Abstract
Human T-lymphotropic virus type-1 (HTLV-1), the first human retrovirus discovered, is the causative agent of adult T-cell leukemia/lymphoma (ATL) and a number of lymphocyte-mediated inflammatory conditions including HTLV-1–associated myelopathy/tropical spastic paraparesis. Development of animal models to study the pathogenesis of HTLV-1–associated diseases has been problematic. Mechanisms of early infection and cell-to-cell transmission can be studied in rabbits and nonhuman primates, but lesion development and reagents are limited in these species. The mouse provides a cost-effective, highly reproducible model in which to study factors related to lymphoma development and the preclinical efficacy of potential therapies against ATL. The ability to manipulate transgenic mice has provided important insight into viral genes responsible for lymphocyte transformation. Expansion of various strains of immunodeficient mice has accelerated the testing of drugs and targeted therapy against ATL. This review compares various mouse models to illustrate recent advances in the understanding of HTLV-1–associated ATL development and how improvements in these models are critical to the future development of targeted therapies against this aggressive T-cell lymphoma.
Keywords: animal models, HTLV-1, lymphoma, review, retrovirus, mouse
Human T-lymphotropic virus type-1 (HTLV-1) is a member of the deltaretroviridae, a family of retroviruses that includes both simian T-lymphotropic virus and bovine leukemia virus.51 These virus genera share common mechanisms of replication, transmission, and pathogenic mechanisms of disease. Based on epidemiology studies it has been estimated that approximately 15 to 20 million HTLV-1 carriers exist throughout the world, with endemic foci in Japan, the Caribbean, and Africa.16 HTLV-1 is spread through contact with bodily fluids containing infected cells, most often from mother to child through breast milk and parenterally via blood transfusion. After prolonged latency periods (as long as 60 years), approximately 5% of HTLV-1–infected individuals will develop either adult T-cell leukemia/lymphoma (ATL) or other lymphocyte-mediated disorders such as HTLV-1–associated myelopathy/tropical spastic paraparesis (HAM/TSP) (Table 1). ATL is an aggressive T-cell malignancy with a poor prognosis and survival times typically less than 1 year.105 HAM/TSP is a chronic inflammatory disease that results in progressive neurologic dysfunction and damage to the thoracic spinal cord. The factors favoring the development of ATL or HAM/TSP are not completely understood but have been linked to factors regarding host (eg, immune response) and virus (eg, viral load).51 The route of exposure to the virus may influence the clinical outcome of HTLV-1 infection. Individuals subjected to mucosal exposure appear more likely to develop ATL, whereas blood-borne exposure favors the development of HAM/TSP.89 The complex and prolonged pathogenesis of disease following HTLV-1 infection presents unique challenges to those seeking to develop animal models of HTLV-1–associated lesions or therapeutic interventions to prevent disease.
Table 1.
HTLV-1–Associated Diseases in Humansa
| Disease | Pathologic and Clinical Characteristics |
|---|---|
| ATL | Aggressive malignancy of T-lymphocytes, immunophenotypically characterized by multiple distinct cell surface markers, including CD3+/CD4+/CD8−/CD25+/HLA-DR+ T cells. Clinical symptoms and presentations may include malaise, fever, lymphoadenopathy, hepatosplenomegaly, hypercalcemia, lytic bone lesions, elevated lactate dehydrogenase, increased IL-2 receptor in serum, lymphomatous skin infiltrates, jaundice, weight loss, and various opportunistic infections, such as Pneumocystis carinii. Four classifications based on clinical signs: asymptomatic, preleukemic, chronic smoldering, and acute forms. Leukocytosis may include atypical cell morphology, including multilobulated nucleus referred to as “flower cells.” Diagnostic criteria include HTLV-1 seropositivity, leukocytosis, increased serum levels of IL-2 receptor and LDH, demonstration of neoplastic T cells with polylobulated nuclear morphology (flower cells), and the presence of clonally integrated HTLV-1 genomes within the chromosomes of neoplastic lymphocytes |
| HAM/TSP | Progressive chronic myelopathy, with preferential damage of the thoracic spinal cord. Spasticity in the lower extremities, hyperreflexia, muscle weakness, and sphincter disorders, including dysfunction of the urinary bladder and intestines. Rarely, cerebellar syndrome with ataxia and tremor. Characterized by multiple white matter lesions in both the spinal cord and the brain involving perivascular demyelination and axonal degeneration. Early lesion development characterized by infiltrates composed predominantly of CD4+ T cells and macrophages with detectable levels of HTLV-1 tax RNA in lesions. Late lesions (>4 years) predominantly CD8+ T cells with less tax RNA. Cerebrospinal fluid contain high levels of proinflammatory cytokines, including IFN-γ, TNF-α, IL-1, and IL-6, as well as increased numbers of activated lymphocytes. |
| HTLV-1–associated dermatitis | Described in Jamaican children as “infectious dermatitis.” Chronic eczema with refractory Staphylococcus aureus or β-hemolytic streptococcus infections. Patients frequently develop HAM/TSP later in life and may have episodes of severe anemia. |
| Ocular lesions | In endemic regions with uveitis, keratoconjunctivitis sicca, and interstitial keratitis. Chronic course in children may result in retinal degeneration. |
| Inflammatory arthropathy and polymyositis | Japanese patients in regions endemic for HTLV-1 infection may present with chronic inflammatory arthropathy of leg and arm joints or polymyositis. Chronic polymyositis may be presented with neuropathy, joint swelling, chest pain, and dyspnea. Similar lesions have been reproduced in transgenic mouse and rat models. |
ATL, adult T-cell leukemia/lymphoma; HAM/TSP, HTLV-1–associated myelopathy/tropical spastic paraparesis; HTLV-1, human T-lymphotropic virus type-1; IFN, interferon; IL, interleukin; LDH, lactate dehydrogenase; TNF, tumor necrosis factor
Adult T-Cell Leukemia/Lymphoma
Leukemia and lymphoma associated with HTLV-1 infection present in a variety of forms. The acute form of ATL is an aggressive T-cell malignancy characterized by several distinct clinical parameters including a leukemic phase of increased circulating CD4+ CD25+ T cells.110 Patients with the acute form of ATL have a poor prognosis independent of therapy, with mean survival time of less than 1 year.105 Adult T-cell leukemia/lymphoma was first described in 1977, following investigation of a unique geographic clustering of patients with particularly aggressive T-cell malignancies and common clinical presentations.105,119 The original series of patients had adult-onset disease, chronic leukemia with a rapidly fatal progressive course, lymphadenopathy, hepatosplenomegaly, neoplastic T cells with lobulated nuclei, and common geographic distribution.105,119 Cells from patients from these original cases were cultured with human umbilical cord lymphocytes, and cell lines MT-1, MT-2, and MT-4 were generated.64 These cell lines were instrumental in the discovery of HTLV-1 and are still used in HTLV-1 research.
Current diagnostic criteria for ATL include the demonstration of a T-cell malignancy, HTLV-1 antibodies in the patient’s sera, and demonstration of monoclonal integration of HTLV-1 provirus in the leukemic cells.111 Since the original description of ATL, lymphoproliferative diseases associated with HTLV-1 infection have been classified into 4 clinical subtypes. The 2 most aggressive forms are the acute and lymphomatous forms.122 These forms are characterized by humoral hypercalcemia of malignancy (HHM) with subsequent bone lysis, lymphadenopathy, and visceral and skin involvement. The lymphomatous form is distinguished by its normal peripheral white cell count, whereas in the acute form, ATL cells are found in the peripheral blood. The smoldering and chronic forms are the more indolent clinical subtypes. Criteria for diagnosing smoldering ATL include a normal leukocyte count, lack of hypercalcemia, and skin or lung involvement without evidence of other visceral disease. In patients with chronic ATL, the peripheral blood leukocyte count is typically elevated; these patients lack hypercalcemia but may have histologic evidence of involvement of the liver, spleen, or lungs.103,110
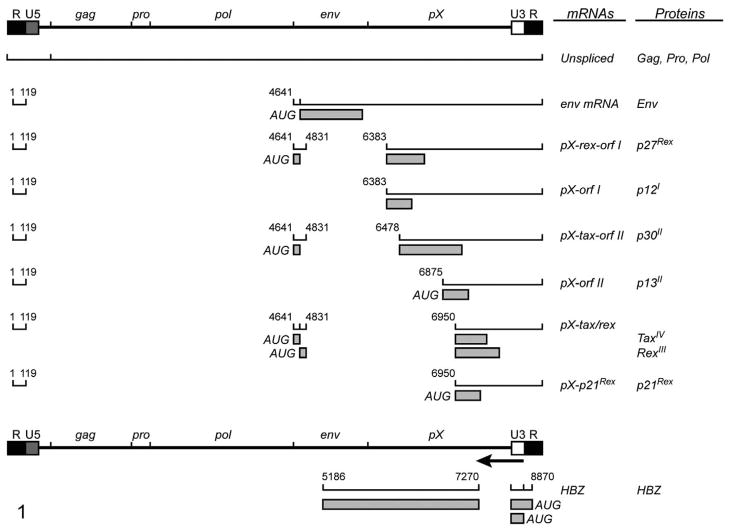
Human T-lymphotropic virus type 1 is a complex retrovirus that relies upon multiple gene products to accomplish replication and disease (Fig. 1). The molecular pathogenesis from HTLV-1 infection to ATL development has not been fully elucidated. The randomly integrated provirus persists for many years, and during this phase infected subjects typically remain asymptomatic. Effective replication of the virus relies on reverse transcription and clonal expansion of infected cells.70 In addition, HTLV-1 encodes for a variety of nonstructural proteins that have been implicated in cell signaling, in transcriptional and posttranscriptional regulation of viral gene expression in vitro, and in viral replication in animal models.3,8,33,80 During the premalignant phase, there is a progression from polyclonal to monoclonal T cells in circulation. Monoclonal T-cell expansion is believed to be promoted by an increasing ability of malignant T cells to escape immune elimination and become less dependent on cell growth factors such as interleukin (IL)-2. Also during this phase, there is an accumulation of somatic mutations both within the provirus and within the flanking regions. Most often, the 5′ portions of the virus are deleted or mutated, and functional infectious virions may not be produced.70 Tax, in the initial phases of transformation, acts as a transcriptional transactivator and causes the simultaneous activation of multiple cellular pathways that disrupt the normal cell cycle and death pathways, leading to the transformation of the T cells. Transformed T cells eventually grow autonomously and the expression of Tax appears to be no longer required for maintenance of the malignancy. Recent evidence indicates that an antisense encoded gene product, HBZ, may have a role in long-term maintenance of HTLV-1–transformed cells.4,60,100
Figure 1.
Diagram of human T-lymphotropic virus type-1 (HTLV-1) full-length provirus, viral transcripts, and corresponding proteins. Open reading frames are indicated by roman numerals, and boxes indicate the protein coding region of the spliced transcripts.
Neurodegenerative and Other Diseases Associated with HTLV-1
The neurologic disease HAM/TSP is a degenerative neurologic condition first described by Gessain et al22 and Osame et al.90 The disease is characterized by lymphocytic meningitis with demyelination and degeneration of the spinal cord. It most commonly occurs in the lumbar spine in patients typically younger than those affected by ATL.71 Transfusion with HTLV-1–contaminated blood has been associated with a more rapid onset of development of HAM/TSP versus ATL.23,88 The pathogenesis of HAM/TSP is thought to involve both molecular mimicry and autoantigens.54,55 A candidate autoantigen was identified from immunoglobulin G of HAM/TSP patients as the heterogeneous nuclear ribonuclear protein-A1 (hnRNP-A1). Cytotoxic T cells (CTLs) directed against HTLV-1 Tax are detectable in the blood and central nervous system of HAM/TSP patients.44 As the disease progresses, the composition of the inflammatory component of the spinal cord lesions progresses from CD4+, CD8+ T cells, B cells, and macrophages to a lesion comprised predominantly of CD8+ T cells.120
Animal Models of HTLV-1 Infection and Disease
Multiple animal models are available to study the infection and transmission of HTLV-1.53 Rabbits, 2,52 some nonhuman primates,72,73 and rats 34,107 can all be infected with HTLV-1 and have been used to monitor the virus spread, determine immune responses against the infection, and develop vaccines against the viral infection. These animal models of HTLV-1 infection or disease have been recently reviewed.53 Rabbits can be consistently infected; however, they remain, in the majority of cases, asymptomatic. Early studies using the rabbit model of HTLV-1 provided important data on the number of cells capable of transmitting the virus infection and effective means to prevent the transmission of the virus.45,65,102,112,115 Infection of rabbits with HTLV-1 parallels the asymptomatic infection of the virus in humans. Rabbits inoculated with HTLV-1–infected cell lines derived from patients with ATL or HAM/TSP demonstrated the heterogeneity in the biological response to HTLV-1 infection.52 Cynomolgus macaques (Macaca fascicularis) and squirrel monkeys (Saimiri sciureus) can be experimentally infected with HTLV-1. The monkeys seroconvert but typically do not demonstrate any clinical signs of disease.66,74,123 Rats, although able to be experimentally infected with HTLV-1,107 exhibit considerable variation in the response to the infection depending on the rat strain used.34,37,49 Wistar-King-Aptekman-Hokudai (WKAH) rats have been used as a model of HAM/TSP. This rat strain, when infected by HTLV-1, develops spastic paraparesis with degenerative thoracic spinal cord and peripheral nerve lesions several months following inoculation.37,49 The lesions (neurologic degeneration with a paucity of lymphocytes) are different than those observed in human HAM/TSP.125 The use of the immunodeficient rat has allowed for some studies of ATL.81
Mouse Models of HTLV-1 Infection and Disease
The need for useful animal models to study the histology and tumorigenesis and to test preclinical efficacy of potential therapeutic agents led to the development of several xenograft and transgenic mouse models. When compared with other available animal models, mouse models are small and comparatively inexpensive and their genetics are easily manipulated. One caveat is that genetically normal, immunocompetent murine cells are not efficiently infected with HTLV-1 and do not develop a natural course of disease.17,48 Mouse xenograft models, although not an exact replica of the human course of disease, demonstrate multiple features in common with the human disease. Xenograft mouse models display multiple organ engraftments with ATL cell lines or patient samples.13,14,19,35,46,47,56,63,91 Large atypical lymphocytes are evident histologically in the peritoneum, liver, spleen, and multiple organ types depending on the inoculum. Xenograft mouse models can also display biochemical characteristics similar to ATL patients. These include PTHrP expression and increasing levels of serum IL-2Rα and β-2 microglobulin that correspond to increasing tumor burden. Transgenic mouse lines permit testing of individual HTLV-1 genes’ contribution to the pathogenesis of ATL and provide evidence of a causal relationship between genes within the HTLV-1 provirus and the development of unregulated cell growth.94
Xenograft Mouse Models of ATL
Transplantation of human cancer cells into immunodeficient mice has been in practice since the late 1960s.99 Use of the xenograft enables investigators to examine human cells in a more physiological relevant context than in vitro systems allow.
The SCID mouse (CB17-Prkdcscid), developed in 1983,9 is commonly used as a model. This mouse contains a spontaneous nonsense mutation in the gene for the protein kinase DNA-activated catalytic polypeptide (Pkrdc) on chromosome 16. The Pkrdc enzyme is necessary for the efficient recombination of the B- and T-cell receptors. Without this enzyme, mature B and T cells do not develop. The SCID mouse retains normal macrophage, antigen presenting cell, and natural killer (NK) cell functions. SCID mice are used extensively in human stem cell and tumor cell engraftment studies. Engraftment of ATL cells and cell lines within the SCID mouse is cell line dependent.19,35 Studies that use ATL cells directly obtained from patients recapitulate more accurately the events of ATL. Cells that engraft and are recovered maintain the genotype and phenotype of the original inoculate.19,35,47 Studies in the SCID mouse also demonstrated that the intact HTLV-1 provirus does not appear necessary for neoplastic cell growth.36 RV-ATL cells, an HTLV-1–positive leukemic cell line derived from a patient sample, were reported to establish tumors readily, but this cell line must be propagated through mice because it does not remain viable in cell culture.56 Whole-body irradiation or administration of antibodies to abrogate NK-cell function proved necessary to establish engraftment of nonleukemic cell lines such as SLB-1 cells.18,56,118 MT-2 cells developed tumors at the site of injection in SCID mice treated with anti-asialo GM-1 antibody, which functionally inactivates NK cells.38 Stewart et al106 demonstrated that NK cells of the SCID mouse mediated specific lysis of HTLV-1–expressing cell lines, suggesting that the absence of HTLV-1 expression in patient derived ATL lines allows these cells to evade immune surveillance.
Further refinement of mouse models with selected genetic defects in innate immune function are needed to explore the role of specific effecter cells in tumor engraftment. This is particularly relevant because recent studies of ATL patients indicate that invariant NK T cells (iNKT) are inversely correlated to proviral loads in ATL patients.5 The SCID/beige mouse (CB17.B6-Prkdcscid/Lystbg) is a double mutant mouse in which the SCID mutation is retained, but these mice have an additional beige mutation in the Lyst gene (mouse chromosome 13) that results in altered lysosomal trafficking. These mice have defective B- and T-cell function, NK cell activity, and granulocyte properties. The RV-ATL cell line was reported to engraft in approximately 75% of the SCID/beige mice, whereas transformed cells (HT-1-RV, SLB-1, MT-2, ACH, and ACH.p12) were unable to establish engraftment.56 These results illustrate the significant difference between ATL cell lines derived from patients versus those transformed ex vivo by HTLV-1. Selection of tumor cells within patients under the pressure of the immune system likely accounts for these discrepant results. Thus, mouse models that use ATL cells directly from patients have an implied advantage to understand factors important in tumor engraftment and spread.
SCID mice used in xenograft studies have been found to display “leakiness” (partial immune function). Leakiness allows for spontaneous rearrangement of antigen receptors and development of functional lymphocytes in aged mice.10 To ablate this partial immune restoration, the SCID mouse was crossed onto the NOD/Lt background. NOD (nonobese diabetic) mice are a model used to study the development of autoimmune-mediated insulin-dependent diabetes mellitus. The resultant NOD/SCID mice lack functional B and T cells, have low NK cell activity, lack complement activity (due to a lack of complement component 5 from the NOD/Lt background), and have impaired macrophage and antigen-presenting cell function. When compared with the SCID mouse and the SCID/bg mouse, the NOD/SCID mouse (NOD.CB17-Prkdcscid/NCrCrl) appeared to be more susceptible to engraftment with the HTLV-1–transformed cell lines.56 Sublethal whole-body irradiation of NOD/SCID mice 1 day prior to inoculation improved engraftment and tumorigenesis and decreased time to clinical signs. Tumor engraftment was described as a lymphoblastic lymphoma with tumor development in the peritoneal cavity, spleen, and mesenteric lymph nodes. Lymphoblasts had large irregular nuclei and large prominent nucleoli. Abnormal mitotic figures were also observed. Tumor cells invaded and displaced multiple abdominal organs.56
Inoculation of MET-1 cells into NOD/SCID mice provides a model system for slowly developing T-cell leukemia with multiple organ involvement.113 In a comparative study by our group, leukemic mice had an increase in serum calcium levels that correlated with expression of receptor activator of nuclear factor-κB ligand (RANKL) on leukemic cells and secretion of PTHrP and IL-6.57 MET-1 cells expressed the adhesion molecules CD11a (LFA-1α) and CD49d (VLA-4α) and produced or induced expression of matrix metalloproteinases 1, 2, 3, and 9.
Further immunodeficiency was produced with development of the NOD/SCID mouse containing a targeted mutation in the β-2 microglobulin gene, a protein necessary for the presentation of antigens via major histocompatibility class (MHC) I. These mice lack all the immune functions that their less immunodeficient NOD/SCID predecessors also lack but have more complete elimination of NK-cell function. ATL cells derived directly from patients were able to engraft in these mice.46 This strain of mouse offers the advantage of an increased percentage of tumor engraftment and a reduced time to clinical signs compared with NOD/SCID mice.
NOD/SCID γ c null (NOG; NOD/Shi-scid/IL-2Rγnull) mice are homozygous for the SCID mutation and a targeted disruption of the interleukin (IL)-2Rγ gene mutation.82 The γ-chain is common to the receptors for IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21. NOG mice are easily transplanted with human cells that would not normally transplant with the same efficiency in the more immunocompetent mouse models.42 NOG mice lack B- and T-cell development as well as NK-cell function and have a severe reduction in interferon-γ production from dendritic cells.42 As a result, NOG mice can be used to relatively easily transplant cells directly from ATL patients95 and to expand peripheral blood mononuclear cells (PBMCs) from asymptomatic carriers.108
The NOG mouse model is advantageous in studies with the goal of comparing the degree of engraftment of ATL cells and gene expression. Tumor suppressor lung cancer 1 (TSLC1) is aberrantly expressed in acute ATL cells and some cell lines. NOG mice inoculated with TSLC1 expressing ED-40515 cells formed larger tumors than their non–TLSC1-expressing counterparts.13 Another advantage of the NOG mouse model is as a tool to study early infection in which PBMCs and lethally irradiated MT-2 cells are both inoculated in NOG mice and HTLV-1 infection in expanded PBMC populations is followed.63
Paraneoplastic syndromes confound the clinical syndromes associated with HTLV-1 infection and some can be studied using mouse models. HHM is an important paraneoplastic syndrome that occurs frequently in ATL. It has clinical implications with regard to organ damage and chemotherapy. Mouse models of ATL that demonstrate HHM include the SCID mouse injected with human lymph node cells109 and SCID/bg mice injected with RV-ATL cells.97 NOD/SCID mice xenografted with ATL cells and treated with a proteasomal inhibitor (PS-341) and zoledronic or a combination of both drugs exhibited a significant reduction in tumor burden and reduced the plasma calcium concentrations illustrating new therapeutic directions against complications associated with ATL.104
Transgenic Mouse Models of HTLV-1
Various transgenic mouse models have compared the influence of viral and cellular promoters to evaluate the role of HTLV-1 gene products on the development of lymphocyte transformation and other lesions attributable to HTLV-1 infection (Table 2). None of the transgenic mouse models fully recapitulate HTLV-1–associated disease, but many have been useful to investigate Tax-mediated disruption of lymphocyte function or provide evidence that Tax is an oncoprotein.
Table 2.
Summary of Transgenic Mouse Models of Adult T-Cell Leukemia/Lymphomaa
| Promoter | Transgene | Disease/Lesion | Relevance to Human HTLV-1–Associated Disease | Reference |
|---|---|---|---|---|
| HTLV-LTR | tax | Neurofibromatosis | Not observed | 32,78 |
| HTLV-LTR | tax | Muscle fiber degeneration | Myositis | 79 |
| HTLV-LTR | tax | Skeletal lesions with increased bone turnover | Lytic bone lesions | 98 |
| HTLV-LTR | tax | Exocrinopathy | Sjogren disease, ocular lesions | 26 |
| HTLV-LTR | tax | Fibrosarcoma | NF-κB–mediated tumors | 12 |
| HTLV-LTR | tax | Arthritis | Arthritis | 28,43 |
| CD4 | tax | Arthritis | Arthritis | 28 |
| Lck | tax | Thymic lymphoma | Lymphoma | 31 |
| Granzyme B | tax | Large granular leukemia/lymphoma, osteolytic bone lesions | Lymphoma and lyrics bone lesions | 21,27 |
| HTLV-LTR | pX (including tax) | Thymic atrophy | Not observed | 20 |
| Immunoglobulin-SV-40 | pX (including tax) | Thymic atrophy | Not observed | 20 |
| Mouse mammary tumor virus LTR | pX (including tax) | Thymic atrophy | Not observed | 20 |
| Lymphocyte-restricted (EmuSR α promoter-enhancer) | tax and tax mutants | CD4+ lymphocytic skin lesions, lymphadenopathy, splenomegaly | Skin lesions and preleukemic forms of ATL | 50 |
| Granzyme B crossed with γ-interferon knockout mice | tax | Accelerated large granular leukemia/lymphoma, osteolytic bone lesions | Lymphoma and lytic bone lesions | 61 |
| HTLV-LTR crossed with LTR-β-gal | tax and β-gal | Transgene expression in multiple tissues without disease | Tax tissue expression | 7 |
| HTLV-LTR | c-myc | Occasional lymphoma | Lymphoma | 6 |
| Immunoglobulin promoter/enhancer | tax | Transgene expression in multiple tissues without disease | Tax tissue expression | 6 |
| HTLV-LTR-c-myc crossed with immunoglobulin promoter/enhancer | tax and c-myc | CD4+ lymphomas with central nervous system involvement | Lymphoma | 6 |
ATL, adult T-cell leukemia/lymphoma; HTLV-1, human T-lymphotropic virus type-1; NF-κB, nuclear factor-κB.
Tax, the 40-kDa transactivating protein of HTLV-1, alters cellular gene expression and promotes cell transformation in vitro.1,24,25,93,114 Tax is widely studied as a prototypic viral transactivating protein because it has a multitude of effects on cellular pathways controlling lymphocyte proliferation, including the NF-κB and serum response factor pathways. Through these signaling pathways, Tax can cause alteration of expression in genes involved in apoptosis, cell cycle, proliferation, and DNA repair. Tax in transgenic models is sufficient to cause oncogenesis; however, leukemia and lymphoma are rare. Serendipitously, the mouse models often recapitulate other chronic conditions associated with HTLV-1.
The first transgenic mice expressing the HTLV-1 tax gene (originally called HTLV-1 tat when mice were produced) under the control of the LTR promoter (Tg(HIV-tat)6-2Gja) developed multicentric mesenchymal tumors of the nose, ear, mouth, tail, and foot (Table 2).78 These tumors were characterized by a typical spindle cell component with infiltration of granulocytes. Although this transgenic system is not appropriate for the study of ATL, it proved that the tax gene encodes an oncoprotein. The close association of the mesenchymal tumors with peripheral and cranial nerves led to their identification as neurofibromas, and it was concluded that this model may be useful in the study of neurofibromatosis, an inherited disorder of humans.32 In addition to the mesenchymal tumors described above, these transgenic mice developed a wasting disease characterized by degeneration of oxidative muscle fibers. As such, this transgenic mouse helps us understand aspects of HTLV-1–associated myopathies.79 LTR-tax mice showed both gross and microscopic evidence of skeletal abnormalities. Bones were grossly thicker and more fragile, whereas histologically they exhibited high bone turnover characterized by increases in osteoclasts and osteoblasts. These lesions were without evidence of PTHrP expression.98 These LTR-tax mice (no genotype reported) also developed a salivary and lacrimal exocrinopathy with lesions similar to humans suffering from Sjögren syndrome.26 Last, LTR-tax transgenic mice derived by injection of C3H/HeN ova were shown to have ankylosing of multiple joints28 and to carry potential benefits as models of other human diseases such as rheumatoid arthritis.43
Combining the LTR-tax mice with LTR-βgal (β galactosidase) mice generated a bitransgenic mouse in which the trans-activator protein acts on the LTR to increase expression of βgal. The enzyme was detected in bone, muscle, cartilage, exocrine glands, and mesenchymal tumors.7 This further supported the notion that the tax transgene was active in multiple tissues that developed lesions in this mouse model.
Leukemia and lymphoma have been reported in tax transgenic mice in which the transgene expression was restricted to the thymus by the Lck promoter (C57BL/6-Tg(Lck-HTLV-1 Tax).31 This thymus-derived, pre–T-cell tumor was associated with constitutive NF-κB activation. This model verified that Tax expression alone is sufficient to cause leukemia and lymphoma.
The development of the tax transgenic mouse (C57BL/6TgN(huGMZBTax) under the control of the granzyme B promoter restricted expression of Tax to CD4+, CD8+, NK cells, and lymphokine-activated killer cells.27 These mice exhibit a large granular lymphocytic leukemia and neutrophilic-dominated inflammation at sites of trauma admixed with LGL leukemic cells. These mice also developed splenomegaly, lymphadenopathy, and masses on the ears, legs, and tail.27 Similar to ATL, these mice had humoral hypercalcemia of malignancy and osteolytic bone lesions associated with metastasis.21 When these mice are crossed with an interferon-γ knockout strain, there is enhanced rate of lesion development.61 This model is advantageous in studies seeking to test the role of inflammation in tumor development. Recent refinement of this model evaluated Tax-mediated activation of luciferase in LTR-luciferase (LTR-LUC) mice (C57BL/6TgN(LtrLuc)) for imaging tumor engraftment in vivo.96 In this model it was recently reported that microscopic intraepithelial lesions precede the onset of peripheral subcutaneous tumors and that Tax is sufficient for inducing tumors. These results suggest that the viral oncoprotein activates lymphocytes to cause NK/T-cell recruitment, activation, and subsequent transformation.
Several attempts have been made to more specifically target Tax expression to leukocytes. Bitransgenic doxycycline-inducible mice (Tg(EmuSR-tTa)83Bop) were generated to specifically control expression of wild-type or selected tax mutants in the lymphocyte compartment.50 Using these transgenic model, tax gene mutants in which the ability to activate NF-κB was maintained developed a fatal dermatologic disease characterized by infiltration of Tax-positive T cells into the dermis and epidermis. Addition of doxycycline (suppression of Tax expression) resulted in the resolution of lesions. Although suggestive of cutaneous lymphoma observed in some ATL patients, the infiltrates in the skin were oligoclonal or polyclonal and not monoclonal as in ATL.50 Several transgenic C57/CBA mouse strains were generated with tax under the regulatory control of CD-3ε promoter enhancer sequence designed to target expression to leukocytes. These mice developed mesenchymal tumors at wound sites and mammary and salivary adenomas.29
Treatment of Adult T-cell Leukemia
A distinct advantage of mouse models of HTLV-1 ATL is the ability to use mice to test the preclinical efficacy of therapeutics against a highly aggressive T-cell lymphoma. This tool provides insight to clinicians before the development of phase I clinical trials. Xenograft mouse models have been used to test conventional and targeted therapeutics as well as monoclonal antibodies (Table 3).
Table 3.
Preclinical Efficacy Studies Using Mouse Models of HTLV-1 ATL: End Points for Studies Included Tumor Burden (Measured Histologically, Volumetrically, or Biochemically) or Survivala
| Cell Line | Mouse Strain | Treatment | Treatment Target | Reference |
|---|---|---|---|---|
| MET-1 | NOD/SCID | Monoclonal antibodies, HAT, MAT, and 7G7/B6 | IL-2 receptor | 91 |
| MET-1 | NOD/SCID | Campath-1H | CD52 | 128 |
| MET-1 | NOD/SCID | MEDI-507 | CD2 | 128 |
| ED | SCID | Bortezomib | Proteasome | 101 |
| HUT102 | SCID | FR901228 | Histone deacetylases | 69 |
| HUT102 | SCID | Fucoidan (natural product–seaweed) | NF-κB, survivin | 30 |
| MT-2 | SCID | DHMEQ | NF-κB | 121 |
| MT-2 and HUT102 | SCID | DHMEQ | NF-κB | 84 |
| MET-1 | NOD/SCID | Flavopiridol and anti-CD25 | Cyclin-dependent kinases, CD25 | 127 |
| Primary ATL cells | NOG | Ritonavir | Retroviral Protease | 15 |
| HUT102 | SCID | Curcumin | NF-κB | 117 |
| MET-1 | NOD/SCID | Dispeptidase +/− daclizumab (anti-human IL-2Rα) | HDAC and IL-2 receptor | 11 |
| HUT102 | SCID | Incadronate bisphosphonate | Mevalonate pathway | 39 |
| HUT102 | SCID | NIK-333 (synthetic retinoid) | NF-κB | 87 |
| MT-2 | NOD/SCID | Herpes simplex vector thymidine kinase and ganciclovir | Viral DNA polymerase | 62 |
| HUT102 | SCID | hG9NC (modified galectin) | NF-κB and AP-1 | 86 |
| TL-Om1 and MT-1 | NOD/SCID β-2 null | DHMEQ (dehydroxymethylepoxyquinomycin) | NF-κB | 85 |
| HUT102 | SCID | Tamibarotene (retinoic acid receptor α/β agonist) | NF-κB and AP-1 | 75 |
| HUT102 | SCID | Fucoxanthinol (carotenoid) | NF-κB and AP-1 | 40 |
IL, interleukin; NF-κB, nuclear factor-κB; DHMEQ, dehydroxymethylepoxyquinomycin.
The finding that NF-κB is constitutively active in ATL led to several models to investigate the outcome of therapy that uses the blockade of NF-κB through multiple different mechanisms. These have proven especially promising in reducing tumor size of subcutaneous xenografts. In vivo preclinical efficacy has been studied with arsenic trioxide,76 proteasome inhibitors,101 all-trans retinoic acid (ATRA),77 curcumin,117 and ritonovir.15 The proteasome inhibitor PS-341 has been the focus of several studies because it has been efficacious against other cancers and promotes the degradation NF-κB leading to apoptosis in human ATL-derived and Tax transgenic mouse cells.92 An inhibitor of NF-κB DNA-binding activity, BAY 11-7082, was also shown to block NF-κB activity and resulted in tumor regression in ATL transplanted NOG mice.
The expression of markers on the cell surface of ATL cells implanted in mice has made them an excellent target for preclinical trials with monoclonal antibodies directed against IL-2Rα,91,127 CD52,128,129 and CD2.129 The outcome of these mouse studies may be predictive of successful therapy for human patients, because a complete response has been reported in an ATL patient treated with alemtuzumab (anti-CD52).67 Treatment of ATL-bearing mice with a humanized anti-CD2 monoclonal antibody led to tumor regression.129 The activity of the monoclonal antibody was most likely due to antibody-dependent cellular cytotoxicity, because expression of Fcγ receptors on neutrophils and monocytes was required for activity.
Treatment of ATL-bearing NOD-SCID mice with an α-emitting radionuclide, bismuth 213, conjugated to an antibody to the IL-2 receptor proved to be highly effective in inducing tumor regression.126 The activity of bismuth 213 in this model was greater than that of unconjugate antibody or radionuclide or antibody conjugate to β-emitting radionuclide yttrium 90. Flavopiridol, an inhibitor of cyclin-dependent kinases, was tested for its therapeutic efficacy alone and in combination with humanized anti-Tac antibody, which recognizes CD25, in a murine model of human ATL using MET-1 leukemic cells.127 Either flavopiridol or humanized anti-Tac antibody inhibited tumor growth and prolonged survival of the leukemia-bearing mice.127 Collectively, these studies provide hope that ATL transplant and Tax transgenic models will provide new directions in the development of effective therapies against HTLV-1–induced lymphoproliferative diseases including ATL.
Summary
ATL is a refractory CD4/CD25+ CD8–T-cell malignancy caused by HTLV-1.116 Despite advances in the understanding of the pathogenesis of HTLV-1–associated diseases and the development of new therapeutic agents, the prognosis for patients with ATL remains poor.41,68,83,124 ATL is classified into 4 primary clinical subtypes, the 2 most aggressive of which are the acute and the lymphomatous forms.59 These forms are associated with HHM, bone lysis, lymphadenopathy, and visceral and skin involvement. The lymphomatous form typically is associated with a normal peripheral blood leukocyte cell count, whereas in the acute form, ATL cells are found in high numbers in the peripheral blood. The more indolent clinical subtypes are the smoldering and chronic forms. Models of ATL are limited, in part, by incomplete knowledge of mechanism of carcinogenesis induced by HTLV-1 infection. Recent progress from studies of viral-induced cell dysregulation offers new potential targets for therapeutic intervention.
The number of available mouse models of ATL is limited, in part, by the lack of ATL cell lines obtained directly from patients. The use of ATL cells directly from patients, such as the MET-1 cell model in NOD/SCID mice, has provided useful models to study specific questions related to tumor engraftment in vivo. A distinct advantage of this type of animal model is the use of a specific human biomarker to noninvasively measure tumor burden. Unfortunately, the MET-1 cell line, like many ATL-derived cell lines, does not grow in vitro, so compounds cannot be tested in this cell line prior to administration in the mouse. An ideal mouse model would be one in which compounds can be tested in vitro to investigate mechanisms of action prior to the use of the same cell line in the immunodeficient mouse. Generation of new mouse models of ATL can be difficult and is dependent on procuring leukemic cells from infected patients and systematically inoculating them into immunodeficient mice. The development of the NOG mice has provided a tractable model to more efficient transplant cells directly from ATL patients and creates more ATL cell lines more closely resembling the neoplasm in humans. Immunodeficient mouse models, although providing the ability to perform preclinical testing of therapeutic agents, do not effectively model therapy in context to ATL patients who have functional immune systems. Considering the recent focus on the innate immune system in HTLV-1 viral spread and ATL development, future models should be directed at mouse models with selected genetic defects in innate immune function to test the role of specific effecter cells, such as invariant NK T cells, in tumor engraftment. Transgenic mouse models, although unraveling the role of the HTLV-1 Tax oncoprotein, will need to be refined to test other potential oncogenic HTLV-1 proteins, such as HBZ,58 and need to be designed to be more specific in targeting viral protein expression in CD4+ lymphocytes or their precursor cells in bone marrow and gut-associated lymphoid tissues.
Collectively, recent advances in the development of mouse models of HTLV-1 ATL have extended opportunities to use mice to test the preclinical efficacy of drugs and targeted therapies against this highly aggressive T-cell lymphoma. Continued progress in understanding the multiple genetic and cellular aberrations that occur during the progression of HTLV-1 ATL will provide new therapeutic targets for drug development. Translating knowledge from the laboratory bench to appropriate animal models and subsequently to ATL patients provides hope for the development of targeted and efficacious treatments against this highly refractory T-cell neoplasm.
Acknowledgments
Funding
The author(s) disclosed receipt of the following financial support for the research and/or authorship of this article: National Institute of Health Career Development Award K01 RR 023965 awarded to B. Zimmerman and National Cancer Institute Program Grant P01CA100730 awarded to M. Lairmore.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no conflicts of interest with respect to the authorship and/or publication of this article.
Reprints and permission: sagepub.com/journalsPermissions.nav
References
- 1.Akagi T, Shimotohno K. Proliferative response of Tax1-transduced primary human T cells to anti-CD3 Antibody stimulation by an interleukin-2-independent pathway. J Virol. 1993;67:1211–1217. doi: 10.1128/jvi.67.3.1211-1217.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akagi T, Takeda I, Oka T, Ohtsuki Y, Yano S, Miyoshi I. Experimental infection of rabbits with human T-cell leukemia virus type 1. Jpn J Cancer Res. 1985;76:86–94. [PubMed] [Google Scholar]
- 3.Albrecht B, Lairmore MD. Critical role of human T-lymphotropic virus type 1 accessory proteins in viral replication and pathogenesis. Microbiol Mol Biol Rev. 2002;66:396–406. doi: 10.1128/MMBR.66.3.396-406.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arnold J, Zimmerman B, Li M, Lairmore MD, Green PL. Human T-cell leukemia virus type-1 antisense-encoded gene, Hbz, promotes T lymphocyte proliferation. Blood. 2008;112:3788–3797. doi: 10.1182/blood-2008-04-154286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Azakami K, Sato T, Araya N, Utsunomiya A, Kubota R, Suzuki K, Hasegawa D, Izumi T, Fujita H, Aratani S, Fujii R, Yagishita N, Kamijuku H, Kanekura T, Seino KI, Nishioka K, Nakajima T, Yamano Y. Severe loss of invariant NKT cells exhibiting anti-HTLV-1 activity in patients with HTLV-1-associated disorders. Blood. 2009;114:3208–3215. doi: 10.1182/blood-2009-02-203042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benvenisty N, Ornitz DM, Bennett GL, Sahagan BG, Kuo A, Cardiff RD, Leder P. Brain tumours and lymphomas in transgenic mice that carry HTLV-I LTR/c- myc and Ig/tax genes. Oncogene. 1992;7:2399–2405. [PubMed] [Google Scholar]
- 7.Bieberich CJ, King CM, Tinkle BT, Jay G. A transgenic model of transactivation by the Tax protein of HTLV−. Virology. 1993;196:309–318. doi: 10.1006/viro.1993.1481. [DOI] [PubMed] [Google Scholar]
- 8.Bindhu M, Nair A, Lairmore MD. Role of accessory proteins of HTLV-1 in viral replication, T cell activation, and cellular gene expression. Front Biosci. 2005;10:17–37. doi: 10.2741/1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bosma GC, Custer RP, Bosma MJ. A severe combined immunodeficiency mutation in the mouse. Nature. 1983;301:527–530. doi: 10.1038/301527a0. [DOI] [PubMed] [Google Scholar]
- 10.Bosma M, Schuler W, Bosma G. The scid mouse mutant. Curr Top Microbiol Immunol. 1988;137:197–202. doi: 10.1007/978-3-642-50059-6_29. [DOI] [PubMed] [Google Scholar]
- 11.Chen J, Zhang M, Ju W, Waldmann TA. Effective treatment of a murine model of adult T-cell leukemia using depsipeptide and its combination with unmodified daclizumab directed toward CD25. Blood. 2009;113:1287–1293. doi: 10.1182/blood-2008-04-149658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coscoy L, Gonzalezdunia D, Tangy F, Syan S, Brahic M, Ozden S. Molecular mechanism of tumorigenesis in mice transgenic for the human T cell leukemia virus Tax gene. Virology. 1998;248:332–341. doi: 10.1006/viro.1998.9298. [DOI] [PubMed] [Google Scholar]
- 13.Dewan MZ, Takamatsu N, Hidaka T, Hatakeyama K, Nakahata S, Fujisawa J, Katano H, Yamamoto N, Morishita K. Critical role for TSLC1 expression in the growth and organ infiltration of adult T-cell leukemia cells in vivo. J Virol. 2008;82:11958–11963. doi: 10.1128/JVI.01149-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dewan MZ, Terashima K, Taruishi M, Hasegawa H, Ito M, Tanaka Y, Mori N, Sata T, Koyanagi Y, Maeda M, Kubuki Y, Okayama A, Fujii M, Yamamoto N. Rapid tumor formation of human T-cell leukemia virus type 1-infected cell lines in novel NOD-SCID/gammac(null) mice: suppression by an inhibitor against NF-kappaB. J Virol. 2003;77:5286–5294. doi: 10.1128/JVI.77.9.5286-5294.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dewan MZ, Uchihara JN, Terashima K, Honda M, Sata T, Ito M, Fujii N, Uozumi K, Tsukasaki K, Tomonaga M, Kubuki Y, Okayama A, Toi M, Mori N, Yamamoto N. Efficient intervention of growth and infiltration of primary adult T-cell leukemia cells by an HIV protease inhibitor, ritonavir. Blood. 2006;107:716–724. doi: 10.1182/blood-2005-02-0735. [DOI] [PubMed] [Google Scholar]
- 16.Edlich RF, Hill LG, Williams FM. Global epidemic of human T-cell lymphotrophic virus type-I (HTLV-I): an update. J Long Term Eff Med Implants. 2003;13:127. doi: 10.1615/jlongtermeffmedimplants.v13.i2.70. [DOI] [PubMed] [Google Scholar]
- 17.Fang J, Kushida S, Feng R, Tanaka M, Kawamura T, Abe H, Maeda N, Onobori M, Hori M, Uchida K, Miwa M. Transmission of human T-cell leukemia virus type 1 to mice. J Virol. 1998;72:3952–3957. doi: 10.1128/jvi.72.5.3952-3957.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feuer G, Stewart SA, Baird SM, Lee F, Feuer R, Chen IS. Potential role of natural killer cells in controlling tumorigenesis by human T-cell leukemia viruses. J Virol. 1995;69:1328–1333. doi: 10.1128/jvi.69.2.1328-1333.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feuer G, Zack JA, Harrington WJ, Jr, Valderama R, Rosenblatt JD, Wachsman W, Baird SM, Chen IS. Establishment of human T-cell leukemia virus type I T-cell lymphomas in severe combined immunodeficient mice. Blood. 1993;82:722–731. [PubMed] [Google Scholar]
- 20.Furuta Y, Aizawa S, Suda Y, Ikawa Y, Kishimoto H, Asano Y, Tada T, Hikikoshi A, Yoshida M, Seiki M. Thymic atrophy characteristic in transgenic mice that harbor pX genes of human T-cell leukemia virus type I. J Virol. 1989;63:3185–3189. doi: 10.1128/jvi.63.7.3185-3189.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao L, Deng H, Zhao H, Hirbe A, Harding J, Ratner L, Weilbaecher K. HTLV-1 Tax transgenic mice develop spontaneous osteolytic bone metastases prevented by osteoclast inhibition. Blood. 2005;106:4294–4302. doi: 10.1182/blood-2005-04-1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gessain A, Barin F, Vernant J, Gout O, Maurs L, Calender A, Dethe G. Antibodies to human T lymphotropic virus type 1 in patients with tropical spastic paresis. Lancet. 1985;2:407–410. doi: 10.1016/s0140-6736(85)92734-5. [DOI] [PubMed] [Google Scholar]
- 23.Gout O, Baulac M, Gessain A, Semah F, Saal F, Peries J, Cabrol C, Foucaultferez C, Laplane D, Sigaux F, de The G. Medical inteligence—rapid development of myelopathy after HTLV-I infection acquired by transfusion during cardiac transplantation. N Engl J Med. 1990;332:383–388. doi: 10.1056/NEJM199002083220607. [DOI] [PubMed] [Google Scholar]
- 24.Grassmann R, Berchtold S, Radant I, Alt M, Fleckenstein B, Sodroski JG, Haseltine WA, Ramstedt U. Role of human T-cell leukemia virus type 1 X region proteins in immortalization of primary human lymphocytes in culture. J Virol. 1992;66:4570–4575. doi: 10.1128/jvi.66.7.4570-4575.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grassmann R, Dengler C, Muller-Fleckenstein I, Fleckenstein B, McGuire K, Dokhelar MC, Sodroski JG, Haseltine WA. Transformation to continuous growth of primary human T lymphocytes by human T-cell leukemia virus type I X-region genes transduced by a Herpesvirus saimiri vector. Proc Natl Acad Sci U S A. 1989;86:3351–3355. doi: 10.1073/pnas.86.9.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Green JE, Hinrichs SH, Vogel J, Jay G. Exocrinopathy resembling Sjogren’s syndrome in HTLV-1 tax transgenic mice. Nature. 1989;341:72–74. doi: 10.1038/341072a0. [DOI] [PubMed] [Google Scholar]
- 27.Grossman WJ, Kimata JT, Wong FH, Zutter M, Ley TJ, Ratner L. Development of leukemia in mice transgenic for the tax gene of human T-cell leukemia virus type I. Proc Natl Acad Sci U S A. 1995;92:1057–1061. doi: 10.1073/pnas.92.4.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Habu K, Nakayama-Yamada J, Asano M, Saijo S, Itagaki K, Horai R, Yamamoto H, Sekiguchi T, Nosaka T, Hatanaka M, Iwakura Y. The human T cell leukemia virus type I-tax gene is responsible for the development of both inflammatory polyarthropathy resembling rheumatoid arthritis and noninflammatory ankylotic arthropathy in transgenic mice. J Immunol. 1999;162:2956–2963. [PubMed] [Google Scholar]
- 29.Hall AP, Irvine J, Blyth K, Cameron ER, Onions DE, Campbell ME. Tumours derived from HTLV-I tax transgenic mice are characterized by enhanced levels of apoptosis and oncogene expression. J Pathol. 1998;186:209–214. doi: 10.1002/(SICI)1096-9896(1998100)186:2<209::AID-PATH162>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 30.Haneji K, Matsuda T, Tomita M, Kawakami H, Ohshiro K, Uchihara JN, Masuda M, Takasu N, Tanaka Y, Ohta T, Mori N. Fucoidan extracted from Cladosiphon okamuranus Tokida induces apoptosis of human T-cell leukemia virus type 1-infected T-cell lines and primary adult T-cell leukemia cells. Nutr Cancer. 2005;52:189–201. doi: 10.1207/s15327914nc5202_9. [DOI] [PubMed] [Google Scholar]
- 31.Hasegawa H, Sawa H, Lewis MJ, Orba Y, Sheehy N, Yamamoto Y, Ichinohe T, Tsunetsugu-Yokota Y, Katano H, Takahashi H, Matsuda J, Sata T, Kurata T, Nagashima K, Hall WW. Thymus-derived leukemia-lymphoma in mice transgenic for the Tax gene of human T-lymphotropic virus type I. Nat Med. 2006;12:466–472. doi: 10.1038/nm1389. [DOI] [PubMed] [Google Scholar]
- 32.Hinrichs SH, Nerenberg M, Reynolds RK, Khoury G, Jay G. A transgenic mouse model for human neurofibromatosis. Science. 1987;237:1340–1343. doi: 10.1126/science.2888191. [DOI] [PubMed] [Google Scholar]
- 33.Hiraragi H, Kim SJ, Phipps AJ, Silic-Benussi M, Ciminale V, Ratner L, Green PL, Lairmore MD. Human T-lymphotropic virus type 1 mitochondrion-localizing protein p13ii is required for viral infectivity in vivo. J Virol. 2006;80:3469–3476. doi: 10.1128/JVI.80.7.3469-3476.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ibrahim F, Fiette L, Gessain A, Buisson N, Dethe G, Bomford R. Infection of rats with human T-cell leukemia virus type-1: susceptibility of inbred strains, antibody response and provirus location. Int J Cancer. 1994;58:446–451. doi: 10.1002/ijc.2910580324. [DOI] [PubMed] [Google Scholar]
- 35.Imada K, Takaorikondo A, Akagi T, Shimotohno K, Sugamura K, Hattori T, Yamabe H, Okuma M, Uchiyama T. Tumorigenicity of human T-cell leukemia virus type I-infected cell lines in severe combined immunodeficient mice and characterization of the cells proliferating in vivo. Blood. 1995;86:2350–2357. [PubMed] [Google Scholar]
- 36.Imada K, Takaorikondo A, Sawada H, Imura A, Kawamata S, Okuma M, Uchiyama T. Serial transplantation of adult T cell leukemia cells into severe combined immunodeficient mice. Jpn J Cancer Res. 1996;87:887–892. doi: 10.1111/j.1349-7006.1996.tb02116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ishiguro N, Abe M, Seto K, Sakurai H, Ikeda H, Wakisaka A, Togashi T, Tateno M, Yoshiki T. A rat model of human T lymphocyte virus type 1 (HTLV-1) infection, 1: humoral antibody response, provirus integration, and YTLV-1-associated myelopathy/tropical spastic paraparesis-like myelopathy in seronegative HTLV-1 carrier rats. J Exp Med. 1992;176:981–989. doi: 10.1084/jem.176.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ishihara S, Tachibana N, Okayama A, Murai K, Tsuda K, Mueller N. Successful graft of HTLV-1-transformed human T-cells (MT-2) in severe combined immunodeficiency mice treated with anti-asialo GM-1 antibody. Jpn J Cancer Res. 1992;83:320–323. doi: 10.1111/j.1349-7006.1992.tb00108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ishikawa C, Matsuda T, Okudaira T, Tomita M, Kawakami H, Tanaka Y, Masuda M, Ohshiro K, Ohta T, Mori N. Bisphosphonate incadronate inhibits growth of human T-cell leukaemia virus type I-infected T-cell lines and primary adult T-cell leukaemia cells by interfering with the mevalonate pathway. Br J Haematol. 2007;136:424–432. doi: 10.1111/j.1365-2141.2006.06445.x. [DOI] [PubMed] [Google Scholar]
- 40.Ishikawa C, Tafuku S, Kadekaru T, Sawada S, Tomita M, Okudaira T, Nakazato T, Toda T, Uchihara JN, Taira N, Ohshiro K, Yasumoto T, Ohta T, Mori N. Anti-adult T-cell leukemia effects of brown algae fucoxanthin and its deacetylated product, fucoxanthinol. Int J Cancer. 2008;123:2702–2712. doi: 10.1002/ijc.23860. [DOI] [PubMed] [Google Scholar]
- 41.Ishitsuka K, Tamura K. Treatment of adult T-cell leukemia/lymphoma: past, present, and future. Eur J Haematol. 2008;80:185–196. doi: 10.1111/j.1600-0609.2007.01016.x. [DOI] [PubMed] [Google Scholar]
- 42.Ito M, Hiramatsu H, Kobayashi K, Suzue K, Kawahata M, Hioki K, Ueyama Y, Koyanagi Y, Sugamura K, Tsuji K, Heike T, Nakahata T. NOD/SCID/gamma(c)(null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood. 2002;100:3175–3182. doi: 10.1182/blood-2001-12-0207. [DOI] [PubMed] [Google Scholar]
- 43.Iwakura Y, Tosu M, Yoshida E, Takiguchi M, Sato K, Kitajima I, Nishioka K, Yamamoto K, Takeda T, Hatanaka M, Yamamoto H, Sekiguchi T. Induction of inflammatory arthropathy resembling rheumatoid arthritis in mice transgenic for HTLV-1. Science. 1991;253:1026–1028. doi: 10.1126/science.1887217. [DOI] [PubMed] [Google Scholar]
- 44.Jacobson S, Shida H, Mcfarlin DE, Fauci AS, Koenig S. Circulating CD8+ cytotoxic T lymphocytes specific for HTLV-I pX in patients with HTLV-I associated neurological disease. Nature. 1990;348:245–248. doi: 10.1038/348245a0. [DOI] [PubMed] [Google Scholar]
- 45.Kataoka R, Takehara N, Iwahara Y, Sawada T, Ohtsuki Y, Dawei Y, Hoshino H, Miyoshi I. Transmission of HTLV-I by blood transfusion and its prevention by passive immunization in rabbits. Blood. 1990;76:1657–1661. [PubMed] [Google Scholar]
- 46.Kawano N, Ishikawa F, Shimoda K, Yasukawa M, Nagafuji K, Miyamoto T, Baba E, Tanaka T, Yamasaki S, Gondo H, Otsuka T, Ohshima K, Shultz LD, Akashi K, Harada M. Efficient engraftment of primary adult T-cell leukemia cells in newborn NOD/SCID/beta2-microglobulin(null) mice. Leukemia. 2005;19:1384–1390. doi: 10.1038/sj.leu.2403829. [DOI] [PubMed] [Google Scholar]
- 47.Kondo A, Imada K, Hattori T, Yamabe H, Tanaka T, Miyasaka M, Okuma M, Uchiyama T. A model of in vivo cell proliferation of adult T-cell leukemia. Blood. 1993;82:2501–2509. [PubMed] [Google Scholar]
- 48.Kushida S, Maeda N, Fang J, Uchida K, Miwa M. Establishment of HTLV-1 carrier mice by injection with HTLV-1-producing T cells. Leukemia. 1997;11(suppl 3):260–262. [PubMed] [Google Scholar]
- 49.Kushida S, Matsumura M, Tanaka H, Ami Y, Hori M, Kobayashi M, Uchida K, Yagami K, Kameyama T, Yoshizawa T, et al. HTLV-1-associated myelopathy/tropical spastic paraparesis-like rats. Jpn J Cancer Res. 1993;84:831–833. doi: 10.1111/j.1349-7006.1993.tb02052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kwon H, Ogle L, Benitez B, Bohuslav J, Montano M, Felsher DW, Greene WC. Lethal cutaneous disease in transgenic mice conditionally expressing HTLV-I tax. J Biol Chem. 2005;280:35713–35722. doi: 10.1074/jbc.M504848200. [DOI] [PubMed] [Google Scholar]
- 51.Lairmore M, Franchini G. Human T-cell leukemia virus types 1 and 2. In: Knipe DM, editor. Fields Virology. Wolters Kluwer/Lippincott Williams & Wilkins; Baltimore, MD: 2007. pp. 2071–2105. [Google Scholar]
- 52.Lairmore MD, Roberts B, Frank D, Rovnak J, Weiser MG, Cockerell GL. Comparative biological responses of rabbits infected with human T-lymphotropic virus Type I isolates from patients with lymphoproliferative and neurodegenerative disease. Int J Cancer. 1992;50:124–130. doi: 10.1002/ijc.2910500125. [DOI] [PubMed] [Google Scholar]
- 53.Lairmore MD, Silverman L, Ratner L. Animal models for human T-lymphotropic virus type 1 (HTLV-1) infection and transformation. Oncogene. 2005;24:6005–6015. doi: 10.1038/sj.onc.1208974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Levin MC, Lee SM, Kalume F, Morcos Y, Dohan FC, Jr, Hasty KA, Callaway JC, Zunt J, Desiderio D, Stuart JM. Autoimmunity due to molecular mimicry as a cause of neurological disease. Nat Med. 2002;8:509–513. doi: 10.1038/nm0502-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Levin MC, Lee SM, Morcos Y, Brady J, Stuart J. Cross-reactivity between immunodominant human T lymphotropic virus type I tax and neurons: implications for molecular mimicry. J Infect Dis. 2002;186:1514–1517. doi: 10.1086/344734. [DOI] [PubMed] [Google Scholar]
- 56.Liu Y, Dole K, Stanley JR, Richard V, Rosol TJ, Ratner L, Lairmore M, Feuer G. Engraftment and tumorigenesis of HTLV-1 transformed T cell lines in SCID/bg and NOD/SCID mice. Leuk Res. 2002;26:561–567. doi: 10.1016/s0145-2126(01)00169-2. [DOI] [PubMed] [Google Scholar]
- 57.Parrula C, Zimmerman B, Nadella M, Shu S, Rosol T, Fernandez S, Lairmore M, Niewiesk S. Expression of tumor invasion factors determines systemic engraftment and induction of humoral hypercalcemia in a mouse model of adult T cell leukemia. Vet Pathol. 2009;46:1003–1014. doi: 10.1354/vp.08-VP-0254-N-FL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Matsuoka M, Green PL. The HBZ gene, a key player in HTLV-1 pathogenesis. Retrovirology. 2009;6:71. doi: 10.1186/1742-4690-6-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Matsuoka M, Jeang KT. Human T-cell leukaemia virus type 1 (HTLV-1) infectivity and cellular transformation. Nat Rev Cancer. 2007;7:270–280. doi: 10.1038/nrc2111. [DOI] [PubMed] [Google Scholar]
- 60.Mesnard JM, Barbeau B, Devaux C. HBZ, a new important player in the mystery of adult T-cell leukemia. Blood. 2006;108:3979–3982. doi: 10.1182/blood-2006-03-007732. [DOI] [PubMed] [Google Scholar]
- 61.Mitra-Kaushik S, Harding J, Hess J, Schreiber R, Ratner L. Enhanced tumorigenesis in HTLV-1 tax-transgenic mice deficient in interferon-gamma. Blood. 2004;104:3305–3311. doi: 10.1182/blood-2004-01-0266. [DOI] [PubMed] [Google Scholar]
- 62.Miyake K, Inokuchi K, Miyake N, Dan K, Shimada T. HIV vector-mediated targeted suicide gene therapy for adult T-cell leukemia. Gene Ther. 2007;14:1662–1667. doi: 10.1038/sj.gt.3303024. [DOI] [PubMed] [Google Scholar]
- 63.Miyazato P, Yasunaga J, Taniguchi Y, Koyanagi Y, Mitsuya H, Matsuoka M. De novo human t-cell leukemia virus type 1 infection of human lymphocytes in NOD-SCID, common-γ-chain knockout mice. J Virol. 2006;80:10683–10691. doi: 10.1128/JVI.01009-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Miyoshi I, Kubonishi I, Yoshimoto S, Akagi T, Ohtsuki Y, Shiraishi Y, Nagata K, Hinuma Y. Type C virus particles in a cord T-cell line derived by co-cultivating normal human cord leukocytes and human leukaemic T cells. Nature. 1981;294:770–771. doi: 10.1038/294770a0. [DOI] [PubMed] [Google Scholar]
- 65.Miyoshi I, Takehara N, Sawada T, Iwahara Y, Kataoka R, Yang D, Hoshino H. Immunoglobulin prophylaxis against HTLV-I in a rabbit model. Leukemia. 1992;6(suppl 1):24–26. [PubMed] [Google Scholar]
- 66.Miyoshi I, Yoshimoto S, Fujishita M, Kubonishi I, Taguchi H, Ohtsuki Y. Infectious transmission of human T-cell leukemia virus to animals. Princess Takamatsu Symp. 1984;15:121–127. [PubMed] [Google Scholar]
- 67.Mone A, Puhalla S, Whitman S, Baiocchi R, Cruz J, Vukosavljevic T, Banks A, Eisenbeis CF, Byrd JC, Caligiuri MA, Porcu P. Durable hematological complete response and suppression of HTLV-1 viral load following alemtuzumab in AZT/IFN{alpha}-refractory adult T-cell leukemia. Blood. 2005;106:3380–3382. doi: 10.1182/blood-2005-01-0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mori N. Cell signaling modifiers for molecular targeted therapy in ATLL. Front Biosci. 2009;14:1479–1489. doi: 10.2741/3319. [DOI] [PubMed] [Google Scholar]
- 69.Mori N, Matsuda T, Tadano M, Kinjo T, Yamada Y, Tsukasaki K, Ikeda S, Yamasaki Y, Tanaka Y, Ohta T, Iwamasa T, Tomonaga M, Yamamoto N. Apoptosis induced by the histone deacetylase inhibitor FR901228 in human T-cell leukemia virus type 1-infected T-cell lines and primary adult T-cell leukemia cells. J Virol. 2004;78:4582–4590. doi: 10.1128/JVI.78.9.4582-4590.2004. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 70.Mortreux F, Gabet AS, Wattel E. Molecular and cellular aspects of HTLV-1 associated leukemogenesis in vivo. Leukemia. 2003;17:26–38. doi: 10.1038/sj.leu.2402777. [DOI] [PubMed] [Google Scholar]
- 71.Mueller NE, Blattner WA. Retroviruses—Human T-Cell Lymphotropic Virus. 4. Plenum; New York, NY: 1997. [Google Scholar]
- 72.Murata N, Hakoda E, Machida H, Ikezoe T, Sawada T, Hoshino H, Miyoshi I. Prevention of human T cell lymphotropic virus type 1 infection in Japanese macaques by passive immunization. Leukemia. 1996;10:1971–1974. [PubMed] [Google Scholar]
- 73.Nakamura H, Hayami M, Ohta Y, Ishikawa K, Tsujimoto H, Kiyokawa T, Yoshida M, Sasagawa A, Honjo S. Protection of cynomolgus monkeys against infection by human T-cell leukemia virus type-1 by immunization with viral env gene products produced in Escherichia coli. Int J Cancer. 1987;40:403–407. doi: 10.1002/ijc.2910400320. [DOI] [PubMed] [Google Scholar]
- 74.Nakamura H, Tanaka Y, Tsujimoto AK, Ishikawa K, Takadaya KI, Tozawa H, Tsujimoto H, Honjo S, Hayami M. Experimental inoculation of monkeys with autologous lymphoid cell lines immortalized by and producing human T-cell leukemia virus type-I. Int J Cancer. 1986;38:867–875. doi: 10.1002/ijc.2910380614. [DOI] [PubMed] [Google Scholar]
- 75.Nakazato T, Okudaira T, Ishikawa C, Nakama S, Sawada S, Tomita M, Uchihara JN, Taira N, Masuda M, Tanaka Y, Ohshiro K, Takasu N, Mori N. Anti-adult T-cell leukemia effects of a novel synthetic retinoid, Am80 (Tamibarotene) Cancer Sci. 2008;99:2286–2294. doi: 10.1111/j.1349-7006.2008.00917.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 76.Nasr R, Rosenwald A, El Sabban ME, Arnulf B, Zalloua P, Lepelletier Y, Bex F, Hermine O, Staudt L, de The H, Bazarbachi A. Arsenic/interferon specifically reverses 2 distinct gene networks critical for the survival of HTLV-1-infected leukemic cells. Blood. 2003;101:4576–4582. doi: 10.1182/blood-2002-09-2986. [DOI] [PubMed] [Google Scholar]
- 77.Nawata H, Maeda Y, Sumimoto Y, Miyatake J, Kanamaru A. A mechanism of apoptosis induced by all-trans retinoic acid on adult T-cell leukemia cells: a possible involvement of the Tax/NF-kappaB signaling pathway. Leuk Res. 2001;25:323–331. doi: 10.1016/s0145-2126(00)00126-0. [DOI] [PubMed] [Google Scholar]
- 78.Nerenberg M, Hinrichs SH, Reynolds RK, Khoury G, Jay G. The tat gene of human T-lymphotropic virus type 1 induces mesenchymal tumors in transgenic mice. Science. 1987;237:1324–1329. doi: 10.1126/science.2888190. [DOI] [PubMed] [Google Scholar]
- 79.Nerenberg MI, Wiley CA. Degeneration of oxidative muscle fibers in HTLV-1 tax transgenic mice. Am J Pathol. 1989;135:1025–1033. [PMC free article] [PubMed] [Google Scholar]
- 80.Nicot C, Harrod RL, Ciminale V, Franchini G. Human T-cell leukemia/lymphoma virus type 1 nonstructural genes and their functions. Oncogene. 2005;24:6026–6034. doi: 10.1038/sj.onc.1208977. [DOI] [PubMed] [Google Scholar]
- 81.Ohashi T, Hanabuchi S, Kato H, Koya Y, Takemura F, Hirokawa K, Yoshiki T, Tanaka Y, Fujii M, Kannagi M. Induction of adult T-cell leukemia-like lymphoproliferative disease and its inhibition by adoptive immunotherapy in T-cell-deficient nude rats inoculated with syngeneic human T-cell leukemia virus type 1-immortalized cells. J Virol. 1999;73:6031–6040. doi: 10.1128/jvi.73.7.6031-6040.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ohbo K, Suda T, Hashiyama M, Mantani A, Ikebe M, Miyakawa K, Moriyama M, Nakamura M, Katsuki M, Takahashi K, Yamamura K, Sugamura K. Modulation of hematopoiesis in mice with a truncated mutant of the interleukin-2 receptor gamma chain. Blood. 1996;87:956–967. [PubMed] [Google Scholar]
- 83.Ohshima K. Pathological features of diseases associated with human T-cell leukemia virus type I. Cancer Sci. 2007;98:772–778. doi: 10.1111/j.1349-7006.2007.00456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ohsugi T, Horie R, Kumasaka T, Ishida A, Ishida T, Yamaguchi K, Watanabe T, Umezawa K, Urano T. In vivo antitumor activity of the NF-kappaB inhibitor dehydroxymethylepoxyquinomicin in a mouse model of adult T-cell leukemia. Carcinogenesis. 2005;26:1382–1388. doi: 10.1093/carcin/bgi095. [DOI] [PubMed] [Google Scholar]
- 85.Ohsugi T, Kumasaka T, Okada S, Ishida T, Yamaguchi K, Horie R, Watanabe T, Umezawa K. Dehydroxymethylepoxyquinomicin (DHMEQ) therapy reduces tumor formation in mice inoculated with tax-deficient adult T-cell leukemia-derived cell lines. Cancer Lett. 2007;257:206–215. doi: 10.1016/j.canlet.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 86.Okudaira T, Hirashima M, Ishikawa C, Makishi S, Tomita M, Matsuda T, Kawakami H, Taira N, Ohshiro K, Masuda M, Takasu N, Mori N. A modified version of galectin-9 suppresses cell growth and induces apoptosis of human T-cell leukemia virus type I-infected T-cell lines. Int J Cancer. 2007;120:2251–2261. doi: 10.1002/ijc.22534. [DOI] [PubMed] [Google Scholar]
- 87.Okudaira T, Tomita M, Uchihara JN, Matsuda T, Ishikawa C, Kawakami H, Masuda M, Tanaka Y, Ohshiro K, Takasu N, Mori N. NIK-333 inhibits growth of human T-cell leukemia virus type I-infected T-cell lines and adult T-cell leukemia cells in association with blockade of nuclear factor-kappaB signal pathway. Mol Cancer Ther. 2006;5:704–712. doi: 10.1158/1535-7163.MCT-05-0434. [DOI] [PubMed] [Google Scholar]
- 88.Osame M, Izumo S, Igata A, Matsumoto M, Matsumoto T, Sonoda S, Tara M, Shibata Y. Blood transfusion and HTLV-I-associated myelopathy. Lancet. 1986;2:104–105. doi: 10.1016/s0140-6736(86)91636-3. [DOI] [PubMed] [Google Scholar]
- 89.Osame M, Janssen R, Kubota H, Nishitani H, Igata A, Nagataki S, Mori M, Goto I, Shimabukuro H, Khabbaz R, Kaplan J. Nationwide survey of HTLV-I-associated myelopathy in Japan: association with blood transfusion. Ann Neurol. 1990;28:50–56. doi: 10.1002/ana.410280110. [DOI] [PubMed] [Google Scholar]
- 90.Osame M, Usuku K, Izumo S, Ijichi N, Amitani H, Igata A, Matsumoto M, Tara M. HTLV-I associated myelopathy, a new clinical entity. Lancet. 1986;i:1031–1032. doi: 10.1016/s0140-6736(86)91298-5. [DOI] [PubMed] [Google Scholar]
- 91.Phillips KE, Herring B, Wilson LA, Rickford MS, Zhang M, Goldman CK, Tso JY, Waldmann TA. IL-2R alpha-directed monoclonal antibodies provide effective therapy in a murine model of adult T-cell leukemia by a mechanism other than blockade of IL-2/IL-2Ralpha interaction. Cancer Res. 2000;60:6977–6984. [PubMed] [Google Scholar]
- 92.Portis T, Harding JC, Ratner L. The contribution of NF-kappa B activity to spontaneous proliferation and resistance to apoptosis in human T-cell leukemia virus type 1 Tax-induced tumors. Blood. 2001;98:1200–1208. doi: 10.1182/blood.v98.4.1200. [DOI] [PubMed] [Google Scholar]
- 93.Pozzatti r, Vogel J, Jay G. The human T-lymphotropic virus type 1 tax gene can cooperate with the ras oncogene to induce neoplastic transformation of cells. Mol Cell Biol. 1990;10:413–417. doi: 10.1128/mcb.10.1.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ratner L. Pathogenesis and treatment of human T-cell leukemia virus infection. Immunol Res. 2005;32:217–223. doi: 10.1385/IR:32:1-3:217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ratner L, Grant C, Zimmerman B, Fritz J, Weil G, Denes A, Suresh R, Campbell N, Jacobson S, Lairmore M. Effect of treatment of Strongyloides infection on HTLV-1 expression in a patient with adult T-cell leukemia. Am J Hematol. 2007;82:929–931. doi: 10.1002/ajh.20929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rauch D, Gross S, Harding J, Niewiesk S, Lairmore M, Piwnica-Worms D, Ratner L. Imaging spontaneous tumorigenesis: inflammation precedes development of peripheral NK tumors. Blood. 2009;113:1493–1500. doi: 10.1182/blood-2008-07-166462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Richard V, Lairmore MD, Green PL, Feuer G, Erbe RS, Albrecht B, D’Souza C, Keller ET, Dai J, Rosol TJ. Humoral hypercalcemia of malignancy: severe combined immunodeficient/beige mouse model of adult T-cell lymphoma independent of human T-cell lymphotropic virus type-1 tax expression. Am J Pathol. 2001;158:2219–2228. doi: 10.1016/S0002-9440(10)64694-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ruddle NH, Li CB, Horne WC, Santiago P, Troiano N, Jay G, Horowitz M, Baron R. Mice transgenic for HTLV-I LTR-tax exhibit tax expression in bone, skeletal alterations, and high bone turnover. Virology. 1993;197:196–204. doi: 10.1006/viro.1993.1580. [DOI] [PubMed] [Google Scholar]
- 99.Rygaard J, Povlsen CO. Heterotransplantation of a human malignant tumour to “Nude” mice. Acta Pathol Microbiol Scand. 1969;77:758–760. doi: 10.1111/j.1699-0463.1969.tb04520.x. [DOI] [PubMed] [Google Scholar]
- 100.Saito M, Matsuzaki T, Satou Y, Yasunaga J, Saito K, Arimura K, Matsuoka M, Ohara Y. In vivo expression of the HBZ gene of HTLV-1 correlates with proviral load, inflammatory markers and disease severity in HTLV-1 associated myelopathy/tropical spastic paraparesis (HAM/TSP) Retrovirology. 2009;6:19. doi: 10.1186/1742-4690-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Satou Y, Nosaka K, Koya Y, Yasunaga JI, Toyokuni S, Matsuoka M. Proteasome inhibitor, bortezomib, potently inhibits the growth of adult T-cell leukemia cells both in vivo and in vitro. Leukemia. 2004;18:1357–1363. doi: 10.1038/sj.leu.2403400. [DOI] [PubMed] [Google Scholar]
- 102.Sawada T, Iwahara Y, Ishii K, Taguchi H, Hoshino H, Miyoshi I. Immunoglobulin prophylaxis against milkborne transmission of human T cell leukemia virus type 1 in rabbits. J Infect Dis. 1991;164:1193–1196. doi: 10.1093/infdis/164.6.1193. [DOI] [PubMed] [Google Scholar]
- 103.Shimoyama M. Diagnostic criteria and classification of clinical subtypes of adult T- cell leukaemia-lymphoma: a report from the Lymphoma Study Group (1984–87) Br J Haematol. 1991;79:428–437. doi: 10.1111/j.1365-2141.1991.tb08051.x. [DOI] [PubMed] [Google Scholar]
- 104.Shu ST, Nadella MV, Dirksen WP, Fernandez SA, Thudi NK, Werbeck JL, Lairmore MD, Rosol TJ. A novel bioluminescent mouse model and effective therapy for adult T-cell leukemia/lymphoma. Cancer Res. 2007;67:11859–11866. doi: 10.1158/0008-5472.CAN-07-1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Siegel R, Gartenhaus R, Kuzel T. HTLV-I associated leukemia/lymphoma: epidemiology, biology, and treatment. Cancer Treat Res. 2001;104:75–88. doi: 10.1007/978-1-4615-1601-9_3. [DOI] [PubMed] [Google Scholar]
- 106.Stewart SA, Feuer G, Jewett A, Lee FV, Bonavida B, Chen ISY. HTLV-I gene expression in adult T-cell leukemia cells elicits an NK cell response in vitro and correlates with cell rejection in SCID mice. Virology. 1996;226:167–175. doi: 10.1006/viro.1996.0643. [DOI] [PubMed] [Google Scholar]
- 107.Suga T, Kameyama T, Shimotohno K, Matsumura M, Tanaka H, Kushida S, Ami Y, Uchida M, Uchida K, Miwa M. Infection of rats with HTLV-1: a small-animal model for HTLV-1 carriers. Int J Cancer. 1991;49:764–769. doi: 10.1002/ijc.2910490522. [DOI] [PubMed] [Google Scholar]
- 108.Takajo I, Umeki K, Morishita K, Yamamoto I, Kubuki Y, Hatakeyama K, Kataoka H, Okayama A. Engraftment of peripheral blood mononuclear cells from human T-lymphotropic virus type 1 carriers in NOD/SCID/gammac(null) (NOG) mice. Int J Cancer. 2007;121:2205–2211. doi: 10.1002/ijc.22972. [DOI] [PubMed] [Google Scholar]
- 109.Takaorikondo A, Imada K, Yamamoto I, Kunitomi A, Numata Y, Sawada H, Uchiyama T. Parathyroid hormone-related protein-induced hypercalcemia in SCID mice engrafted with adult T-cell leukemia cells. Blood. 1998;91:4747–4751. [PubMed] [Google Scholar]
- 110.Takatsuki K. Adult T-cell leukemia. Intern Med. 1995;34:947–952. doi: 10.2169/internalmedicine.34.947. [DOI] [PubMed] [Google Scholar]
- 111.Takatsuki K. Discovery of adult T-cell leukemia. Retrovirology. 2005;2:16. doi: 10.1186/1742-4690-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Takehara N, Iwahara Y, Uemura Y, Sawada T, Ohtsuki Y, Iwai H, Hoshino H, Miyoshi I. Effect of immunization on HTLV-1 infection in rabbits. Int J Cancer. 1989;44:332–336. doi: 10.1002/ijc.2910440224. [DOI] [PubMed] [Google Scholar]
- 113.Tan C, Waldmann TA. Proteasome inhibitor PS-341, a potential therapeutic agent for adult T-cell leukemia. Cancer Res. 2002;62:1083–1086. [PubMed] [Google Scholar]
- 114.Tanaka A, Takahashi C, Yamaoka S, Nosaka T, Maki M, Hatanaka M. Oncogenic transformation by the tax gene of human T-cell leukemia virus type 1 in vitro. Proc Natl Acad Sci U S A. 1990;87:1071–1075. doi: 10.1073/pnas.87.3.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tanaka Y, Tanaka R, Terada E, Koyanagi Y, Miyanokurosaki N, Yamamoto N, Baba E, Nakamura M, Shida H. Induction of antibody responses that neutralize human T-cell leukemia virus type I infection in vitro and in vivo by peptide immunization. J Virol. 1994;68:6323–6331. doi: 10.1128/jvi.68.10.6323-6331.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Taylor GP, Matsuoka M. Natural history of adult T-cell leukemia/lymphoma and approaches to therapy. Oncogene. 2005;24:6047–6057. doi: 10.1038/sj.onc.1208979. [DOI] [PubMed] [Google Scholar]
- 117.Tomita M, Kawakami H, Uchihara JN, Okudaira T, Masuda M, Takasu N, Matsuda T, Ohta T, Tanaka Y, Ohshiro K, Mori N. Curcumin (diferuloylmethane) inhibits constitutive active NF-kappaB, leading to suppression of cell growth of human T-cell leukemia virus type I-infected T-cell lines and primary adult T-cell leukemia cells. Int J Cancer. 2006;118:765–772. doi: 10.1002/ijc.21389. [DOI] [PubMed] [Google Scholar]
- 118.Uchiyama T. ATL and HTLV-I: in vivo cell growth of ATL cells. J Clin Immunol. 1996;16:305–314. doi: 10.1007/BF01541665. [DOI] [PubMed] [Google Scholar]
- 119.Uchiyama T, Yodoi J, Sagawa K, Takatsuki K, Uchino H. Adult T-cell leukemia: clinical and hematologic features of 16 cases. Blood. 1977;50:481–492. [PubMed] [Google Scholar]
- 120.Umehara F, Izumo S, Nakagawa M, Ronquillo AT, Takahashi K, Matsumoto K, Sato E, Osame M. Immunocytochemical analysis of the cellular infiltrate in the spinal cord lesions in HTLV-I-associated myelopathy. J Neuropathol Exp Neurol. 1993;52:424–430. doi: 10.1097/00005072-199307000-00010. [DOI] [PubMed] [Google Scholar]
- 121.Watanabe M, Ohsugi T, Shoda M, Ishida T, Aizawa S, Maruyama-Nagai M, Utsunomiya A, Koga S, Yamada Y, Kamihira S, Okayama A, Kikuchi H, Uozumi K, Yamaguchi K, Higashihara M, Umezawa K, Watanabe T, Horie R. Dual targeting of transformed and untransformed HTLV-1-infected T cells by DHMEQ, a potent and selective inhibitor of NF-kappaB, as a strategy for chemoprevention and therapy of adult T-cell leukemia. Blood. 2005;106:2462–2471. doi: 10.1182/blood-2004-09-3646. [DOI] [PubMed] [Google Scholar]
- 122.Yamaguchi K. Human T-lymphotropic virus type I in Japan. Lancet. 1994;343:213–216. doi: 10.1016/s0140-6736(94)90994-6. [DOI] [PubMed] [Google Scholar]
- 123.Yamamoto N, Hayami M, Komuro A, Schneider J, Hunsmann G, Okada M, Hinuma Y. Experimental infection of cynomolgus monkeys with a human retrovirus, adult T-cell leukemia virus. Med Microbiol Immunol (Berl) 1984;173:57–64. doi: 10.1007/BF02123570. [DOI] [PubMed] [Google Scholar]
- 124.Yasunaga J, Matsuoka M. Leukaemogenic mechanism of human T-cell leukaemia virus type I. Rev Med Virol. 2007;17:301–311. doi: 10.1002/rmv.548. [DOI] [PubMed] [Google Scholar]
- 125.Yoshiki T. Chronic progressive myeloneuropathy in WKAH rats induced by HTLV- I infection as an animal model for HAM/TSP in humans. Intervirology. 1995;38:229–237. doi: 10.1159/000150437. [DOI] [PubMed] [Google Scholar]
- 126.Zhang M, Yao Z, Garmestani K, Axworthy DB, Zhang Z, Mallett RW, Theodore LJ, Goldman CK, Brechbiel MW, Carrasquillo JA, Waldmann TA. Pretargeting radioimmunotherapy of a murine model of adult T-cell leukemia with the alpha-emitting radionuclide, bismuth 213. Blood. 2002;100:208–216. doi: 10.1182/blood-2002-01-0107. [DOI] [PubMed] [Google Scholar]
- 127.Zhang M, Zhang Z, Goldman CK, Janik J, Waldmann TA. Combination therapy for adult T-cell leukemia-xenografted mice: flavopiridol and anti-CD25 monoclonal antibody. Blood. 2005;105:1231–1236. doi: 10.1182/blood-2004-05-1709. [DOI] [PubMed] [Google Scholar]
- 128.Zhang Z, Zhang M, Goldman CK, Ravetch JV, Waldmann TA. Effective therapy for a murine model of adult T-cell leukemia with the humanized anti-CD52 monoclonal antibody, Campath-1H. Cancer Res. 2003;63:6453–6457. [PubMed] [Google Scholar]
- 129.Zhang Z, Zhang M, Ravetch JV, Goldman C, Waldmann TA. Effective therapy for a murine model of adult T-cell leukemia with the humanized anti-CD2 monoclonal antibody, MEDI-507. Blood. 2003;102:284–288. doi: 10.1182/blood-2002-11-3601. [DOI] [PubMed] [Google Scholar]