Abstract
Integrins are ubiquitous transmembrane mechanoreceptors that elicit changes in intracellular biochemistry in response to mechanical force application, but these alterations generally proceed over seconds to minutes. Stress-sensitive ion channels represent another class of mechanoreceptors that are activated much more rapidly (within msec), and recent findings suggest that calcium influx through Transient Receptor Potential Vanilloid-4 (TRPV4) channels expressed in the plasma membrane of bovine capillary endothelial cells is required for mechanical strain-induced changes in focal adhesion assembly, cell orientation and directional migration. However, whether mechanically stretching a cell’s extracellular matrix (ECM) adhesions might directly activate cell surface ion channels remains unknown. Here we show that forces applied to β1 integrins result in ultra-rapid (within 4 msec) activation of calcium influx through TRPV4 channels. The TRPV4 channels were specifically activated by mechanical strain in the cytoskeletal backbone of the focal adhesion, and not by deformation of the lipid bilayer or submembranous cortical cytoskeleton alone. This early-immediate calcium signaling response required the distal region of the β1 integrin cytoplasmic tail that contains a binding site for the integrin-associated transmembrane CD98 protein, and external force application to CD98 within focal adhesions activated the same ultra-rapid calcium signaling response. Local direct strain-dependent activation of TRPV4 channels mediated by force transfer from integrins and CD98 may therefore enable compartmentalization of calcium signaling within focal adhesions that is critical for mechanical control of many cell behaviors that underlie cell and tissue development.
Introduction
Physical forces are fundamental regulators of cell and tissue development, yet little is known about the earliest mechanotransduction events that first convert mechanical signals exerted on the cell surface into changes in intracellular biochemistry. Cell surface integrins that mediate cell adhesion are mechanoreceptors that transfer mechanical forces from extracellular matrix (ECM) to the cytoskeleton, and activate various intracellular signaling pathways in a stress-dependent manner.1–6 However, mechanochemical conversion supported by integrins is usually measured over seconds to minutes and thus, the proximal integrin-dependent transduction events remain unknown7–10. Stress-activated (SA) membrane ion channels are also ubiquitous mechanotransducers that support the conversion of mechanical force signals applied to the cell surface into transmembrane ion gradients. When measured using the patch clamp technique in isolated membranes,11, 12 the generation of ion current through SA channels in response to negative pressure applied to the isolated membrane occurs within milliseconds (msec). However, patch clamp technology is limited in its ability to study the immediate effects of mechanical stimulation of ion channels through integrins, as technologies capable of applying force directly on integrins such as magnetic pulling cytometry8, 13, 14 and/or optical laser tweezers15 interfere with the sensitive transducers and amplifiers used to record ion currents during patch clamp measurements. However, use of general SA channel blockers, such as gadolinium chloride (Gd+3), in experiments with whole cells and tissues suggests that SA channels become activated when forces are transferred from ECM to integrins.8, 16, 17 Moreover, stress-dependent calcium influx through SA channels is required for later changes in gene expression, cell shape and cytoskeletal structure that enable changes in cell movement and tissue morphogenesis.18–20 Although some SA channels (e.g., ENaC, polycystin) have been reported to colocalize with β1 integrins,21, 22 immediate activation of SA channels by forces applied to integrins has never been demonstrated. Thus, it is not known whether SA channels respond directly to force applied to integrins, nor is the mechanism clear by which these two fundamental mechanical signaling pathways might first converge at the cell surface.
Mechanical forces applied to cell surface integrin receptors are transferred across discrete cytoskeletal elements over long distances throughout the cytoplasm in living adherent cells. 23, 24 As such, force can induce near instantaneous mechano-chemical signaling at remote locations throughout the cell.7, 25, 26 We recently showed that cyclic strain of cultured bovine capillary endothelial cells applied through their ECM-integrin adhesions activates calcium influx through Transient Receptor Potential Vanilloid 4 (TRPV4) ion channels expressed in their plasma membrane. 20 This calcium signal triggers subsequent cytoskeletal remodeling and movement necessary for cell realignment in the vasculature.20 We hypothesized that TRPV4 channels in these cells can respond directly to force applied to integrins and that mechanical force induced calcium signaling through TRPV4 requires focal adhesion proteins that structurally link integrins with the ion channel in the plasma membrane. To test these hypotheses in these intact cells, we used magnetic pulling cytometry (MPC),8, 14, 27 a technique whereby mechanical force can be directly applied to cell surface integrin receptors using ligand coated magnetic beads and an automated electromagenetic needle,8, 27 and high speed microfluorimetric calcium imaging to gauge the intracellular consequences of localized force application through integrins. In addition, we used a molecular biology approach to transfect cells with specially designed chimeric integrin receptors through which mechanical force can be applied to the cells. The integrin chimeras contain portions of intracellular and transmembrane domains of β1 integrin that are connected to an extracellular domain containing carbonic anhydrase IV (CAIV) at the terminus. 28 The extracellular CAIV protein selectively binds to anti-CAIV antibody used to coat magnetic beads, and thus provides specificity through which mechanical force is applied to cells. Importantly, upon binding, the chimeric receptor participates intracellularly as a β1 integrin in focal adhesion formation and signaling. 28 This approach enables the identification of structural components within focal adhesions that are required for force transfer from integrins to TRPV4 channels in the plasma membrane.
Our studies reveal that mechanical force applied through integrins induces a near instantaneous and localized transient TRPV4 mediated calcium influx in intact capillary endothelial cells expressing both native and genetically engineered integrin receptors. We used a novel image analysis strategy to show that stress-induced displacements and mechanical strain of phase-dense cytoplasmic constituents in the region of the focal adhesion surrounding stressed beads were required for the rapid calcium signaling. Studies using integrin chimera suggest that the distal most region of the β1 integrin cytoplasmic domain that contains a binding site for the transmembrane amino acid transporter CD98 mediates this near instantaneous integrin dependent force induced calcium signal. Therefore, these cytoskeletal linkages likely provide the continuity between integrin mediated focal adhesions and force sensitive ion channels that enables direct mechanotransduction between these ubiquitous molecules in the plasma membrane.
Results
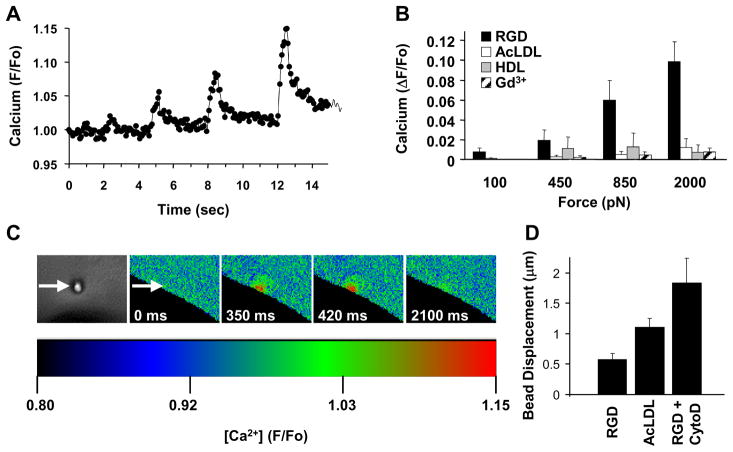
To begin these studies, four brief (500 msec) pulses of increasing tensional force (100, 450, 850 and 2000 pN) were applied to surface integrins on cultured cells through bound magnetic microbeads (4.5 μm diameter) coated with anti-β1 integrin antibody (12G10) or synthetic RGD peptide using magnetic field gradients applied with an electromagnetic needle.8 Dose-dependent, transient increases in intracellular calcium could be detected as quickly as 4 msec after force application and peaked within 250 to 350 msec (Fig. 1A–C and Electronic Supplementary Information (ESI) Fig. S1; ESI Movies S1 and S2). This nearly instantaneous signaling response was inhibited by removing extracellular calcium from the medium or adding Gd+3 to block SA channels (Fig. 1B). Mechanically-induced calcium influx localized within the cytoplasm precisely at the site of force application beneath the bead-membrane interface (Fig. 1C; ESI Movies S1 and S2) where the bound and clustered integrins induce focal adhesion formation, as confirmed by recruitment of zyxin (ESI Fig. S2), paxillin, vinculin, α-actinin, talin, FAK and F-actin in these capillary cells.29
Fig. 1. Ultra-rapid integrin-mediated mechanochemical conversion.
(A) Localized calcium increases induced by applying four 500 msec force pulses (arrows) of increasing magnitude (100, 450, 850 and 200pN, from left to right) while taking Fluo-4 measurements every 70 msec (F/Fo = ratio of normalized fluorescence intensity relative to time 0). (B) Calcium increases induced by force application through microbeads coated with RGD in the presence or absence of gadolinium chloride (Gd3+, 25 μM), AcLDL or HDL. (C) Phase contrast (left) and pseudocolored ratiometric fluorescence images at indicated times following application of the 4th force pulse shown in a (arrowheads indicate bead position). (D) Displacement of surface-bound beads coated with RGD (in the absence and presence of cytochalasin D (CytoD, 2 μg/mL) or AcLDL in response to 2000 pN force (*, p < 0.05 relative to RGD bead).
Transmembrane integrin receptors might activate SA channels by deforming the lipid bilayer or distorting the submembranous cytoskeleton, two mechanisms previously proposed to mediate SA channel activation.11, 12 However, the submembranous cytoskeletal proteins fodrin and ankryin were excluded from focal adhesions at bead binding sites, as well as from basal zyxin-containing focal adhesions (ESI Fig. S2), as shown in other cell types.29, 30 Similar studies were carried out with magnetic microbeads coated with acetylated low density lipoprotein (AcLDL) or high density lipoprotein (HDL) that bind metabolic receptors which span the lipid bilayer and interact with the submembranous cytoskeleton, but do not form focal adhesions in these cells.29 Force application to these transmembrane receptors produced even greater bead displacement and membrane deformation than when stresses were applied to integrin-bound beads (Fig. 1D); yet, it did not induce calcium influx (Fig. 1B).
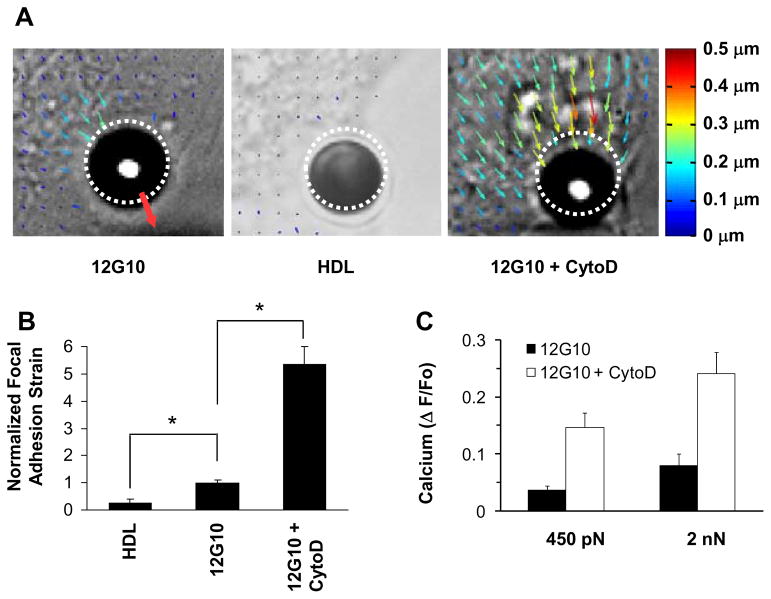
To confirm that integrin-cytoskeleton connections were required, we mapped stress-induced displacements and mechanical strain in the region of the focal adhesion within the cytoplasm directly beneath the bound bead using computerized image analysis. Localized force application to β1 integrins induced mechanical strain of the focal adhesion in a stress-dependent manner, and produced detectable displacements of distant phase-dense cytoplasmic constituents as far as 10–15 μm away (Fig. 2A; ESI Movie S3). This action-at-a-distance has been shown to be mediated by force channeling over discrete cytoskeletal linkages in the living cytoplasm.24–26, 31 In contrast, there was neither significant displacement of distant cytoplasm (Fig. 2A), focal adhesion strain (Fig. 2B), nor calcium signaling (Fig. 1C) when the same forces were applied to HDL or Acetylated Low Density Lipoprotein (AcLDL) receptors that do not form focal adhesions.32–34 Importantly, force application to these beads produced greater bead displacement than beads bound to integrins (Fig. 2A; ESI Movie S4), and hence increased distortion of the surface membrane bilayer, without inducing calcium signaling. Generalized deformation of the plasma membrane and submembranous cytoskeleton alone is therefore not sufficient to induce rapid calcium signaling in these cells.
Fig. 2. Activation of calcium signaling by mechanical strain within focal adhesions.
(A) Maps of cytoplasmic displacement directly beneath bound microbeads immediately after application of a force pulse (2000 pN, 500 msec). Dashed circles indicate bead starting position; size and color of arrows are scaled with degree of local deformation relative to time 0; red arrow indicates direction of force. (B) Normalized focal adhesion strain (+ S.E.M.) within a 3 × 3 μm2 region of interest located directly above the submembranous focal adhesion within 1 μm from the bead-membrane interface (p <0.05). (C) Intracellular calcium increases measured in response to force application through β1 integrin (12G10 antibody) in the absence and presence of cytochalasin D (2 μg/ml).
A subset (10–15%) of beads coated with integrin ligands formed such stiff cytoskeletal connections that there was no detectable movement during the force pulses. Interestingly, these beads that did not strain (distort) focal adhesions also failed to induce calcium influx, even though high levels (2 nanoNewton (nN)) of isometric tension were exerted on the bound integrin receptors. Conversely, when the actin cytoskeleton was partially disrupted using cytochalasin D under conditions that do not interfere with bead binding and that leave focal adhesions intact (ESI Fig. S3), greater bead displacement (Fig. 1D), increased focal adhesion strain (Fig. 2A–B; ESI Movie S5) and enhanced calcium influx (Fig. 2C) were observed; and the correlation between calcium signaling and focal adhesion distortion was highly significant (p < 0.007). These data therefore support a mechanism of early-immediate signal activation that involves physical distortion of the cytoskeletal backbone of the focal adhesion, rather than deformation of integrins, the lipid bilayer or the submembranous cortical cytoskeleton.
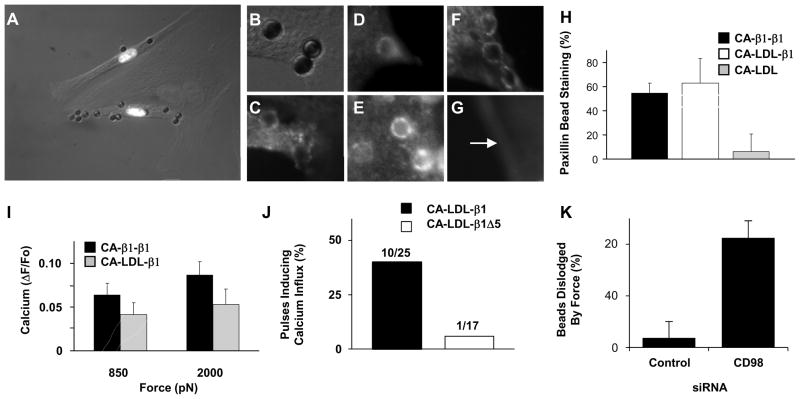
To analyze the molecular mechanism by which stresses transmitted through integrins activate rapid calcium influx, we used MPC to apply tensional forces to cells via a transfected single chain β1 integrin chimera that contains the extracellular domain of carbonic anhydrase IV (CA) fused to the transmembrane and intracellular domains of β1 integrin (CA–β1-β1)28 (ESI Fig. S4). The CA–β1-β1 construct supported focal adhesion formation, spreading and cytoskeletal reorganization in capillary cells cultured on CA-antibody coated substrates (ESI Fig. S5A, B) as it does in other cell types, even though the CA adhesive ligand it binds does not interact with endogenous α-integrins.28 Cells expressing CA–β1-β1 also bound to magnetic beads coated with anti-CA antibody (Fig. 3A) and formed focal adhesions containing talin, paxillin and vinculin at sites of bead binding (Fig. 3B–E, H). Importantly, force application through CA–β1-β1 induced rapid calcium influx (Fig. 3I) confirming that the β1 integrin chain alone is sufficient to mediate stress-dependent activation of calcium influx.
Fig. 3. Distal integrin cytoplasmic domain is necessary for rapid force-induced calcium signaling.
(A) Merged phase contrast and immunofluorescence photomicrographs of three BCE cells demonstrating that only cells transfected with the CA-β1-β1 construct bind beads coated with anti-CA antibody (white nuclei indicate cells transfected with chimera and histone 2B Ds-red; an adjacent non-transfected cells is outlined in white). (A, C) High magnification phase contrast (B) and fluorescence (C) view of beads shown in A after cells expressing CA-β1-β1 were stained for paxillin (C). Fluorescence imaging of GFP-talin (D) and actin (E), in similarly treated cells. (F, G) Fluorescence views of beads in cells transfected with CA-LDL-β1 (F) or CA-LDL (G) stained for paxillin. Arrow indicates bead position. (H) Quantitation of paxillin staining results, and of calcium signaling responses (I), for the different integrin chimeras. (J) Percentage of 3 sec tensional force pulses (350 pN) applied via anti-CA antibody coated microbeads to BCE cells transfected with the indicated integrin chimeras inducing calcium influx. (K) Percentage of RGD coated microbeads bound to BCE cells treated with indicated siRNA that were dislodged by 2000 pN force.
We next generated integrin chimeras containing only the transmembrane domain of the LDL receptor (CA-LDL) or this domain fused to the β1 integrin cytoplasmic tail (CA-LDL-β1; ESI Fig. S4) to determine whether the internal portion of the integrin chain is sufficient to mediate this mechanical signaling response. Cells expressing either of these chimeras bound to anti-CA-coated beads (ESI Fig. S5C,D), but only cells transfected with CA-LDL-β1 formed focal adhesions (Fig. 3F–H). Consistent with this observation, when tensional force (2 nN) was applied to these surface-bound beads, virtually all beads were dislodged from cells expressing the CA-LDL chimera (43 out of 45). In contrast, most (64 to 79%) beads bound to the other chimera containing the cytoplasmic β1 integrin tail resisted these stresses, and force application induced similar levels of rapid calcium influx in cells expressing both the CA-LDL-β1 and CA–β1-β1 chimeras (Fig. 3I). Hence, mechanical stresses must be specifically transmitted to the β1 integrin cytoplasmic tail that links to the actin cytoskeleton to activate this nearly instantaneous mechanotransduction response.
To determine which regions of the integrin cytoplasmic tail are necessary for localized calcium influx, a series of integrin deletion mutants was engineered in which sequential regions of the integrin cytoplasmic domain were eliminated from the CA-LDL-β1 chimera (ESI Fig. S4). The mechanical strength of adhesions formed between cells transfected with each of these mutants and anti-CA antibody-coated beads was weaker than that in cells expressing the full length construct when measured using 2 nN force (ESI Fig. S6). Interestingly, all bound CA-antibody-coated beads were physically dislodged from cells expressing the construct lacking the final 6 amino acid residues of the cytoplasmic domain (CA-LDL-β1Δ5) (ESI Fig. S6), yet focal adhesions containing paxillin and vinculin formed at the sites of bead binding (ESI Fig. S7). Moreover, when lower force levels were applied to the CA-LDL-β1Δ5 receptors that did not dislodge the beads from the cell surface, displacement of distant cytoplasmic elements was observed (i.e., functional cytoskeletal connections remained intact); yet the calcium response was almost completely abolished in these cells (Fig. 3J).
This distal region of the β1 integrin cytoplasmic tail has been previously shown to bind to the transmembrane amino acid transporter heavy chain, CD98, which is also required for adhesion strengthening through integrins and cytoskeletal tension-dependent fibronectin fibrillogenesis.35 We therefore investigated its role in mediating this ultra-rapid, strain-induced calcium signaling response. CD98 protein appeared in focal adhesions formed at sites of cell binding to CA-antibody-coated beads in cells expressing CA-LDL-β1, whereas it was significantly reduced (p < 0.005) in similar bead-membrane adhesions in cells transfected with the CA-LDL-β1Δ5 mutant (ESI Fig. S8). Cell binding to beads coated with an antibody to the extracellular domain of CD98 also induced focal adhesion formation at the bead-membrane interface (ESI Fig. S9A). Interestingly, force application to cells via these CD98-bound beads also stimulated local calcium influx (ESI Fig. S9B), much as we observed when we pulled on native β1 integrins (Fig. 1A). Moreover, knocking down CD98 protein expression using siRNA (ESI Fig. S9C,D) significantly decreased the adhesion strength of native β1 integrins, with almost all beads detaching at a force level (2 nN) that control cells could easily support (Fig. 3K). Unfortunately, these beads detached even at the lowest force levels, and hence the effects of CD98 knock down on calcium signaling could not be analyzed directly. Nevertheless, these data confirm that CD98 is required for this nearly instantaneous mechanical signaling response, and suggest that lateral binding of transmembrane CD98 proteins to the cytoplasmic portion of adjacent integrins is required for integrin-dependent mechanical triggering of nearby strain-sensitive calcium channels on the surface membrane.
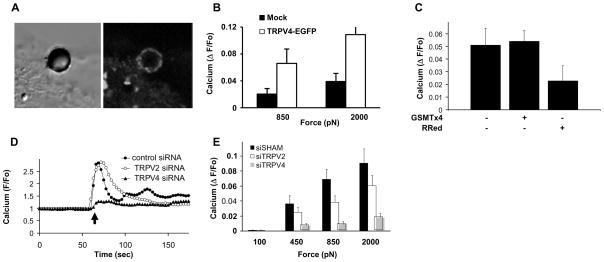
Although no calcium channel has been shown to be directly mechanically-gated in endothelial cells, the non-selective TRPV4 ion channel has been reported to mediate slower responses of these cells to fluid shear stress36 and cyclic strain20 of a time course of seconds to minutes. Stimulation of the same capillary cells used here which have been shown to express functionally active TRPV420 with the TRPV4 activator, 4α-phorbol 12,13-didecanoate (PDD) produces a rapid increase in intracellular calcium necessary for cell realignment in response to mechanical strain, and this can be inhibited by removing calcium from the medium, pre-treating cells with the TRPV blocker, ruthenium red, or knocking down its expression with siRNA directed against TRPV4, but not against TRPV2 or TRPC1.20 To explore the proximal signaling mechanism through which integrins activate TRPV4 channels in greater detail, we transfected these capillary cells with a TRPV4-Enhanced Green Fluorescent Protein (TRPV4-EGFP), which was shown to express functional TRPV4 channels, as measured in whole cell patch clamp studies.20 Fluorescence microscopic analysis revealed that when the cells bound to beads coated with β1 integrin ligands, the TRPV4-EGFP ion channels concentrated within focal adhesions at the bead-membrane interface, even before forces were applied (Fig. 4A). Moreover, when we transfected TRPV4-EGFP into Chinese Hamster Ovary (CHO) cells that do not normally express this channel,37 and applied stress to integrins, a robust (3.5-fold) increased calcium response was observed (Fig. 4B), thus confirming that TRPV4 channels function as mechanosensitive ion channels.
Fig. 4. TRPV4 channels mediate rapid force-induced calcium signaling.
(A) Phase contrast (left) and fluorescence (right) views of a bound RGD-bead on a cell transfected with TRPV4-EGFP. (B) Calcium increases induced by force application (850 and 2000 pN) through RGD coated microbeads to CHO cells transfected with indicated DNA. (C) Effects of the SA channel blocker GSMTx4 and RRed on calcium influx stimulated by force application through β1 integrin (12G10 antibody). (D) Changes of intracellular Ca2+ induced by the TRPV4 activator PDD (black arrow) in BCE cells transfected with TRPV4, TRPV2 or sham siRNAs. (E) Inhibition of increases in Ca2+ induced by force application to β1 integrin (12G10 antibody) by treatment of cells with TRPV4 siRNA, but not TRPV2 or sham siRNAs.
The possibility that endogenous TRPV4 channels might be responsible for instantaneous mechanical-induced calcium influx in capillary cells was further supported by the finding that rapid force-induced calcium signaling was inhibited in these cells by addition of ruthenium red, whereas GsMTx4, a peptide blocker of other types of SA channels,38 had no effect (Fig. 4C). Moreover, when TRPV4 mRNA and protein expression were suppressed using specific siRNA (ESI Fig. S10),20 calcium influx induced by both the TRPV4 activator 4α-PDD (Fig. 4E) and by stress application to integrins (Fig. 4F, p < 0.05) was greatly inhibited. Our results therefore reveal a novel biophysical mechanism whereby local mechanical stresses applied to β1 integrins elicit a nearly instantaneous (within 4 msec) calcium signaling response mediated by TRPV4 ion channels within the same focal adhesion at the surface membrane. To the best of our knowledge, this is the fastest integrin-mediated mechanical signaling event detected to date.
Discussion
Ion current through mechanically gated SA channels is perhaps the most rapid form of mechanotransduction, and recent work suggests that integrins might harness this mechanism for various types of physiologically relevant control mechanisms. 8, 16, 17, 20 However, the extremely rapid response time between mechanical perturbation and ion current in these channels has only been observed in patch clamp experiments by applying negative pressure mechanical force to isolated plasma membranes. Thus, the immediate and local effects of mechanical stimulation on cells when force is applied through the cell’s ECM adhesions can not be studied with this technique, and hence, the mechanism by which integrins might regulate the earliest phases of SA channel activation in intact cells remains unclear. Furthermore, the magnetic fields and electric currents needed to apply forces via integrin-bound beads using magnetic pulling cytometry can interfere with the monitoring and recording of ion currents using the patch clamp technology. As a result, we turned to high speed microfluorimetric imaging of intact cells using the highly sensitive intracellular calcium fluorophore Fluo-4 to study whether mechanical stimulation of surface integrins directly controls calcium signaling by ion channels. Although it has been recently shown that mechanical forces applied to cell surface integrin receptors transfer across discrete cytoskeletal elements over long distances throughout the cytoplasm in living adherent cells,23, 24 integrins have only been reported to mediate ion channel activation over slower time scales indirectly through biochemical signaling and production of secondary messengers.39, 40 Nevertheless, studies on intact cells whereby force was applied directly to cell surface integrins using magnetic twisting cytometry showed rapid (<0.3 s) activation of the tyrosine kinase Src at distant cytoplasmic sites remote from where force was applied to the cells.25 These data suggest that rapid integrin dependent mechanotransduction can occur away from the site of force application near instantaneously and in the likely absence of secondary messengers.25 The even more rapid response of the calcium signal (within 4 msec) observed here using whole cell calcium imaging strongly supports this notion and suggests that TRPV4 channels are activated in the absence of second messengers, and are directly mechanosensitive. In addition, although the existing mechanical activation paradigm for SA channels involves generalized distortion of the lipid bilayer or the submembranous cytoskeleton,11, 12 we show here that TRPV4 signaling induced by stress application to integrins correlates with the degree of mechanical strain in integrin-cytoskeletal linkages within focal adhesions at the site of force application, and not with membrane deformation. Thus, this nearly instantaneous calcium influx is a direct and immediate mechanotransduction response that likely represents the first mechanochemical conversion event that takes place inside these cells when mechanical stresses are applied through cell-integrin-ECM adhesions. The ability to restrict this mechanical signaling response spatially to local sites of force application within focal adhesions may be critical for coordinating complex global cell behaviors that require constant and immediate feedback, such as focal adhesion assembly, cell orientation and directional cell migration in response to mechanical strain16, 41, which may all be mediated by early integrin-dependent channel activation.20
Our studies also revealed that CD98-mediated associations with β1 integrins in focal adhesions are necessary to support mechanical force transfer required for ultra-rapid mechanical activation of calcium signaling through nearby TRPV4 channels on the surface membrane. Although an interaction between integrins and TRPV4 has been recently demonstrated in nerve cells42, it is not known whether this is through direct binding or mediated by other focal adhesion proteins. Integrin-linked kinase (ILK) associates with integrins through an intermediary binding protein43, and so TRPV4 channels may similarly bind integrins indirectly through CD98; however, this remains to be demonstrated experimentally. CD98 binding to the β1-integrin cytoplasmic tail also promotes the expression and clustering of integrins, mediates their ligand affinity, alters integrin signaling, and modulates both focal adhesion formation and the level of traction forces cells exert on their ECM adhesions44. This may explain why cell-bead adhesions deficient in CD98 break during high tensional stress. Moreover, calcium signaling increased with greater mechanical strain of focal adhesions in cytochalasin D-treated cells, whereas similar mechanical deformation of focal adhesions that lacked CD98 failed to produce calcium influx. Thus, focal adhesion strengthening facilitated by binding of CD98 to the distal integrin tail in the cytoplasm appears to be critical to maintain the mechanical connectivity within the focal adhesion necessary for TRPV4 channels to sense stresses applied to the extracellular domain of adjacent cell surface integrins. Force transfer between these cell surface molecules – β1 integrins, CD98 and TRPV4 channels – then enables nearly instantaneous localized mechanochemical transduction within focal adhesions that are required for control of more complex cell and tissue behaviors.
Materials and Methods
General Methods and Reagents
Methods for culturing bovine capillary endothelial (BCE) cells and human dermal microvascular endothelial (HMVE) cells, coating magnetic microbeads (4.5 μm diameter; Dynal M-450 beads) with adhesive ligands (including 12G10 activating β1 integrin antibodies (Serotec), RGD peptide (Peptite-2000, Integra Life Sciences), acetylated-low density lipoprotein (AcLDL) and high density lipoprotein (HDL)(both from Biomedical Technologies Inc), analysis of GFP-focal adhesion and cytosketal proteins (talin, zyxin, actin), carrying out magnetic pulling cytometry, RT-PCR, cytohistochemistry and immunofluorescence microscopy, and real-time optical analysis of bead translocation in response to force, all have been published previously8, 14, 27, 45.
Plasmids
A single chain β1 integrin chimera containing the extracellular domain of carbonic anhydrase IV (CA) fused to the transmembrane and intracellular domains of β1 integrin (CA–β1TM-β1IC)28 were kindly provided by Dr. Milan Mrksich (University of Chicago). Additional integrin receptor chimeras containing the CA external domain linked to the metabolic LDL receptor transmembrane domain, either alone (CA-LDLTM) or fused to the β1 integrin cytoplasmic tail (CA-LDLTM- β1IC) were generated using standard molecular biology techniques. TRPV4-EGFP was a gift from Dr. Bereiter-Hahn and Dr. Marina Jendrach.
Immunocytochemistry
Immunocytochemical staining was performed as previously described 45 for antibodies against talin, paxillin, zyxin (all from Abcam), fodrin and ankyrin (both from Chemicon). The polyclonal antibodies against TRPV4 were obtained from Affinity BioReagents and MBL International Corporation and CD98 antibody was from Santa Cruz Biotechnology. Actin was visualized with Phalloidin (Molecular Probes); primary antibodies were visualized with FITC or TRITC labeled secondary antibodies (Invitrogen).
Calcium Experiments
To measure rapid calcium responses over the time course of msec, cells were loaded with the single wavelength calcium dye Fluo-4 (1 μM, Molecular Probes) for 30 min in the presence of 0.01% Pluronic F127 (Molecular Probes) and 2.5 mM probenecid (Sigma-Aldrich) in serum-free DMEM with 0.1% BSA (Sigma) at 37ºC. During the final 10 min of incubation with Fluo-4, the cells were allowed to bind ligand-coated magnetic microbeads as described8. The cells were then washed 3 times with PBS and kept in a standard calcium solution containing 127 mM NaCl, 3 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM Hepes, pH 7.4 for the duration of the pulling experiments. For experiments with cytochalasin D, cells were incubated with Fluo-4 and ligand-coated beads for 10 min, and then exposed to cytochalasin D (2 μg/mL, Sigma) in the presence of Fluo-4 for 20 min prior to washing. Change in the fluorescence intensity at a single wavelength (488 nm excitation) in response to force was measured using a Nikon Eclipse TE-2000-E Fluorescence microscope equipped with a 1000 W High Pressure Mercury Burner (Olympus Optical Company). Alterations in intracellular calcium induced by force application were determined by measuring changes in Fluo-4 intensity in a 7 μm diameter circular region of interest (ROI) centered on each bead. Results are presented as F/Fo, where F is the average fluorescence intensity in the ROI at each time point during time lapse imaging, and Fo is the fluorescence intensity in the ROI at time=0. Images (exposed for 50 ms) were recorded every 70 ms using a Coolsnap HQ CCD camera (Photometrics) and IPLab imaging software (BD Biosciences Bioimaging). Gadolinium chloride (25 μM) was from (Sigma); GsMTx4 (5 μM) was kindly provided from Dr. Fred Sachs (SUNY Buffalo).
Calcium imaging experiments on BCE cell monolayers with TRPV channel activators were performed in cells cultured in the standard calcium solution on Mattek No. 1 glass bottom dishes and viewed using a Leica DMIRE2 inverted microscope equipped with a Spectral Confocal Leica TCS-SP2 and LCS Imaging software (Leica). 4α-phorbol 12,13-didecanoate (PDD) (2 μM) and Ruthenium Red (2 μM) were purchased from Sigma.
Knockdown experiments using siRNA
siRNA-mediated knockdown was performed in HMVE and BCE cells using the RNA interference technique. Cells were transfected with 10 nM of smart pool siRNAs against TRPV2 or TRPV4 (Dharmacon) or a pool of siRNA duplexes (Dharmacon) using Silentfect (BioRad) according to the manufacturer’s instructions, and cells were analyzed after 48–72 hrs, as described previously.20, 45 CD98 siRNA duplex forward sequences as follows: CUAAAGGAGCGGAUGGAUUUU, GAGAAGAAUGGUCUGGUUAUU, and UAAAGGAGCGGAUGGAUUAUU. siRNA duplex with irrelevant sequence (QIAGEN) was used as another control. We measured the silencing efficiency of TRPV2 and TRPV4 siRNAs in both HMVEC and BCE cells by RT-PCR using species-specific primers as described previously.20 CD98 knockdown was done using a pool of three oligos. Western blotting of CD98 and TRPV4 was used to confirm knockdown of the protein expression.
Strain Mapping
Intracellular deformation maps of cells mechanically stimulated with magnetic pulling cytometry were generated using an optical flow algorithm that was originally designed for computer vision.46 Briefly, the algorithm calculates the deformation vector field between two frames such that the product of the local pixel intensity gradient in the first frame and the deformation vector field equals the time-varying change in pixel intensity between the two frames. The algorithm is programmed in MATLAB (v7), and the deformation vectors are calculated and averaged over a 9×9 pixel neighborhood centered on the pixel of interest. The 2-dimensional finite strain tensor and the corresponding principal strains are calculated using standard techniques,47 and the maximum principal strain (representing the tensile component) near the focal adhesion is averaged within a 3 μm × 3 μm Region of Interest (ROI) positioned within 1 μm from the bead-membrane interface.
Data analysis
Statistical comparisons were performed with two way ANOVA and Student’s t-test. All data are expressed as the mean ± standard error of the mean (SEM).
Supplementary Material
Insight, innovation, integration
The molecular mechanism by which cells first sense mechanical forces and transduce them into intracellular signaling is unclear. By combining a micromagnetic force probing technology with cells expressing genetically engineered, chimeric single chain β1 integrin receptors and high resolution calcium imaging, we show that the TRPV4 membrane ion channel is activated within 4 milliseconds by mechanical strain applied through integrins, but not through generalized membrane distortion. Integration of these technologies led to the discovery of this ultra-rapid integrin-mediated mechanotransduction event that is upstream of most signaling pathways previously studied in the mechanoregulation field. These studies also reveal compartmentalization of calcium signaling within focal adhesions that may be critical for mechanical control of cell and tissue development.
Acknowledgments
We thank Dr. Milan Mrksich for supplying the β1 integrin chimera. This work was supported by grants from NIH (CA45548), AHA (0635095N) and by an NIH postdoctoral fellowship (HL086172). Dr. Ingber is a recipient of a DoD Breast Cancer Innovator Award.
References
- 1.Katsumi A, Orr AW, Tzima E, Schwartz MA. J Biol Chem. 2004;279:12001–4. doi: 10.1074/jbc.R300038200. [DOI] [PubMed] [Google Scholar]
- 2.Bershadsky A, Kozlov M, Geiger B. Curr Opin Cell Biol. 2006;18:472–81. doi: 10.1016/j.ceb.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 3.Wang N, Butler JP, Ingber DE. Science. 1993;260:1124–7. doi: 10.1126/science.7684161. [DOI] [PubMed] [Google Scholar]
- 4.Roca-Cusachs P, Gauthier NC, Del Rio A, Sheetz MP. Proc Natl Acad Sci U S A. 2009;106:16245–50. doi: 10.1073/pnas.0902818106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.del Rio A, Perez-Jimenez R, Liu R, Roca-Cusachs P, Fernandez JM, Sheetz MP. Science. 2009;323:638–41. doi: 10.1126/science.1162912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwartz MA, DeSimone DW. Curr Opin Cell Biol. 2008;20:551–6. doi: 10.1016/j.ceb.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y, Botvinick EL, Zhao Y, Berns MW, Usami S, Tsien RY, Chien S. Nature. 2005;434:1040–5. doi: 10.1038/nature03469. [DOI] [PubMed] [Google Scholar]
- 8.Matthews BD, Overby DR, Mannix R, Ingber DE. J Cell Sci. 2006;119:508–18. doi: 10.1242/jcs.02760. [DOI] [PubMed] [Google Scholar]
- 9.Sawada Y, Tamada M, Dubin-Thaler BJ, Cherniavskaya O, Sakai R, Tanaka S, Sheetz MP. Cell. 2006;127:1015–26. doi: 10.1016/j.cell.2006.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao G, DeLisser H, Schwartz MA. Nature. 2005;437:426–31. doi: 10.1038/nature03952. [DOI] [PubMed] [Google Scholar]
- 11.Hamill OP. Pflugers Arch. 2006;453:333–51. doi: 10.1007/s00424-006-0131-0. [DOI] [PubMed] [Google Scholar]
- 12.Sukharev S, Corey DP. Sci STKE. 2004;2004:re4. doi: 10.1126/stke.2192004re4. [DOI] [PubMed] [Google Scholar]
- 13.Alenghat FJ, Fabry B, Tsai KY, Goldmann WH, Ingber DE. Biochem Biophys Res Commun. 2000;277:93–9. doi: 10.1006/bbrc.2000.3636. [DOI] [PubMed] [Google Scholar]
- 14.Matthews BD, Overby DR, Alenghat FJ, Karavitis J, Numaguchi Y, Allen PG, Ingber DE. Biochem Biophys Res Commun. 2004;313:758–64. doi: 10.1016/j.bbrc.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 15.Choquet D, Felsenfeld DP, Sheetz MP. Cell. 1997;88:39–48. doi: 10.1016/s0092-8674(00)81856-5. [DOI] [PubMed] [Google Scholar]
- 16.Munevar S, Wang YL, Dembo M. J Cell Sci. 2004;117:85–92. doi: 10.1242/jcs.00795. [DOI] [PubMed] [Google Scholar]
- 17.McMahon LA, Campbell VA, Prendergast PJ. J Biomech. 2008;41:2055–9. doi: 10.1016/j.jbiomech.2008.03.027. [DOI] [PubMed] [Google Scholar]
- 18.Naruse K, Yamada T, Sokabe M. Am J Physiol. 1998;274:H1532–8. doi: 10.1152/ajpheart.1998.274.5.H1532. [DOI] [PubMed] [Google Scholar]
- 19.Sasamoto A, Nagino M, Kobayashi S, Naruse K, Nimura Y, Sokabe M. Am J Physiol Cell Physiol. 2005;288:C1012–22. doi: 10.1152/ajpcell.00314.2004. [DOI] [PubMed] [Google Scholar]
- 20.Thodeti CK, Matthews B, Ravi A, Mammoto A, Ghosh K, Bracha AL, Ingber DE. Circ Res. 2009;104:1123–30. doi: 10.1161/CIRCRESAHA.108.192930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shakibaei M, Mobasheri A. Histol Histopathol. 2003;18:343–51. doi: 10.14670/HH-18.343. [DOI] [PubMed] [Google Scholar]
- 22.Wilson PD, Geng L, Li X, Burrow CR. Lab Invest. 1999;79:1311–23. [PubMed] [Google Scholar]
- 23.Hu S, Chen J, Fabry B, Numaguchi Y, Gouldstone A, Ingber DE, Fredberg JJ, Butler JP, Wang N. Am J Physiol Cell Physiol. 2003;285:C1082–90. doi: 10.1152/ajpcell.00159.2003. [DOI] [PubMed] [Google Scholar]
- 24.Hu S, Wang N. Mol Cell Biomech. 2006;3:49–60. [PubMed] [Google Scholar]
- 25.Na S, Collin O, Chowdhury F, Tay B, Ouyang M, Wang Y, Wang N. Proc Natl Acad Sci U S A. 2008;105:6626–31. doi: 10.1073/pnas.0711704105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang N, Tytell JD, Ingber DE. Nat Rev Mol Cell Biol. 2009;10:75–82. doi: 10.1038/nrm2594. [DOI] [PubMed] [Google Scholar]
- 27.Overby DR, Matthews BD, Alsberg E, Ingber DE. Acta Biomater. 2005;1:295–303. doi: 10.1016/j.actbio.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 28.Kato M, Mrksich M. J Am Chem Soc. 2004;126:6504–5. doi: 10.1021/ja039058e. [DOI] [PubMed] [Google Scholar]
- 29.Plopper GE, McNamee HP, Dike LE, Bojanowski K, Ingber DE. Mol Biol Cell. 1995;6:1349–65. doi: 10.1091/mbc.6.10.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katoh K, Kano Y, Masuda M, Fujiwara K. Cell Struct Funct. 1996;21:27–39. doi: 10.1247/csf.21.27. [DOI] [PubMed] [Google Scholar]
- 31.Maniotis AJ, Chen CS, Ingber DE. Proc Natl Acad Sci U S A. 1997;94:849–54. doi: 10.1073/pnas.94.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miyamoto S, Akiyama SK, Yamada KM. Science. 1995;267:883–5. doi: 10.1126/science.7846531. [DOI] [PubMed] [Google Scholar]
- 33.Miyamoto S, Teramoto H, Coso OA, Gutkind JS, Burbelo PD, Akiyama SK, Yamada KM. J Cell Biol. 1995;131:791–805. doi: 10.1083/jcb.131.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Plopper G, Ingber DE. Biochem Biophys Res Commun. 1993;193:571–8. doi: 10.1006/bbrc.1993.1662. [DOI] [PubMed] [Google Scholar]
- 35.Cantor JM, Ginsberg MH, Rose DM. Immunol Rev. 2008;223:236–51. doi: 10.1111/j.1600-065X.2008.00640.x. [DOI] [PubMed] [Google Scholar]
- 36.Kohler R, Heyken WT, Heinau P, Schubert R, Si H, Kacik M, Busch C, Grgic I, Maier T, Hoyer J. Arterioscler Thromb Vasc Biol. 2006;26:1495–502. doi: 10.1161/01.ATV.0000225698.36212.6a. [DOI] [PubMed] [Google Scholar]
- 37.Liedtke W, Choe Y, Marti-Renom MA, Bell AM, Denis CS, Sali A, Hudspeth AJ, Friedman JM, Heller S. Cell. 2000;103:525–35. doi: 10.1016/s0092-8674(00)00143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suchyna TM, Tape SE, Koeppe RE, 2nd, Andersen OS, Sachs F, Gottlieb PA. Nature. 2004;430:235–40. doi: 10.1038/nature02743. [DOI] [PubMed] [Google Scholar]
- 39.Gao X, Wu L, O’Neil RG. J Biol Chem. 2003;278:27129–37. doi: 10.1074/jbc.M302517200. [DOI] [PubMed] [Google Scholar]
- 40.Vriens J, Watanabe H, Janssens A, Droogmans G, Voets T, Nilius B. Proc Natl Acad Sci U S A. 2004;101:396–401. doi: 10.1073/pnas.0303329101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xia N, Thodeti CK, Hunt TP, Xu Q, Ho M, Whitesides GM, Westervelt R, Ingber DE. FASEB J. 2008;22:1649–59. doi: 10.1096/fj.07-090571. [DOI] [PubMed] [Google Scholar]
- 42.Alessandri-Haber N, Dina OA, Joseph EK, Reichling DB, Levine JD. J Neurosci. 2008;28:1046–57. doi: 10.1523/JNEUROSCI.4497-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li F, Zhang Y, Wu C. J Cell Sci. 1999;112(Pt 24):4589–99. doi: 10.1242/jcs.112.24.4589. [DOI] [PubMed] [Google Scholar]
- 44.Kim SM, Hahn JH. Exp Mol Med. 2008;40:261–70. doi: 10.3858/emm.2008.40.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mammoto A, Huang S, Ingber DE. J Cell Sci. 2007;120:456–67. doi: 10.1242/jcs.03353. [DOI] [PubMed] [Google Scholar]
- 46.Lucas BD, Kanade T. Proceedings of Imaging Understanding Workshop. 1981:121–130. [Google Scholar]
- 47.Mase GE, Mase GT. Continuum Mechanics for Engineers. CRC Press; Boca Raton: 1992. p. 190. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.