaxation was defined as the percent rise in diastolic tension from 60/min to 180/min. For group statistical comparisons, the onset-rate was considered to be 180/min if incomplete relaxation was not observed.
b. Post-Rest Contractions and Rapid Cooling Contracture
In order to evaluate sarcoplasmic reticulum (SR) calcium retention and release, we utilized a post-rest contraction (PRC) protocol. After steady-state conditions were reached at 60/min, resting intervals of 5, 20, 40, 80, and 120 seconds were introduced. The resting periods were followed by a single applied stimulus prior to resuming continuous stimulation. This protocol was then repeated at 180/min. The varying developed tension of the PRC is a marker of SR calcium release. The time dependent decay of the PRCs reflect calcium leakage from the SR (9,10).
The SR calcium content was assessed by rapid cooling contractures (RCC), a semi-quantitative physiological measure of total SR calcium content (10). A rapid switch to a RCC solution at 1°C induces a complete SR calcium release leading to a contracture. The contracture amplitude is a measure of SR calcium content. RCCs were induced after 5 and 120 seconds of rest at two preceding pacing rates of 60/min and 180/min (11).
c. Pharmacological Cross Bridge Inhibition
To estimate resting tone, the non-stimulated muscle strip was exposed to a circulating solution of Tyrode solution containing 30mmol/L 2,3-butanedione monoxime (BDM). BDM is a potent, fully reversible inhibitor of crossbridge cycling (7). Resting tone was thus considered to be the difference between baseline resting diastolic tension and resting diastolic tension after addition of BDM.
d. Sarcolemmal Contribution to Contraction and Relaxation
To test the contribution of sarcolemmal calcium transport we chemically inhibited SR with 20μmol/L cyclopiazonic acid, an inhibitor of the SR calcium ATPase, and 1μmol/L ryanodine, an inhibitor of the SR calcium release channels. These were added to the perfusate of the strip while it was paced at 60/min. The combination of both compounds has been demonstrated to abolish SR function in human myocardium (12). This was confirmed by the absence of rapid cooling contractures (data not shown).
e. Myofilament Calcium Sensitivity
To determine the potential role of myofilament calcium sensitivity, the myofilament response to varying calcium concentration was studied as previously described (13). The negative logarithm of the calcium concentration at half maximal force generation (EC50) was used to compare the groups.
Statistical Analysis
After the initial group assignment a repeat measure ANOVA followed by a student t-test without corrections for multiplicity was used to assess the rise in diastolic tension. For between and within group comparisons unpaired and paired student t-tests were used. Values in the graphs are plotted ± standard error (SE). Values in the text and tables are presented ± standard deviations (SD), if not otherwise indicated. p values <0.05 were considered statistically significant. The alpha was set at 0.05 to calculate confidence intervals.
RESULTS
Incomplete Relaxation
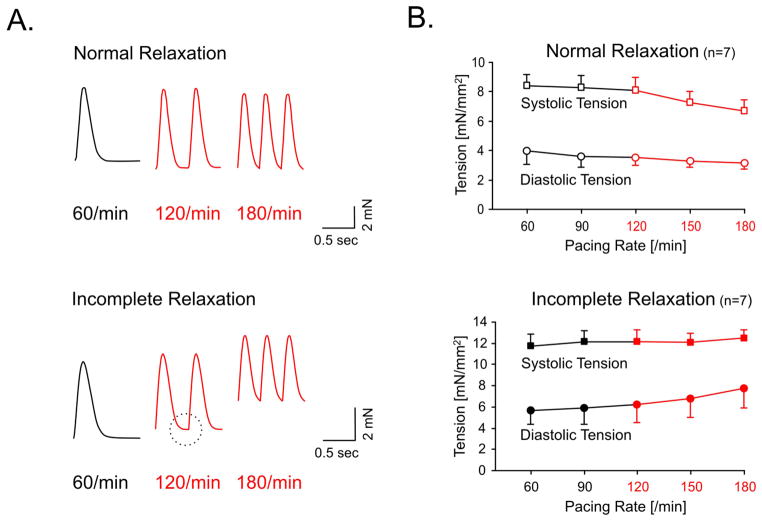
The experimental data were grouped based on the presence or absence of incomplete relaxation, as shown in Figure 1A. This resulted in two groups of 7 experiments each. The developed tension at 60/min was 6.0±1.1mN/mm2 (SE) in the incomplete relaxation group and 4.5±1.1mN/mm2 in the normal relaxation group (p=0.2). In the incomplete relaxation group diastolic tension increased from 5.4±1.3 mN/mm2 at 60/min to 7.8±1.8mN/mm2 at 180/min (p<0.05), whereas no significant change in diastolic tension was found in the normal relaxation group. Average diastolic tensions and peak systolic tensions are shown in Figure 1B. This graph demonstrates that incomplete relaxation is associated with a progressive rise in diastolic tension as the rate is increased. Although we observed a directional trend towards prolonged relaxation indices at 60/min in the incomplete relaxation group none of the indices reached statistical significance (Table 1). This result suggests that the relaxation characteristics at low resting rates can not predict the development of incomplete relaxation as the rate is increased.
Figure 1.
Panel A depicts two force tracings of paced human myocardial strips. The upper tracing shows a normal relaxation up to the highest rate. The lower tracing demonstrates incomplete relaxation that is first apparent at 120/min (circle). At 180/min diastolic tension is markedly increased resulting in a diastolic contracture. Panel B shows average peak systolic force (■) and average diastolic tension (●) after grouping the experiments according to their relaxation behavior (normal vs. incomplete). The group of preparations that displayed incomplete relaxation showed a rise in diastolic tension as the rate was increased (p<0.05). Rates typical for tachycardia are in red.
Table 1. Contraction and Relaxation Time Intervals.
Time indices of contraction and relaxation at 60/min and 180/min.
| TPT [ms] | RT50 [ms] | RT90 [ms] | Tau [ms] | ||
|---|---|---|---|---|---|
| Normal Relaxation (n=7) | 60/min | 152 ± 21 | 280 ± 34 | 416 ± 54 | 8 ± 1 |
| 180/min | 113 ± 11 | 200 ± 11 | 284 ± 9 | 7 ± 1 | |
|
| |||||
| Incomplete Relaxation (n=7) | 60/min | 155 ± 24 | 287 ± 29 | 454 ± 79 | 12 ± 7 |
| 180/min | 114 ± 12 | 206 ± 17 | 287 ± 2 | 7 ± 1 | |
TPT, time to peak tension; RT50, time to half maximal relaxation, RT90, time to 90% relaxation; Tau, time index of relaxation after RT50. No significant changes between the groups were found.
Clinical Data Correlations
The baseline clinical data and relevant clinical correlations categorized by the principle experimental finding of incomplete relaxation at increasing rates are shown in Table 2. All patients with aortic stenosis displayed incomplete relaxation, whereas clinical documentation of hypertension was not different between the groups. Most importantly the results demonstrate that the presence of incomplete relaxation is associated with a greater LV mass and left atrial volume, a marker of chronic diastolic dysfunction (14). Trends in age, E/E’ and LVEDP towards higher values were also evident in the incomplete relaxation group. The presence of incomplete relaxation predicted or excluded left ventricular hypertrophy in all but one patient, a male with a mass index of 89g/m2.
Table 2. Clinical Characteristics.
Characteristics of patients grouped by normal versus incomplete relaxation characteristics.
| Incomplete Relaxation (n=7) | Normal Relaxation (n=7) | |||
|---|---|---|---|---|
| Age (years) | 76 ± 14 | 62 ± 15 | p = 0.10 | |
| Gender (number of patients) | male | 4 | 7 | |
| female | 3 | – | ||
| Aortic Stenosis | 3 | – | ||
| Hypertension(medical history) | 3 | 4 | ||
| Blood Pressure (mmHg) | 133±12 / 74±6 | 134±9 / 75±8 | n.s. | |
| LVEF (%) | 61 ± 8 | 62 ± 5 | n.s. | |
| LV mass (gr/m2) | 132 ± 47 | 78 ± 14 | p < 0.01 | |
| LA volume (mL/m2) | 43 ± 15 | 23 ± 7 | p < 0.01 | |
| E/E’ | 11 ± 5 | 6 ± 1 | p = 0.11 | |
| LVEDP (mmHg) | 16 ± 5 | 13 ± 3 | p = 0.18 | |
| Comorbidities | PVD | 2 | – | |
| COPD | 2 | – | ||
| AF | 1 | 1 | ||
| VT | – | 1 |
AF, atrial fibrillation; COPD, chronic obstructive pulmonary disease; PVD, peripheral vascular disease; VT, ventricular tachycardia. LVEF, left ventricular ejection fraction; LVmass, left ventricular mass index, LA volume, left atrial volume index; E/E’, early peak mitral inflow/early peak lateral mitral annulus tissue Doppler ratio; LVEDP, left ventricular end-diastolic pressure. Values are reported ± SD.
Resting Tone
Use of the reversible crossbridge inhibitor BDM allowed us to evaluate if unstimulated preparations have an active resting tone due to crossbridge formation. These experiments demonstrated that active resting tone was detectable in all of the strips with incomplete relaxation but none of the strips from the normal relaxation group. The average resting tone in the preparations with incomplete relaxation was 1.3±0.6mN/mm2 (p<0.01). The presence of an active resting tone in a preparation with incomplete relaxation at 180/min is shown in the lower panel of Figure 2.
Figure 2. Tracings from two preparations with normal and incomplete relaxation.
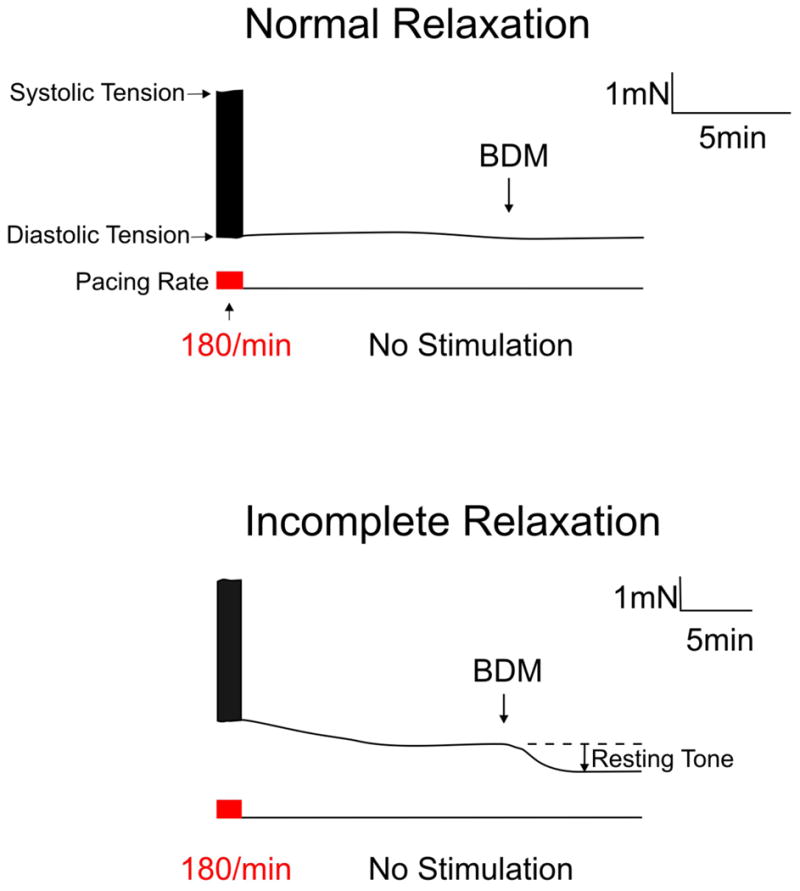
Both preparations were paced at 180/min when the stimulation was stopped. After allowing the preparation to establish a stable resting tension, BDM, a reversible crossbridge inhibitor, was added to the circulating solution. This revealed a substantial resting tone in the preparation with incomplete relaxation. No resting tone was found in preparations that showed normal relaxation characteristics with tachycardia.
Evaluation of Mechanisms
The experimental design of this study allowed us to evaluate the mechanism of incomplete relaxation and resting tone by adding a sequence of experiments in each preparation. For clarity the results are not reported in sequence of performance:
Myofilamental Calcium Sensitivity
As shown in Figure 3, there was no difference in calcium sensitivity between preparations with and without incomplete relaxation (EC50 values: incomplete relaxation 6.4±0.4, normal relaxation 6.4±0.2). Thus, incomplete relaxation is not associated with increased myofilament calcium sensitivity.
Figure 3. Calcium-force relationship in skinned preparations from the preparations with normal and incomplete relaxation.
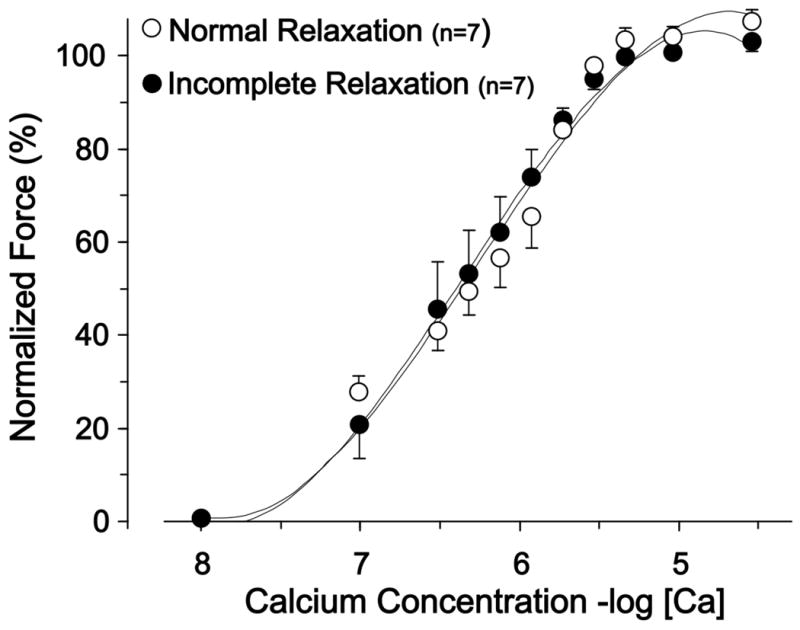
There was no difference in the calcium concentrations at half maximal force (EC50). This indicates that calcium sensitivity is unchanged in preparations that display incomplete relaxation.
Post-Rest Contractions and Rapid Cooling Contractures
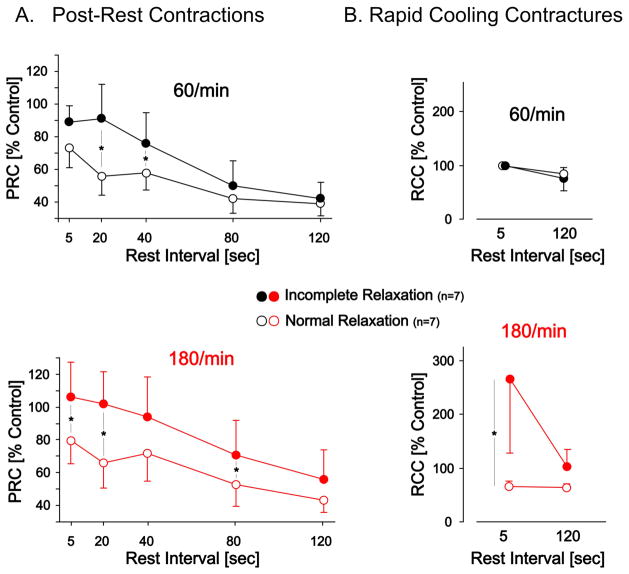
The developed tension of post-rest contractions (PRCs) after varying resting intervals were used to quantify sarcoplasmic reticulum (SR) calcium release and retention (Figure 4A). PRCs after short resting intervals were more pronounced in preparations that displayed incomplete relaxation (p<0.05). The time dependent decay in PRCs was similar in both groups. These results suggest greater SR calcium release in preparations that display incomplete relaxation at both rates.
Figure 4.
Panel A. Post-Rest Contractions (PRCs) and the time dependent decay of PRCs at two preceding pacing rates (60/min and 180/min). The rest intervals were incrementally increased as indicated. The developed tension of the post-rest contraction is a measure of sarcoplasmic reticulum (SR) calcium release. This data demonstrates an increase in SR mediated contractility in the group that displays incomplete relaxation. Panel B. Rapid Cooling Contractures (RCCs) as a measure of the total SR calcium content at 60/min and 180/min in the normal and incomplete relaxation group. Rapid switching to a solution at 1°C releases all calcium from the SR which then activates the myofilaments. The group with incomplete relaxation demonstrates a disproportional increase in SR calcium content at 180/min that recovers to more normal levels after a prolonged resting interval. * indicates p<0.05 for between group comparisons at the respective rates.
Rapid cooling contractures (RCCs) provide a semi-quantitative assessment of SR calcium content (10). As shown in Figure 4b there appears to be a disproportionate increase in SR calcium content at 180/min, which re-equilibrates to more normal levels after a prolonged rest interval. This slow re-equilibration is likely also responsible for the delay in diastolic tension readjustment after the abrupt cessation of tachycardia, as shown in the lower panel of Figure 2. These data suggest that tachycardia dramatically increases SR calcium content in the group with incomplete relaxation. It is important to note that this increase in SR calcium did not translate into an increase in developed tension at 180/min, as shown in Figure 1B.
Complete Sarcoplasmic Reticulum Inhibition
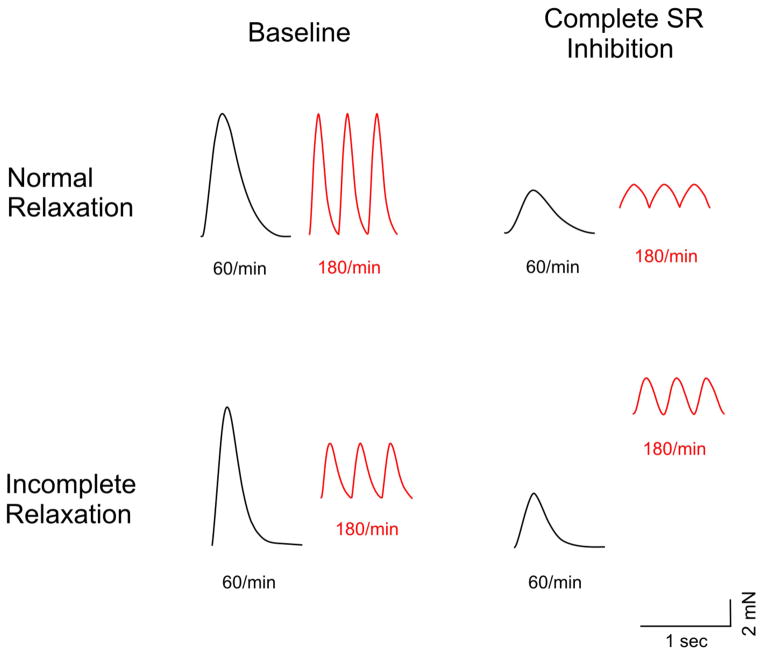
In preparations without functioning SR cytosolic calcium elimination from the cytoplasm is almost exclusively dependent on the sarcolemmal sodium-calcium exchanger (10). After SR inhibition the contraction developed tension (at 60/min) decreased by 47±24 percent in the normal relaxation group versus 57±24 percent in the group that displayed incomplete relaxation (p=0.2). Due to the general prolongation of the mechanical twitch incomplete relaxation and the rate-dependent rise in diastolic tension was more pronounced in both groups after SR inhibition. However, the rise in diastolic tension after SR inhibition was much more pronounced in strips exhibiting incomplete relaxation, as exemplified in Figure 5. After SR inhibition the rise in diastolic tension was 129±44 percent, compared to 36±32 percent with an intact SR, a more than three-fold increase (p<0.01). In contrast, biopsies from patients with normal relaxation behavior displayed only a 12±5 percent rise in diastolic tension after SR inhibition, a major difference compared to the preparations that displayed incomplete relaxation with an intact SR (p<0.01). This suggests that sarcolemmal function can partially compensate for the loss of SR function in preparations with normal relaxation, whereas preparations with incomplete relaxation demonstrated a lack of sarcolemmal calcium extrusion reserve. This is very likely the primary reason for the disproportionate increase in SR calcium load at high rates.
Figure 5. Contractile consequences of complete sarcoplasmatic reticulum (SR) inhibition in preparations with normal and incomplete relaxation.
The preparation that exemplifies normal relaxation shows a small increase in diastolic tension after SR removal when paced at 180/min. This rise in diastolic tension is much more pronounced in the preparation that displays incomplete relaxation at baseline.
DISCUSSION
The present study is the first to systematically relate diastolic function in isolated, contracting myocardium from patients with normal ejection fraction to clinical variables of diastolic function. Our data demonstrate that incomplete relaxation at rates typical for clinical tachycardia was strongly associated with an increased LV mass and an increased left atrial size. Incomplete relaxation was present in all preparations from patients with concentric left ventricular hypertrophy. With incomplete relaxation the myocardium never completely relaxes and remains in an activated state that can be described as a partial or diastolic contracture (15). Tachycardia-induced incomplete relaxation is a physiological finding at very high rates that was first documented by Frank in 1895 (16). His pressure tracings obtained in isolated animal hearts show incomplete relaxation with bursts of ventricular tachycardia that elevated the diastolic pressure baseline.
In our samples from patients with LV hypertrophy incomplete relaxation was observed at substantially lower rates compared to samples from patients with a normal LV mass (Figure 6). Our experiments also indicate that myocardium from patients with incomplete relaxation has a substantial active resting tone due to crossbridge cycling. In other words, complete relaxation can never be achieved even with an indefinitely prolonged diastole. The finding of incomplete relaxation in addition to abnormal resting tone may play a role in the dynamic clinical presentation of patients with acute diastolic heart failure symptoms.
Figure 6. Incomplete relaxation and contracture onset-rates in patients with concentric LV hypertrophy versus patients with a normal LV mass.
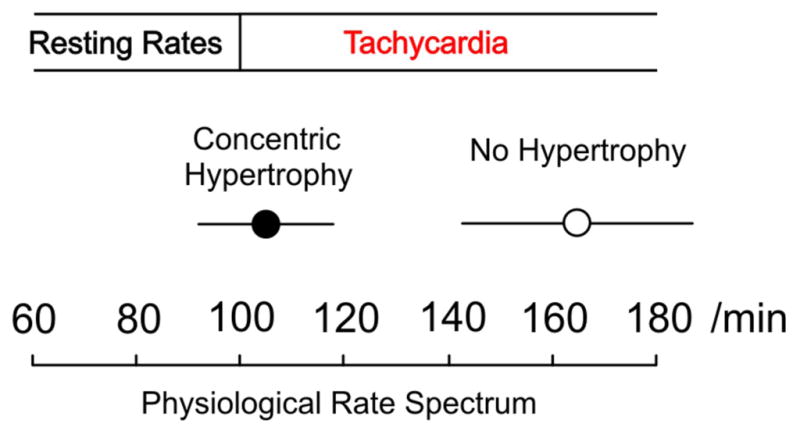
The onset of incomplete relaxation at rates that are just above physiological resting heart rates is demonstrated in myocardium from patients with hypertrophy. Error bars represent confidence intervals.
If our observation of incomplete relaxation is of clinical relevance it would be manifest as a tachycardia induced reduction in LV end-diastolic volumes. This was indeed observed by Westermann et al (17), who measured pressure-volume loops in 70 patients after hospital admissions for heart failure with normal ejection fraction. Compared to 20 control subjects these patients had an increased LV wall thickness and when paced at 120/min the LV end-diastolic volume decreased by 28 percent. In contrast, in control subjects they found an incremental increase in LV end-diastolic volume. Although the authors could not explain this finding, they argued that this observation may play a substantial role in the development of symptoms in these patients.
The mechanisms underlying diastolic dysfunction in patients with left ventricular hypertrophy are unclear. There are a number of possible explanations, including increased mass:volume ratio, increased passive stiffness due to changes in collagen and titin, altered calcium sensitivity of the myofilaments and abnormal calcium handling. Prior to our study, the only pertinent data obtained in human myocardium have been those of Paulus and colleagues (4,5,18). They have studied the passive mechanical characteristics of demembranated cardiomyocytes obtained from patients with heart failure and normal ejection fraction of various etiologies and found an increased myofilamental tension and stiffness. These findings were linked to changes in structural proteins such as titin.
Our results suggest a significant active resting tone in preparations that displayed incomplete relaxation, as shown by the force reduction in unstimulated preparations after crossbridge inhibition. No active resting tone was found in the group with normal relaxation behavior. This observation may be explained by an inability to restore diastolic calcium to levels that prevent force generation or an increase in myofilament calcium sensitivity. However, calcium sensitivity was not found to be altered in these preparations. This suggests that an abnormality of calcium handling is the predominant mechanism. The single most important calcium extruding mechanism in cardiomyocytes is the sarcolemmal sodium calcium exchanger. In concert with the sarcoplasmic reticulum the exchanger restores cytoplasmic calcium to diastolic levels, thus inducing relaxation. Our data demonstrate a tachycardia-induced disproportionate increase in sarcoplasmic reticulum calcium content which did not translate into a stronger contraction. This observation strongly suggests that calcium is released into a high calcium environment, which manifests as diastolic contracture. These data also suggests that the sarcoplasmic reticulum calcium ATPase mediated calcium sequestration is increased in these biopsies.
Elevated diastolic calcium levels at low rates may be beneficial and increase contractility by enhancing sarcoplasmic reticulum dependent calcium cycling. This was evident in stronger post-rest contractions, a trend towards increased developed tension and a trend towards a more substantial loss in contractility after complete sarcoplasmic reticulum inhibition. However, the removal of the sarcoplasmic reticulum revealed a more important difference that became apparent as the preparations were challenged by tachycardia. The group with normal relaxation at baseline developed a minor increase in diastolic tension, suggesting that the exchanger mediated calcium extrusion can largely compensate for the tachycardia mediated increase in cellular calcium influx. In contrast, diastolic tension increased by more than three-fold in the group with incomplete relaxation, suggesting a substantially reduced cellular calcium extrusion reserve. Hence, the principle defect causing incomplete relaxation appears to be at the level of the sarcolemma, i.e., insufficient sodium calcium exchanger mediated calcium extrusion that overwhelms the compensatory calcium sequestration by the sarcoplasmic reticulum.
The energetic implications of tachycardia-induced diastolic contractures in addition to resting tone are likely profound. Our calculations based on the well-established linear relationship between force-time integral and myocardial oxygen consumption indicate that at 180/min more energy is consumed for maintaining contracture and resting tone than for contraction and relaxation (8,18). Such a wasteful energy expense may play a substantial role in the development of ischemia, as described in canine hearts with concentric LV hypertrophy (19).
In summary, our data point to an intrinsic difference in tachycardia induced relaxation characteristics in left ventricular myocardium from patients with normal ejection fraction and increased LV mass. This finding is associated with a substantial active resting tone. These observations may have a role in the pathophysiology of exercise and tachycardia induced symptoms.
Acknowledgments
The assistance of our surgeons Frank P. Ittleman M.D., Joseph D. Schmoker M.D., and Bruce J. Leavitt M.D. in obtaining the biopsies is greatly appreciated.
Funding Sources
This research was supported by a NHLBI Institutional Training Grant (T32 HL07647), American Heart Association Scientist Development Grant 0730056N (MM), and NIH grants R01HL089944 (MML) and R01HL086902 (BMP).
Abbreviations
- BDM
Butanedione monoxime
- PRP
Post Rest Potentiation
- RCC
Rapid Cooling Contracture
- SD
standard deviation
- SEM
standard error of the mean
- SR
sarcoplasmic reticulum
Footnotes
Disclosures and Conflicts
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 2.Paulus WJ, Tschöpe C, Sanderson JE, et al. ; How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28:2539–50. doi: 10.1093/eurheartj/ehm037. [DOI] [PubMed] [Google Scholar]
- 3.Zile MR, Baicu CF, Gaasch WH. Diastolic heart failure - abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med. 2004;350:1953–9. doi: 10.1056/NEJMoa032566. [DOI] [PubMed] [Google Scholar]
- 4.Borbély A, van der Velden J, Papp Z, et al. ; Cardiomyocyte stiffness in diastolic heart failure. Circulation. 2005;111:774–81. doi: 10.1161/01.CIR.0000155257.33485.6D. [DOI] [PubMed] [Google Scholar]
- 5.Borbély A, Falcao-Pires I, van Heerebeek L, et al. ; Hypophosphorylation of the Stiff N2B titin isoform raises cardiomyocyte resting tension in failing human myocardium. Circ Res. 2009;104:780–6. doi: 10.1161/CIRCRESAHA.108.193326. [DOI] [PubMed] [Google Scholar]
- 6.Lang RM, Bierig M, Devereux RB, et al. ; Chamber Quantification Writing Group; American Society of Echocardiography's Guidelines and Standards Committee; European Association of Echocardiography. J Am Soc Echocardiogr. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Mulieri LA, Hasenfuss G, Ittleman F, Blanchard EM, Alpert NR. Protection of human left ventricular myocardium from cutting injury with 2,3-butanedione monoxime. Circ Res. 1989;65:1441–9. doi: 10.1161/01.res.65.5.1441. [DOI] [PubMed] [Google Scholar]
- 8.Meyer M, Keweloh B, Guth K, et al. ; Frequency-dependence of myocardial energetics in failing human myocardium as quantified by a new method for the measurement of oxygen consumption in muscle strip preparations. J Mol Cell Cardiol. 1998;30:1459–70. doi: 10.1006/jmcc.1998.0706. [DOI] [PubMed] [Google Scholar]
- 9.Pieske B, Sütterlin M, Schmidt-Schweda S, et al. Diminished post-rest potentiation of contractile force in human dilated cardiomyopathy. Functional evidence for alterations in intracellular Ca2+ handling. J Clin Invest. 1996;98:764–76. doi: 10.1172/JCI118849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bers DM. Sarcoplasmic reticulum Ca uptake, content and release. In: Bers DM, editor. Excitation-contraction coupling and cardiac contractile force. 2. Dordrecht, The Netherlands: Kluwer Academic Publishers; 2001. pp. 161–202. [Google Scholar]
- 11.Meyer M, Trost SU, Bluhm WF, Knot HJ, Swanson E, Dillmann WH. Impaired sarcoplasmic reticulum function leads to contractile dysfunction and cardiac hypertrophy. Am J Physiol Heart Circ Physiol. 2001;280:H2046–H2052. doi: 10.1152/ajpheart.2001.280.5.H2046. [DOI] [PubMed] [Google Scholar]
- 12.von Levinski D, Stumme B, Fialka F, et al. Functional relevance of the stretch-dependent slow force response in failing human myocardium. Circ Res. 2004;94:1392–8. doi: 10.1161/01.RES.0000129181.48395.ff. [DOI] [PubMed] [Google Scholar]
- 13.Palmer BM, Fishbaugher DE, Schmitt JP, et al. ; Differential cross-bridge kinetics of FHC myosin mutations R403Q and R453C in heterozygous mouse myocardium. Am J Physiol Heart Circ Physiol. 2004;287:H91–9. doi: 10.1152/ajpheart.01015.2003. [DOI] [PubMed] [Google Scholar]
- 14.Pritchett AM, Mahoney DW, Jacobsen SJ, et al. ; Diastolic dysfunction and left atrial volume: a population based study. J Am Coll Cardiol. 2005;45:87–92. doi: 10.1016/j.jacc.2004.09.054. [DOI] [PubMed] [Google Scholar]
- 15.Langer GA. Heart: Excitation-contraction coupling. Ann Rev Physiol. 1973;35:55–86. doi: 10.1146/annurev.ph.35.030173.000415. [DOI] [PubMed] [Google Scholar]
- 16.Frank O. Zur Dynamik des Herzmuskels. Zeitschrift fuer Biologie. 1895;14:370–437. [Google Scholar]
- 17.Westermann D, Kasner M, Steendijk P, et al. ; Role of left ventricular stiffness in heart failure with normal ejection fraction. Circulation. 2008;117:2051–60. doi: 10.1161/CIRCULATIONAHA.107.716886. [DOI] [PubMed] [Google Scholar]
- 18.van Heerebeek L, Hamdani N, Handoko ML, et al. ; Diastolic stiffness of the failing diabetic heart: importance of fibrosis, advanced glycation end products, and myocyte resting tension. Circulation. 2008;117:43–51. doi: 10.1161/CIRCULATIONAHA.107.728550. [DOI] [PubMed] [Google Scholar]
- 19.McDonald RH., Jr Developed tension: a major determinant of myocardial oxygen consumption. J Physiol. 1966;210:351–356. doi: 10.1152/ajplegacy.1966.210.2.351. [DOI] [PubMed] [Google Scholar]
- 20.Hittinger L, Shannon RP, Kohin S, Manders WT, Kelly P, Vatner SF. Exercise-induced subendocardial dysfunction in dogs with left ventricular hypertrophy. Circ Res. 1990;66:329–43. doi: 10.1161/01.res.66.2.329. [DOI] [PubMed] [Google Scholar]