Abstract
Selective serotonin reuptake inhibitors (SSRIs) mediate their antidepressant effects by blocking serotonin transporter (SERT) which, in turn, increases the extracellular serotonin [5-hydroxytryptamine (5-HT)] at neuron synapse. Interestingly, Fluoxetine, one of the SSRIs have been found to possess immune modulation effects. However, it remains unclear if SSRIs can suppress the antigen-presenting function of dendritic cells (DCs). Therefore, Fluoxetine was applied to a co-culture between Aggregatibacter actinomycetemcomitans (Aa)-reactive T cells (×Aa-T) isolated from Aa-immunized mouse and DCs, which resulted in suppressing the proliferation of ×Aa-T stimulated with Aa-antigen presentation by DCs. Fluoxetine increased the extracellular 5-HT in the ×Aa-T/DC co-culture, whereas exogenously applied 5-HT promoted T cell proliferation in the ×Aa-T/DC co-culture, indicating that extracellular 5-HT is not responsible for Fluoxetine-mediated suppression of ×Aa-T/DC responses. Fluoxetine remarkably suppressed the expression of co-stimulatory molecule ICOS-L on DCs. Blocking of ICOS-L expressed on DCs with specific antibody down-modulated the antigen presentation from DCs to ×Aa-T cells. These results suggested that Fluoxetine suppressed the ability of DCs to present bacterial antigens to T cells and resulting T cell proliferation in a SERT/5-HT-independent manner and that diminished expression of ICOS-L on DCs caused by Fluoxetine might be partially associated with Fluoxetine-mediated suppressions on DC/T cell responses.
Keywords: Fluoxetine, serotonine (5-HT), dendritic cells, antigen presentation, ICOS-L
Introduction
Selective serotonin [5-hydroxytryptamine (5-HT)] reuptake inhibitors (SSRIs) are a class of antidepressant drugs used to treat major depression and other related neuronal disorders. In recent years, Fluoxetine, a commonly prescribed SSRI that blocks the serotonin transporter (SERT) in the brain, was revealed to possess host beneficial side effects represented by peripheral anti-inflammatory and immunomodulatory properties (Yaron et al., 1999; Abdel-Salam et al., 2003; Roumestan et al., 2007). It was reported that Fluoxetine down-regulates the Th1-type cytokine productions and proliferation of human blood T cells stimulated with a non-specific T cell mitogen, concanavalin A (Con A) (Diamond et al., 2006). Recently published in vivo studies using rats demonstrated that SSRIs can down-regulate the activation of T lymphocytes (Fazzino et al., 2009). Although antigen presentation to memory T cells from DCs plays a critical role in the induction of adaptive immune responses to non-self organisms, especially to bacteria, it remains unclear if Fluoxetine can affect antigen presentation from DCs to effector T lymphocytes via T-cell receptor (TCR)/MHC-class-II engagement.
Periodontal disease (PD) is a chronic inflammatory disease triggered by bacterial infection that affects the attachment structures of the teeth. PD is one of the most important causes of tooth loss and has been considered a modifying factor of the systemic health of individuals (Seymour et al., 2007). The inflammatory products released by immune cells, such as dendritic cells (DCs) and T cells, after bacterial challenge are strongly related to host tissue destruction (Loesche & Grossman, 2001; Taubman et al., 2005). It is well documented that antigen presentation by DCs plays a pivotal role in regulating the activation of T cells by presenting bacterial antigens in the context of PD (Cutler & Jotwani, 2004; Cutler & Teng, 2007). DCs, which are well-equipped professional antigen-presenting cells, express higher levels of major histocompatibility complexes (MHC) than other antigen-presenting cells, along with permissive co-stimulatory molecules, for the induction of TCR/CD3 activation (Banchereau & Steinman, 1998). In addition to their roles in presenting bacterial antigens to T cells, DCs are also engaged in the production of important pro- and anti-inflammatory cytokines (i.e., IL-12, IL-1β, TNF-α and IL-10) and chemokines (i.e., RANTES and MIP-1α) in response to bacterial stimuli (Banchereau & Steinman, 1998). It has been thought that immune response to periodontal pathogens is host protective. However, in the chronic infection of PD, recent theory supports the idea that insufficiently controlled immune responses elicited by DCs can cause collateral tissue damage by their production of proinflammatory cytokines as well as by induction of overreacting T cells (Cutler & Teng, 2007). Given such possible pathogenic engagement mediated by DCs, they are considered to be interesting targets for the development of pharmacological regimens for chronic infection, especially PD.
Based on the above-noted evidence showing that Fluoxetine can affect DCs and T cells, respectively, the aims of this study were to determine 1) whether Fluoxetine can affect the ability of DCs to present bacterial antigens to T cells in the immune synapse involving TCR and MHC-class-II and 2) whether blocking of SERT expressed in DCs by Fluoxetine is responsible for its effects on antigen-presentation by DCs. Desipramine belongs to another class of antidepressant drug known as Norepinephrine Reuptake Inhibitors (NRIs). Since NRIs are also reported to possess immune suppressive effect (Roumestan et al., 2007; Hashioka et al., 2009), Desipramine was included to compare its effects to Fluoxetine. In this study, the periodontal pathogen Aggregatibacter actinomycetemcomitans (Aa) was used as a model bacterium which can elicit periodontal tissue destruction via activation of bacterial reactive T cells (Taubman et al., 2005; Teng et al., 2005; Kawai et al., 2007). Contrary to our expectation, the results demonstrated that Fluoxetine suppresses the ability of DCs to present bacterial antigens to T cells and resulting T cell proliferation in a SERT/5-HT-independent manner.
Materials and methods
Chemicals
Fluoxetine hydrochloride and Desipramine hydrochloride were obtained from Sigma-Aldrich (St. Louis, MO) and dissolved in water at a high concentration (1 mM, respectively). The drugs were then diluted in fresh RPMI medium containing 10 % FBS to reach final concentrations tested in the present study. Synthetic 5-HT (serotonin hydrochloride) was purchased from Acros Organics USA (Morris Plains, NJ).
Animals
C57BL/6 wild type mice (6–8 weeks old, male, Jackson Laboratory, Bar Harbor, ME) were housed in cages with water and food ad libitum in 12-hour dark-light cycles at constant temperature and maintained in the animal housing facility of The Forsyth Institute. All experiments were performed in compliance with protocols approved by the Forsyth Institutional Animal Care and Use Committee (IACUC).
Bacterial antigens
Aa strain Y4 (ATCC, Manassas, VA) was cultured in trypticase soy broth supplemented with 0.6% yeast extract (TSBY; Difco Laboratories, Detroit, MI) in humidified 5% CO2 atmosphere at 37°C. After cultivation, cells were fixed with formalin following the methods published previously (Kawai et al., 2007).
Development of CD11c+ DCs ex vivo
To prepare primary culture of DCs, bone marrow was obtained from femurs and tibias of normal C57BL/6 wild type mice. The bone marrow mononuclear cells were isolated by density gradient centrifugation with Histopaque™ (Sigma, St. Louis, MO) and cultured ex vivo with recombinant GM-CSF (20 ng/mL, Peprotech, Rocky Hill, NJ) in a complete DMEM medium that contains 10% fetal bovine serum (FBS; Invitrogen, Carlsbad, CA), antibiotics (penicillin, streptomycin, and gentamicin; Invitrogen) and L-glutamine. At the third day, the complete DMEM medium with GM-CSF was partially (50%) replaced. After 7 days, CD11c+ DCs were isolated from the cultures using MACS beads (Miltenyi Biotec, Bergisch Gladbach, Germany). For all experiments, CD11c+ DCs were cultured in a RPMI 1640 medium supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, CA), 50 μmol/L of β-mercaptoethanol, antibiotics (penicillin, streptomycin, and gentamicin) and L-glutamine in 24- or 96-well plates.
Cytotoxicity assay
For evaluation of the drugs’ cytotoxicity, DCs (2 × 104 cells/well in a 96-well plate) were incubated with Fluoxetine or Desipramine at concentrations of 0.01, 0.1 or 1 μM for 24 hours in RPMI medium, and the colorimetric MTT assay was performed. The stock MTT (3[4,5-dimethyl-thiazol-2yl]-2,5-diphenyl-tetrazolium bromide; Sigma-Aldrich) dissolved in PBS at 5 mg/mL was added to all wells (MTT stock 20 μL/90 μL culture medium containing DCs), followed by incubation for 4 hours at 37 °C to form formazan crystals. In order to dissolve the crystals, 0.04 N HCl in propanol solution was added (120 μL/well). The plates were read after 30 minutes at 570 nm. The percentage of viability was calculated based on the control cells (non-treated) as having 100% of viability.
Enzyme immuno-assay to detect 5-HT, cytokines and chemokines
In order to monitor the 5-HT produced during the co-culture between T cells and DC, Serotonin EIA kit (Immuno Biological Laboratories, Inc., Minneapolis, MN) was utilized. To detect the concentration of IL-12, IL-1β, TNF-α, IL-10, RANTES (regulated on activation, normal T cell expressed and secreted or CCL5) and MIP-1α (macrophage inflammatory protein 1α or CCL3), culture supernatants were subjected to ELISA (ELISA development kits; PeproTech, Rocky Hill, NJ).
Detection of serotonin transporter (SERT) mRNA by RT-PCR
For RT-PCR analyses, total RNA was extracted from DCs cultures stimulated or not with LPS for 6 hours as well as from mouse brain (positive control), using RNA-bee™ reagent following the manufacturer’s protocol (Tel. Test, Inc., Friendswood, TX). RT-PCR was performed as previously described (Han et al., 2009). Isolated RNA (1μg) was reverse transcribed with SuperScript-II (Invitrogen, Carlsbad, CA) in the presence of random primers. The resulting cDNA was used as the template DNA for the subsequent PCR performed by the High Fidelity Expand System (Roche, Indianapolis, IN). Designs of primers for serotonin transporter (SERT) and β-actin are as follows: SERT (forward, 5′-acaacatcacctggacactccattc-3′ and reverse, 5′-ccgcatatgtgatgaaaaggaggct-3′), β-actin (forward 5′-gacggggtcacccacactgt-3′, and reverse, 5′-aggagcaatgatcttgatcttc-3′). PCR conditions were as follows: 35 cycles of 94°C for 30 s; 55°C (β-actin) or 58°C (SERT) for 30 s (optimized for each set of primer); 72°C for 1 min. PCR products were separated in 1.5% agarose gels stained with SYBR Safe™.
Flow cytometry to evaluate expression profile of cell surface molecules on DCs
The effects of Fluoxetine or Desipramine on the expression profiles of MHC-class II (I-Ab), CD80, CD86 ICOS-L and PD-L1 on immature DCs were determined using flow cytometry. The ex vivo-developed immature DCs were incubated in the presence or absence of Fluoxetine or Desipramine (1 μM) for 36 hours. After incubation, 5 × 105 cells re-suspended in PBS containing 1% BSA and 0.02% NaN3 were incubated with fluorescein isothiocyanate-conjugated anti-mouse CD11c (FITC-CD11c, BD Pharmingen, San Diego, CA, USA) along with phycoerythrin (PE)-conjugated anti-mouse MHC-class II (PE-I-Ab), PE-conjugated anti-mouse CD80 (PE-CD80) or PE-conjugated anti-mouse CD86 (PE-CD86) MAbs (all MAbs were from BD Pharmingen), with each antibody concentration at 10 μg/mL. After 1 hour of incubation, cells were washed twice and fixed with 2.5% formalin in PBS. For the staining of ICOS-L and PD-L1, the DCs were reacted with rat anti-mouse ICOS-L and anti-mouse PD-L1 MAbs (ICOS-L-MAb and PD-L1-MAb; eBioscience, San Diego, CA), followed by PE-conjugated anti-rat IgG (PE-anti-rat IgG; BD Pharmingen). After removal of PE-anti-rat IgG by washing DCs, FITC-CD11c were reacted to the DCs. Control isotype-matched rat IgG, PE-rat IgG and FITC-rat-IgG antibodies were also used to determine nonspecific staining. The expression profile of each molecule on DCs was determined by flow cytometry.
Co-culture of DCs and T cells
The Aggregatibacter actinomycetemcomitans (Aa)–reactive T cells were developed using C57BL/6 mice following the method published by our group (Kawai et al., 2007). The mononuculear cells were isolated from cervical lymph nodes of mice that received immunization with formalin-fixed Aggregatibacter actinomycetemcomitans (Aa) Y4 (injected 109 bacteria/100 μL in saline, subcutaneously (s.c.) at dorsal skin on days 0, 2 and 4) followed by booster immunization of subcutaneous injection into cheek region (109 bacteria/100 μL in saline on day 10). Mononuclear cells were isolated by density gradient centrifugation with Histopaque™ 1083 (Sigma, St. Louis, MO), and the resulting cells were passed through a nylon- and glass-wool column to enrich T cells. Aa-reactive T cells were co-cultured in the complete RPMI medium along with DCs in the presence or absence of Aa antigen (107 fixed bacteria/mL/well). The CD11c-positive DCs used in the co-cultures were obtained as described above (3. Development of CD11c-DC) and submitted to one of the following treatments: (1) Pre-treatment with drugs: DCs were pretreated with Fluoxetine or Desipramine (1 μM) for 36 h before culturing with T cells. After the pre-treatment period of 36 h, the DCs were treated with mitomycin C (MMC; 20 μg/ml, 1 h, 37°C). It is important to note that MMC treatment (20 μg/ml, 1 h, 37°C) did not change the cell surface expressions of MHC-II and other co-stimulatory molecules (CD80, CD86, ICOS-L and PD-L1) expressed on DCs, as determined at 36 hours after the incubation in complete RPMI1640 medium (cf. Fig 1). After extensive washing, the DCs were co-cultured (2 × 104 cells/well) with Aa-reactive T cells (4 × 105 cells/well) for 3 days. (2) Post-treatment with drugs: DCs (2 × 104 cells/well), which were not pretreated with drugs, were incubated with MMC (1 h, 37°C) and co-cultured with Aa-reactive T cells (4 × 105 cells/well) in the presence or absence of Fluoxetine or Desipramine (1 μM) for 3 days. As noted above, Aa (107 fixed bacteria/mL/well) was applied to these co-culture systems as T cell antigen. After 3 days, the supernatants were collected for the measurement of cytokine production (TNF-α and IL-10) or serotonin by T cells. Proliferation of T cells was assessed as described previously (Kajiya et al., 2009). Briefly, [3H] thymidine (0.5 μCi) was added to each well during the last 16 hours of a total 4-day culture. Cells were harvested, and the incorporated radioactivity in the cells under proliferation (cpm) was measured by a scintillation counter.
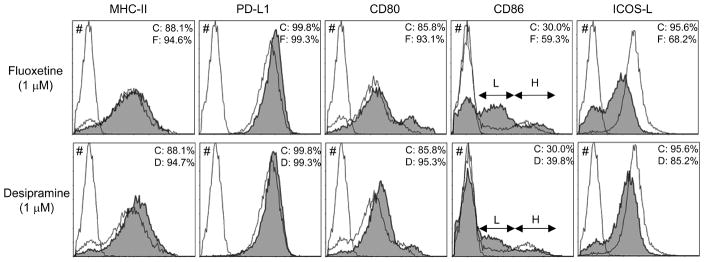
Figure 1. Influence of Fluoxetine and Desipramine on co-stimulatory molecules expressed on DC.
DCs were developed from bone marrow cells by incubation ex vivo in the presence of GM-CSF for 7 days. The developed DCs were treated with or without Fluoxetine or Desipramine for 24 hr. The MHC-class-II and co-stimulatory molecules expression pattern on CD11c+ DCs were monitored using flow cytometry. Without separation of CD11c+ cells using MACS beads, whole bone marrow cell culture containing developed DCs was subjected to flow cytometry. CD11c+ cells were labeled by PE, while remaining cell markers, MHC-class-II, PD-L1, CD80, CD86, and ICOS-L, were labeled with FITC. # shows the non-stained control Bone marrow cells. The open histogram indicates the FITC staining of PE positive (CD11c+) DCs incubated in medium alone. The solid histograms display the FITC staining of PE positive (CD 11c+) DCs stimulated with Fluoxetine (upper panel) or Desipramine (bottom panel), respectively. C: no drug treated control group. F: Fluoxetine treated group. D: Desipramine treated group. The arrows (L and H) shown in CD86-stained cells indicate CD86Low and CD86High populations, respectively.
Statistical analysis
Results were assessed using Student’s t test. Values of P < 0.05 were considered statistically significant.
Results
Effects of Fluoxetine on the antigen-specific T cell proliferation induced by DCs
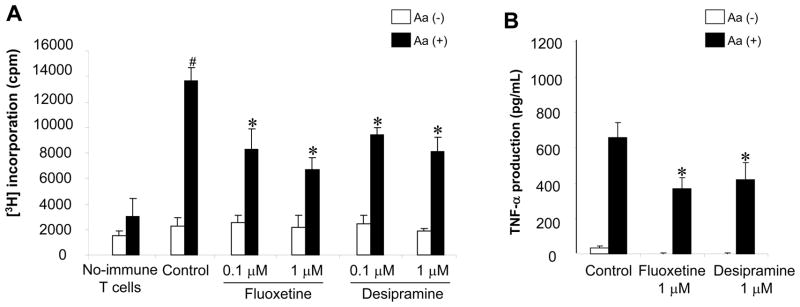
The effects of Fluoxetine and Desipramine on the Aa-reactive T cell proliferation induced by co-culture with bone marrow-derived DCs and Aa-antigen were examined (Figure 2). The lymph node T cells isolated from control non-immunized mice showed little or no proliferation response to the co-culture with DCs and Aa-antigen (Figure 2A). However, in the presence of Aa-antigen, co-culture between DC and the lymph node T cells isolated from Aa-immunized mice showed significantly elevated proliferative response. On the other hand, the addition of Fluoxetine and Desipramine suppressed such ×Aa-T cell proliferation in a dose dependent manner (Figure 2A). Moreover, in response to antigen-presentation by DC, ×Aa-T cells produced TNF-α (Figure 2B), but not IL-4 (data not shown). Importantly, Fluoxetine and Desipramine suppressed the TNF-α production from ×Aa-T cells co-cultured with DC and Aa-antigen (Figure 2B).
Figure 2. The effects of Fluoxetine and Desipramine on the bacteria (Aa)-antigen-reactive T cell (×Aa-T) responses induced by DCs.
(A and B) The effects of Fluoxetine and Desipramine on the Aa-antigen-specific ×Aa-T cell proliferation induced by co-culture with bone marrow derived DCs (treated with MMC) and Aa-antigen were examined. The lymph node T cells isolated from control non-immunized mice or Aa-immunized mice (4 × 105 cells/well, respectively) were co-cultured with DCs (2 × 104 cells/well, pretreated with MMC) in the presence or absence of Aa-antigen (2 × 106 fixed bacteria/well) for 4 days. Proliferation of T cells was determined by adding [3H] thymidine (0.5 μCi) to each well during the last 16 hours of a total 4 day culture (A). Culture supernatants were collected 3 days after co-culture for the measurement of TNF-α production by ELISA (B). Results are expressed as the mean ± SD of incorporated [3H] thymidine (cpm) or concentration of TNF-α (pg/mL). One representative result from three different experiments is shown. #, significantly higher than non-immunized mice by Student t test (P < 0.01). *, significantly lower than control cultured with Aa by Student t test (P < 0.01).
In a separate assay, within the range of concentrations selected (0.1 – 10.0 μM), Fluoxetine and Desipramine did not affect the cell viability of DCs (Supplement Figure 1). It is noteworthy that viability of T cells was not also affected by the same concentrations (0.1 – 10.0 μM) of Fluoxetine or Desipramine (data not shown). These results indicated that the diminished antigen-specific T cell proliferation mediated by Fluoxetine and Desipramine did neither result from the cytotoxicity of these two drugs to DCs nor T cells.
Pre-treatment of DCs with Fluoxetine attenuated their ability to present antigen to T cells
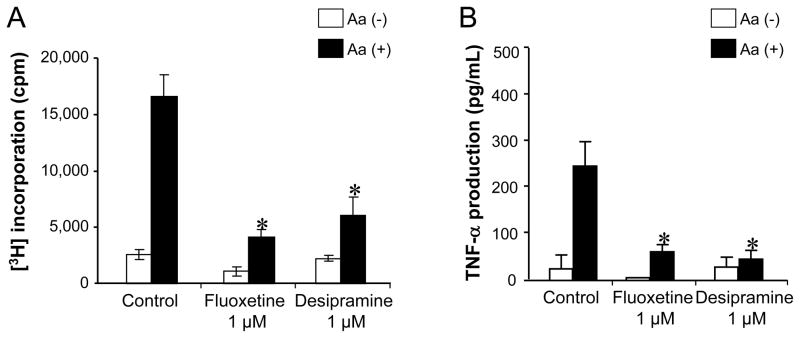
When the DCs were pre-treated with Fluoxetine or Desipramine prior to the co-culture with ×Aa-T cells, antigen-specific T cell proliferation, as well as their TNF-α production, induced by such drug-pretreated DCs were still significantly suppressed compared to the control DCs that were pre-incubated in the culture medium in the absence of drugs (Figure 3A and 3B), suggesting that the change of immunological property of DCs caused by Fluoxetine and Desipramine resulted in the diminished ability of DCs to present bacterial antigen to T cells.
Figure 3. The effects of pre-treatment of DC with Fluoxetine or Desipramine on DC’s ability to present antigen.
(A and B) DCs were pre-treated with Fluoxetine (1 μM) or Desipramine (1 μM) for 24 hours. Subsequently, those DCs were further treated with MMC and co-cultured with ×Aa-T cells in the presence and absence of Aa antigen. Antigen-specific T cell proliferation (A) and their TNF-α production (B) were monitored following the protocol described in Figure 1. Results are expressed as the mean ± SD of incorporated [3H] thymidine (cpm) or concentration of TNF-α (pg/mL). One representative result from three different experiments is shown. *, significantly lower than control (Control) cultured with Aa by Student’s t test (P < 0.01).
Lack of association of 5-HT in the Fluoxetine-mediated suppression of antigen-specific T cell proliferation induced by DCs
Recent findings revealed that activated DCs express SERT (O’Connell et al., 2006), and that ligation of 5-HT receptors expressed on T cells can activate T cells (Aune et al., 1993; Leon-Ponte et al., 2007). Following these two cutting-edge findings, production of 5-HT and effects of exogenously applied 5-HT on the co-culture between Aa-reactive T cells and DCs were examined in the following experiments.
The co-culture between Aa-reactive T cells and DCs in the presence of Aa increased the level of extracellular 5-HT compared to the co-culture in the absence of Aa, suggesting that antigen-presentation from DCs induced 5-HT production by Aa-reactive T cells (Figure 4A). Fluoxetine increased 5-HT production from co-culture with or without Aa-antigen, while the presence of Aa-antigen showed higher 5-HT production than no-Aa control. The addition of Desipramine also enhanced 5-HT production from the co-culture, while the presence or absence of Aa did not alter the level of 5-HT (Figure 4A).
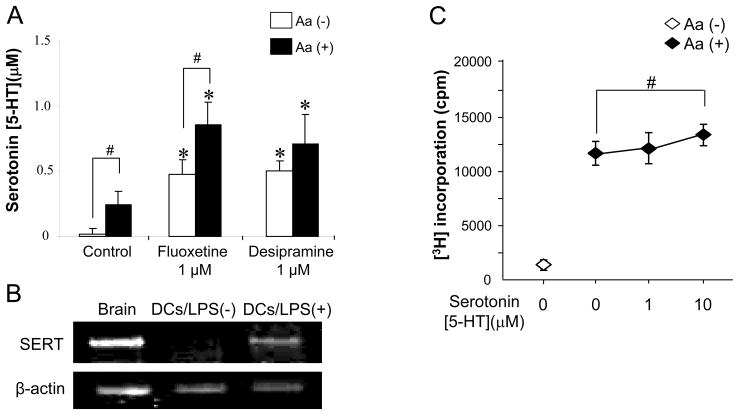
Figure 4. Expressions of 5-HT and SERT in ×Aa-T and DC as well as the effects of synthetic 5-HT on the proliferative response of ×Aa-T cells.
(A) ×Aa-T cells (4 × 105 cells/well) were co-cultured with DCs (2 × 104 cells/well, pretreated with MMC) in the presence and absence of Aa (107 fixed bacteria/mL/well). In addition, Fluoxetine or Desipramine (1 μM) was applied to the co-culture. After 3 days, the supernatants were collected for the measurement of 5-HT. *, higher than control co-culture with Aa (Student’s t test, P < 0.05), #, there is significant difference between the culture with and without Aa (Student’s t test, P < 0.05). (B) mRNA for SERT expressed in DCs was monitored using RT-PCR. Total RNA isolated from DCs incubated with or without LPS for 24 hours was subjected to RT-PCR using PCR primer set specific to SERT orβ-actin. As a positive control, brain tissue isolated from normal C57BL/6 mice was used. (C) Effects of synthetic 5-HT on the proliferative response of ×Aa-T cells in the co-culture with DC were evaluated. ×Aa-T cells (4 × 105 cells/well) were co-cultured with DCs (2 × 104 cells/well, pretreated with MMC) in the presence and absence of Aa (107 fixed bacteria/mL/well). Synthetic 5-HT (1 and 10 μM) was applied to the co-culture. [3H] thymidine (0.5 μCi) was applied to each well during the last 16 hours of a total 4-day culture. #, there is a significant difference between the culture with 5-HT (10 μM) and without 5-HT (Student’s t test, P < 0.05)
The expression of SERT mRNA was confirmed in LPS-stimulated DCs, but not control non-stimulated DCs (Figure 4B), corresponding to the previous report (O’Connell et al., 2006). Very importantly, exogenously applied synthetic 5-HT to the ×Aa-T/DC co-culture showed up-regulation of ×Aa-T cell proliferation induced by DCs (Figure 4C), indicating that 5-HT provided a co-stimulatory, instead of a suppressive, signal to the ×Aa-T cells.
These results suggested, in turn, that while Fluoxetine can increase extracellular 5-HT in the co-culture between T cells and DCs, such increased 5-HT was not associated with Fluoxetine-mediated suppression of DCs’ ability to present bacterial antigens to T cells and resulting T cell proliferation, because it was shown that elevated 5-HT can up-regulate the proliferation of T cells, rather than suppressing it.
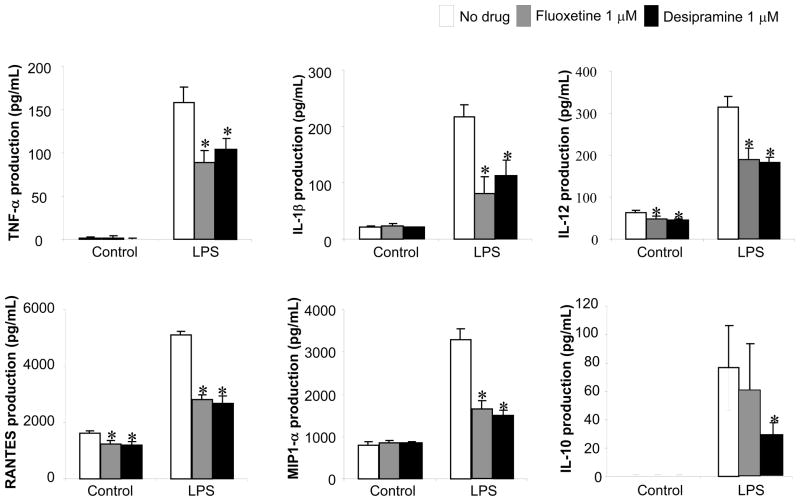
Effects of Fluoxetine on the production of cytokine and chemokine by DCs
The effects of Fluoxetine and Desipramine on expression of immune-suppressive cytokine, IL-10, along with other proinflammatory cytokines from LPS-stimulated DCs were monitored. In particular, LPS was used to stimulate DCs, because Aa is a Gram (-) pathogen which produce LPS and we reported that LPS can elicit in vivo antigen presentation to Aa-reactive T cells (Kawai et al., 2000). The non-stimulated DCs showed modest basal expression of IL-1β, IL-12, MIP-1α and RANTES, whereas TNF-α and IL-10 expression were lower than the detection limit of the ELISA system. Both Fluoxetine and Desipramine suppressed basal level expression (non-stimulated) of IL-12 and RANTES, but not IL-1β, or MIP-1α. Furthermore, while LPS stimulation markedly increased the production of TNF-α, IL-1β, IL-12, RANTES and MIP-1α in the DCs, addition of either Desipramine or Fluoxetine to the cultures resulted in significant suppression (Figure 5). The production of immune suppressive cytokine IL-10 induced by LPS was only suppressed by Desipramine, but not by Fluoxetine (Figure 5). These results showed that both Fluoxetine and Desipramine can suppress the LPS-induced production of anti- and proinflammatory cytokines as well as chemokines from DCs. In terms of the question addressed in this assay, Fluoxetine and Desipramine did not increase IL-10 expression from DCs irrespective of LPS-stimulation, indicating that IL-10 produced from DCs may not be associated with the Fluoxetine- or Desipramine-mediated suppression of proliferation of antigen-specific T cells induced by DCs.
Figure 5. Effects of Fluoxetine and Desipramine on the production of cytokines and chemokines by LPS-stimulated DC.
DCs were stimulated with or without LPS (1μg/mL) in the presence or absence of Fluoxetine or Desipramine (1 μM) for 24 hours. The concentrations of TNF-α, IL-1β, IL-12, RANTES, MIP-1α and IL-10 in the culture supernatant were measured using ELISA. Results are expressed as the mean ± SD of cytokines/chemokines concentrations (pg/mL) of triplicate cultures. One representative result from three different experiments is shown. *, significantly lower than no drug treatment by Student’s t test (P < 0.01).
Effects of Fluoxetine on IL-10 production from the co-culture of T cells and DCs
It was conceivable that Fluoxetine or Desipramine may act on T cells or DCs with regulatory property to induce their expression of IL-10, which, in turn, down regulated the antigen-specific T cell proliferation induced by DCs. However, the addition of Fluoxetine into the co-culture between DCs and Aa-reactive T cells did not alter the production of IL-10 in the culture (Figure 6). In fact, Desipramine suppressed the production of IL-10 in this co-culture system (Figure 6). These results show that neither Fluoxetine nor Desipramine 1) promoted IL-10 expression from DCs (Figure 5) or 2) increased IL-10 production from co-culture of DCs and Aa-reactive T cells (Figure 4), suggesting that IL-10 produced by either T cells or DCs may not be associated with the drug-mediated suppression of antigen-specific T cell proliferation induced by DCs.
Figure 6. Effects of Fluoxetine and Desipramine on IL-10 production from antigen-specific T cell proliferation induced by DCs.
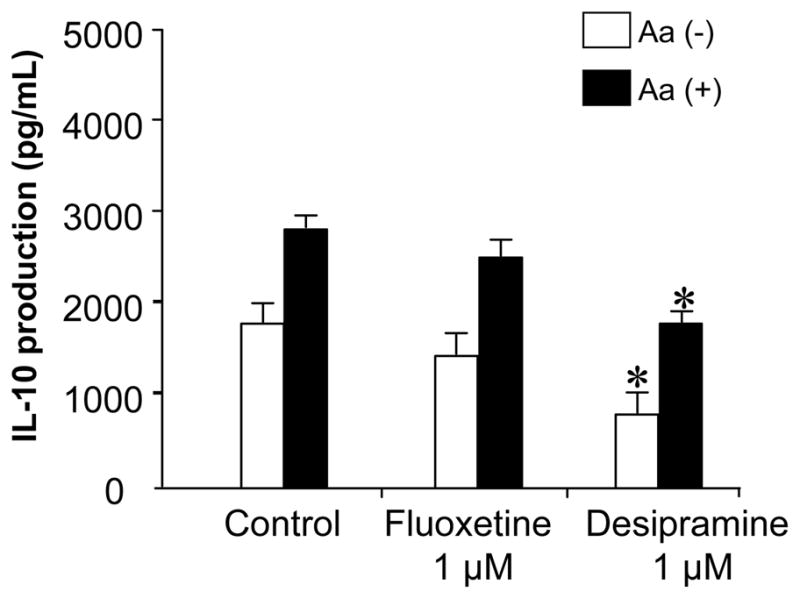
×Aa-T cells (4 × 105 cells/well) were co-cultured with DCs (2 × 104 cells/well, pretreated with MMC) in the presence or absence of Aa (107 fixed bacteria/mL/well). In addition, Fluoxetine or Desipramine (1 μM) was applied to the co-culture. After 3 days, the supernatants were collected from the co-culture for the measurement of IL-10 using ELISA. Results are expressed as the mean ± SD of IL-10 (pg/mL) of triplicate cultures. *, significantly lower than control without drug by Student’s t test (P < 0.01).
Expression profile of DCs surface molecules after exposure to Fluoxetine
The effects of Fluoxetine and Desipramine on the cell surface molecules that are considered to be involved in the antigen presentation by CD11c+ DCs to T cells were monitored using flow cytometry (Figure 1). In the control non-stimulated DCs, the majority (more than 85%) of CD11c cells were positive for CD80, MHC-class-II, ICOS-L and PD-L1, whereas about 30% of CD11c cells expressed CD86, indicating that these CD11c+ DCs induced in ex vivo culture display characteristics of immature DCs. The prevalence of CD86-positive cells in the CD11c+ DCs was increased by addition of Fluoxetine and Desipramine. However, at the same time, among the increased number of total of CD86-positive DCs induced by both drugs, population size of CD86Low cells increased, whereas that of CD86High cells remained same or even diminished (L; CD86Low and H; CD86High, Figure 1), suggesting that both drugs promoted the proportion of immature DCs compared to the mature DCs. Although both drugs appeared to possess marginal effects on the expression of MHC-class-II, PD-L1, and CD80, which showed slight increase, noticeable suppression of ICOS-L was induced by Desipramine and Fluoxetine (Figure 1).
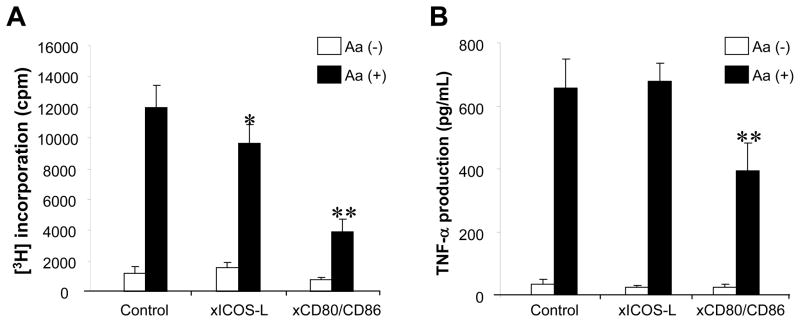
It was questioned if ICOS-L expressed on DCs provides the costimulatory signals to ×Aa-T cells. To test such possibility, Aa–reactive T cells were co-cultured with DCs in the presence or absence of Aa-antigen with or without anti-ICOS-L MAb or anti-CD80/CD86 MAbs. Anti-ICOS-L MAb suppressed antigen-specific T cell growth, albeit to a lesser extent (Student t test, P < 0.05) when compared to anti-CD80/CD86 MAbs that suppressed antigen-specific T cell growth remarkably (Student t test, P < 0.01) (Figure 7A). TNF-α production from Aa–reactive T cells that were co-cultured with DCs and Aa antigen was diminished by anti-CD80/CD86 MAbs, but not by anti-ICOS-L MAb (Figure 7B). These results indicated that down-regulation of ICOS-L expressed on DCs by the anti-depressant drugs might be associated with the drugs’ effects to suppress antigen-specific T cell proliferation, but not the production of TNF-α.
Figure 7. Engagement of ICOS-L co-stimulatory molecule in the ×Aa-T cell responses induced by antigen presentation from DCs.
×Aa-T cells (4 × 105 cells/well) were co-cultured with DCs (2 × 104 cells/well, pretreated with MMC) in the presence or absence of Aa (107 fixed bacteria/mL/well). In addition, anti-mouse MHC-class II Ab MAb (10 μg/mL), anti-ICOS-L MAb (10 μg/mL), a mixture of anti-CD80 MAb and anti-CD86 MAb (10 μg/mL, respectively), or control rat IgG (10μg/mL) was applied to the co-culture. Antigen-specific T cell proliferation (A) and their TNF-α production (B) were monitored following the protocol described in Figure 1. *, **, significantly lower than control cultured with Aa by Student’s t test (P < 0.05, P < 0.01, respectively).
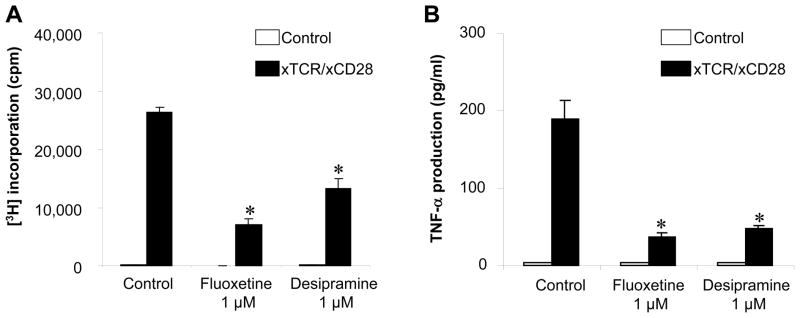
Effects of Fluoxetine on the TCR/CD28-activated T cells responses
Finally, there is a possibility that these drugs also affect T cells directly and suppress their response to antigen-presentation by DCs through TCR/MHC-class-II engagement. Indeed, Fluoxetine and Desipramine significantly inhibited the proliferation of T cells as well as their production of TNF-α in response to TCR/CD28 stimulation (Figure 8). Furthermore, the addition of 5-HT (10 μM) to the TCR/CD28-stimulated T cells did up-regulate T cell proliferation in response to stimulation with anti-TCR/CD28 MAbs (Supplement Figure 2), suggesting that 5-HT can provide additional co-stimulation to the T cell activation induced by TCR/CD28 engagement, but that 5-HT is not responsible for the drug-mediated suppression of TCR/CD28-activated T cells. These results indicated that Fluoxetine and Desipramine appear to down-regulate not only the antigen presenting function of DCs but also suppress the TCR/CD28 elicited T cell activation process by overwhelming the T cell co-stimulatory effects of extracellular 5-HT produced and accumulated by drug-mediated inhibition of 5-HT from SERT.
Figure 8. Effects of Fluoxetine and Desipramine (1 μM) on T-cell proliferation induced by immobilized anti-TCR MAb and anti-CD28 MAb.
Naïve T cells isolated from cervical lymph nodes of C57BL/6 mice were stimulated with immobilized anti-TCRβ MAb (clone: H57-597, Pharmingen) and anti-CD28 MAb (clone: 37.51, Pharmingen) on 9-well culture plate in the presence or absence of Fluoxetine or Desipramine. To measure the proliferation of T cells via engagement of TCR/CD28 activation, 3H-thyimidine was applied to each well for the last 16 hours of a total 4 day culture, and the radio-activities incorporated into the cells under mitosis was monitored by a scintillation counter. TNF-α production from naive T cells stimulated by TCR/CD28 activation was monitored in the culture supernatant harvested after 72 h incubation in the presence of absence of Fluoxetine or Desipramine, using an ELISA for mouse TNF-α. Results are expressed as the mean ± SD of 3H thymidine incorporated into cells (c.p.m.) or cytokines concentrations (pg/mL) of triplicate cultures. No statistical difference was detected between no drug control and the group received each drug. *, significantly lower than control without drug by Student’s t test (P<0.01).
Discussion
The present study demonstrated that Fluoxetine suppresses the ability of DCs to present bacterial (Aa) antigens to Aa-reactive T cells (×Aa-T cells) in a SERT/5-HT-independent manner, as monitored by T cell proliferation and their production of TNF-α, although activated T cells and DC expressed 5-HT and SERT, respectively. SERT/5-HT independency is important, because of following evidence supported that Fluoxetine mediated SERT/5-HT system is vitally affecting the interaction between ×Aa-T cells and DCs; 1) ligation of 5-HT receptors expressed on T cells is reported to activate T cells (Aune et al., 1993; Leon-Ponte et al., 2007), 2) activated DCs express SERT (Figure 4B) (O’Connell et al., 2006), and 3) Fluoxetine increase 5-HT in the co-culture between ×Aa-T cells and DC (Figure 4A), and 4) it is based on the finding that synthetic 5-HT applied to the co-culture of ×Aa-T cells and DCs increased the proliferation and TNF-α production from ×Aa-T cells (Figure 4C), indicating that extracellular 5-HT in the ×Aa-T/DC co-culture can up-regulate T cell proliferation. However, Fluoxetine applied to ×Aa-T/DC co-culture suppressed T cell proliferation and their production of TNF-α by overwhelming the co-stimulatory effects of extracellular 5-HT on T cell response to antigen-presentation by DCs. Similar to Fluoxetine, Desipramine, one of the NRI drugs, also demonstrated suppressive effects on the antigen-presentation function by DCs, supporting a still undefined common nature among antidepressant drugs in the role they play to suppress the ability of DC to present bacterial antigens in a SERT-independent manner. Immune suppressive cytokine IL-10 appeared not to be associated with Fluoxetine- or Desipramine-mediated suppression of proliferation of bacteria-reactive T cells induced by antigen-presentation by DCs because neither drug increased the level of IL-10 produced in the ×Aa-T/DC co-culture. Diminished expression of co-stimulatory molecule ICOS-L on DCs caused by Fluoxetine, as well as Desipramine, appeared to be partially associated with their suppression of antigen-presenting function by DCs.
Initially, the ICOS co-stimulatory molecule was implicated to play a role in Th2-prone T cell activation based on the study using an ICOS-knockout mice (Dong et al., 2001; McAdam et al., 2001; Tafuri et al., 2001). However, subsequent studies demonstrated that ICOS co-stimulation is, indeed, required for both Th1 and Th2 responses (Ozkaynak et al., 2001; Rottman et al., 2001; Smith et al., 2003; Smith et al., 2006). Furthermore, other reports indicated a role of ICOS in supporting memory and effector T cell responses (Shiao et al., 2005; Mahajan et al., 2007). Most recent studies revealed that CXCR5(+)ICOS(+) follicular helper T cells (TFH), a novel T cell subset, play a key helper function to induce B cell differentiation into plasma cells and memory B cells, which, in turn, result in antigen-specific antibody production by B cells (Haynes, 2008). Especially, ICOS expression by CD4 T cells at the time of DC priming is required for TFH differentiation (Crotty, 2011). Knowing that the elevated level of pathogen-specific serum IgG antibody (Ebersole, et al., 1986; Mouton, et al., 1981) and infiltration of RANKL+ B cells in the bone resorption lesion (Kawai, et al., 2006) is characteristic to the periodontal disease, TFH cells are speculated to be involved in the onset and progression of potentially pathogenic B cell-rich lesion in the context of periodontal disease. If such premise, i.e. involvement of TFH in development of pathogenic B cell-rich lesion, is true, Fluoxetine- or Desipramine would offer a novel therapeutic approach for periodontal disease.
Co-stimulation via CD80 and CD86, which interact with CD28 and CTLA-4, is essential for full T-cell activation (Janeway & Bottomly, 1994). Therefore, optimal activation of CD4+ T cells requires the antigen presentation by mature DCs that express MHC-class-II along with sufficient level of CD80/CD86 expression (Harding, et al., 1992). The low level of CD80/CD86 expression is thought to be responsible for diminished antigen presentation capacity to T cells by immature DCs, compared to that mediated by mature DC (Verhoeven, et al., 2000; Delgado, et al., 2004). Immature CD86Low DCs has weaker antigen presenting capacity than mature CD86High DCs (Jin, et al., 2004). Our group also showed that local antigen-specific activation of Th1-type T cells by CD80/CD86 (B7) costimulation appeared to trigger inflammatory bone resorption in a rat model of periodontal disease, whereas inhibition of CD80/CD86 expression by CTLA4-Ig can abrogate the bone resorption induced by Th1-type T cells (Kawai et al., 2000). The results showing that both drugs promoted the proportion of CD86Low DCs compared to CD86High DCs (Figure 1) implicate that increased incidence of immature CD86Low DCs caused by Fluoxetine or Desipramine may be associated with the diminished antigen presentation by the drug-treated DCs to ×Aa-T cells.
Fluoxetine displayed inhibitory effects on the production of TNF-α, IL-1β and IL-12 by immature DCs which were stimulated with LPS (Figure 5). Similar suppressive effects were also detected when Desipramine was applied to the LPS-stimulated immature DCs (Figure 5). Since ×Aa-T cells were Th1 type and since overactivation of Th1-type T cells is associated with RANKL-mediated periodontal bone loss (Taubman et al., 2005), drug-mediated suppression of IL-12, which functions to promote the development and activation of the Th1 type T cells, is considered to contribute to the down-regulation of pathogenic Th1 type responses. These results of Fluoxetine- and Desipramine-mediated suppression of proinflammatory cytokine production by DCs were in accordance with data from previous studies evaluating the influence of NRIs or SSRIs on immune cells (Xia et al., 1996; Maes et al., 1999; Kubera et al., 2001; Roumestan et al., 2007; Guemei et al., 2008). It was also reported that these two drugs can down-regulate the production of pro-inflammatory cytokines from LPS-stimulated monocytes (Roumestan et al., 2007). However, effects of 5-HT on cytokine production by DCs are rather complex. For example, 5-HT decreased TNF-α and IL-12, but promoted IL-1β production, in human DCs (Idzko et al., 2004), which cannot account for the Fluoxetine- and Desipramine-mediated suppression of all three proinflammatory cytokines, i.e., TNF-α, IL-1β, and IL-12, produced by DCs (Figure 4). Indeed, some studies have suggested that there is another possible mechanism inducing Fluoxetine suppression of immune responses in a 5-HT-independent manner (Diamond et al., 2006; Frick et al., 2008). We support the latter theory, i.e., that Fluoxetine suppresses proinflammatory cytokine production from activated DCs in a 5-HT-independent fashion because the addition of exogenous 5-HT to LPS-stimulated DCs did not alter their expression pattern of TNF-α and IL-1β (data not shown).
The present study also analyzed the effects of Fluoxetine and Desipramine on the production of chemokines. Fluoxetine and Desipramine reduced the RANTES and MIP-1α production by LPS-stimulated DCs. RANTES and MIP-1α are produced by variety of immune cells including DCs (Lore et al., 1998), and these two chemokines in concert with other chemotactic factors control chemotaxis of T cells and other leukocytes (Miller andKrangel, 1992; Dieu-Nosjean et al., 1999). Furthermore, chemotaxis of lymphocytes induced by chemokines appears to be engaged in the antigen presentation from DCs to T cells (Caux et al., 2000; Vicari et al., 2004). Therefore, diminished expression of RANTES and MIP-1α from activated DCs in response to Fluoxetine and Desipramine may be also associated with the drug-mediated suppression of antigen-presentation from DCs to T cells.
Based on evidence that 1) antigen-presentation is the key rate-limiting step in the generation of an immune/inflammatory response (Yoshimura et al., 2001) and 2) over-activation of bacteria-reactive Th1-type T cells possibly results in host periodontal tissue destruction (Kawai et al., 2007), regulation of antigen-presenting by DCs, which are the most robust antigen-presenting cells, might be a valid strategy for the down-regulation of tissue destruction caused by immune-associated periodontal disease (Cutler & Jotwani, 2004). The present study demonstrated that Fluoxetine and Desipramine can be potent drugs in down-regulating the activation of bacteria-reactive T cells by suppressing the antigen-presenting function of DCs. Furthermore, Fluoxetine and Desipramine were able to suppress T cell responses induced by TCR/CD28- ligations (Figure 8). Our preliminary study also showed that systemic administration of Fluoxetine inhibited the development of periodontal bone resorption along with the suppression of immune response elicited to oral bacteria (Pasteurella pneumotropica) induced in a mouse model of periodontal disease following the previously published protocol (Kawai et al., 2007).
In conclusion, the present study demonstrated that Fluoxetine and Desipramine suppressed the ability of DCs to present bacterial antigens to T cells and down-regulated the resulting T cell proliferation in a SERT/5-HT-independent manner and that diminished expression of ICOS-L on DCs caused by these two drugs appeared to be partially associated with their suppression of antigen-presenting function by DCs. These findings clearly suggest an interesting potential of such drugs for modulation of the T cell immune responses to bacterial infection in the context of periodontal disease and other conditions, e.g., colitis, where overreaction of T cells to bacteria is thought to be related to host tissue damage.
Supplementary Material
Acknowledgments
This study was supported in part by NIH grants, DE-03420, DE-18499 and DE-19917 from the National Institute of Dental and Craniofacial Research. The authors thank the Brazilian Government Agencies for fellowships to L.S.B.A. (FAPESP 2008/00566-6 and PDEE/CAPES-BEX 4073/08-8).
References
- Abdel-Salam OM, Nofal SM, El-Shenawy SM. Evaluation of the anti-inflammatory and anti-nociceptive effects of different antidepressants in the rat. Pharmacol Res. 2003;48:157–65. doi: 10.1016/s1043-6618(03)00106-3. [DOI] [PubMed] [Google Scholar]
- Aune TM, McGrath KM, Sarr T, Bombara MP, Kelley KA. Expression of 5HT1a receptors on activated human T cells. Regulation of cyclic AMP levels and T cell proliferation by 5-hydroxytryptamine. J Immunol. 1993;151:1175–83. [PubMed] [Google Scholar]
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Caux C, Ait-Yahia S, Chemin K, de Bouteiller O, Dieu-Nosjean MC, Homey B, Massacrier C, Vanbervliet B, Zlotnik A, Vicari A. Dendritic cell biology and regulation of dendritic cell trafficking by chemokines. Springer Semin Immunopathol. 2000;22:345–69. doi: 10.1007/s002810000053. [DOI] [PubMed] [Google Scholar]
- Crotty S. Follicular Helper CD4 T Cells (T(FH)) Annu Rev Immunol. 2011;29:621–63. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- Cutler CW, Jotwani R. Antigen-presentation and the role of dendritic cells in periodontitis. Periodontol 2000. 2004;35:135–57. doi: 10.1111/j.0906-6713.2004.003560.x. [DOI] [PubMed] [Google Scholar]
- Cutler CW, Teng YT. Oral mucosal dendritic cells and periodontitis: many sides of the same coin with new twists. Periodontol 2000. 2007;45:35–50. doi: 10.1111/j.1600-0757.2007.00222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado M, Reduta A, Sharma V, Ganea D. VIP/PACAP oppositely affects immature and mature dendritic cell expression of CD80/CD86 and the stimulatory activity for CD4(+) T cells. J Leukoc Biol. 2004;75:1122–30. doi: 10.1189/jlb.1203626. [DOI] [PubMed] [Google Scholar]
- Diamond M, Kelly JP, Connor TJ. Antidepressants suppress production of the Th1 cytokine interferon-gamma, independent of monoamine transporter blockade. Eur Neuropsychopharmacol. 2006;16:481–90. doi: 10.1016/j.euroneuro.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Dieu-Nosjean MC, Vicari A, Lebecque S, Caux C. Regulation of dendritic cell trafficking: a process that involves the participation of selective chemokines. J Leukoc Biol. 1999;66:252–62. doi: 10.1002/jlb.66.2.252. [DOI] [PubMed] [Google Scholar]
- Dong C, Juedes AE, Temann UA, Shresta S, Allison JP, Ruddle NH, Flavell RA. ICOS co-stimulatory receptor is essential for T-cell activation and function. Nature. 2001;409:97–101. doi: 10.1038/35051100. [DOI] [PubMed] [Google Scholar]
- Ebersole JL, Taubman MA, Smith DJ, Frey DE. Human immune responses to oral microorganisms: patterns of systemic antibody levels to Bacteroides species. Infect Immun. 1986;51:507–513. doi: 10.1128/iai.51.2.507-513.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazzino F, Urbina M, Cedeno N, Lima L. Fluoxetine treatment to rats modifies serotonin transporter and cAMP in lymphocytes, CD4+ and CD8+ subpopulations and interleukins 2 and 4. Int Immunopharmacol. 2009;9:463–7. doi: 10.1016/j.intimp.2009.01.011. [DOI] [PubMed] [Google Scholar]
- Frick LR, Palumbo ML, Zappia MP, Brocco MA, Cremaschi GA, Genaro AM. Inhibitory effect of fluoxetine on lymphoma growth through the modulation of antitumor T-cell response by serotonin-dependent and independent mechanisms. Biochem Pharmacol. 2008;75:1817–26. doi: 10.1016/j.bcp.2008.01.015. [DOI] [PubMed] [Google Scholar]
- Guemei AA, El Din NM, Baraka AM, El Said Darwish I. Do desipramine [10,11-dihydro-5-[3-(methylamino) propyl]-5H-dibenz[b,f]azepine monohydrochloride] and fluoxetine [N-methyl-3-phenyl-3-[4-(trifluoromethyl)phenoxy]-propan-1-amine] ameliorate the extent of colonic damage induced by acetic acid in rats? J Pharmacol Exp Ther. 2008;327:846–50. doi: 10.1124/jpet.108.141259. [DOI] [PubMed] [Google Scholar]
- Han X, Lin X, Seliger AR, Eastcott J, Kawai T, Taubman MA. Expression of receptor activator of nuclear factor-kappaB ligand by B cells in response to oral bacteria. Oral Microbiol Immunol. 2009;24:190–6. doi: 10.1111/j.1399-302X.2008.00494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding FA, McArthur JG, Gross JA, Raulet DH, Allison JP. CD28-mediated signaling co-stimulates murine T cells and prevents induction of anergy in T-cell clone. Nature. 1992;356:607–609. doi: 10.1038/356607a0. [DOI] [PubMed] [Google Scholar]
- Hashioka S, McGeer PL, Monji A, Kanba S. Anti-inflammatory effects of antidepressants: possibilities for preventives against Alzheimer’s disease. Cent Nerv Syst Agents Med Chem. 2009;9:12–9. doi: 10.2174/187152409787601897. [DOI] [PubMed] [Google Scholar]
- Haynes NM. Follicular associated T cells and their B-cell helper qualities. Tissue Antigens. 2008;71:97–104. doi: 10.1111/j.1399-0039.2007.00995.x. [DOI] [PubMed] [Google Scholar]
- Idzko M, Panther E, Stratz C, et al. The serotoninergic receptors of human dendritic cells: identification and coupling to cytokine release. J Immunol. 2004;172:6011–9. doi: 10.4049/jimmunol.172.10.6011. [DOI] [PubMed] [Google Scholar]
- Janeway CA, Jr, Bottomly K. Signals and signs for lymphocyte responses. Cell. 1994;76:275–85. doi: 10.1016/0092-8674(94)90335-2. [DOI] [PubMed] [Google Scholar]
- Jin Y, Fuller L, Ciancio G, Burke GW, 3rd, Tzakis AG, Ricordi C, Miller J, Esquenzai V. Antigen presentation and immune regulatory capacity of immature and mature-enriched antigen presenting (dendritic) cells derived from human bone marrow. Hum Immunol. 2004;65:93–103. doi: 10.1016/j.humimm.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Kajiya M, Sato K, Silva MJ, Ouhara K, Do PM, Shanmugam KT, Kawai T. Hydrogen from intestinal bacteria is protective for Concanavalin A-induced hepatitis. Biochem Biophys Res Commun. 2009;386:316–21. doi: 10.1016/j.bbrc.2009.06.024. [DOI] [PubMed] [Google Scholar]
- Kawai T, Eisen-Lev R, Seki M, Eastcott JW, Wilson ME, Taubman MA. Requirement of B7 costimulation for Th1-mediated inflammatory bone resorption in experimental periodontal disease. J Immunol. 2000;164:2102–9. doi: 10.4049/jimmunol.164.4.2102. [DOI] [PubMed] [Google Scholar]
- Kawai T, Matsuyama T, Hosokawa Y, Makihira S, Seki M, Karimbux NY, Goncalves RB, Valverde P, Dibart S, Li YP, Miranda LA, Ernst CW, Izumi Y, Taubman MA. B and T lymphocytes are the primary Sources of RANKL in the bone resorptive lesion of periodontal isease. Am J Pathol. 2006;169:987–998. doi: 10.2353/ajpath.2006.060180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T, Paster BJ, Komatsuzawa H, Ernst CW, Goncalves RB, Sasaki H, Ouhara K, Stashenko PP, Sugai M, Taubman MA. Cross-reactive adaptive immune response to oral commensal bacteria results in an induction of receptor activator of nuclear factor-kappaB ligand (RANKL)-dependent periodontal bone resorption in a mouse model. Oral Microbiol Immunol. 2007;22:208–15. doi: 10.1111/j.1399-302X.2007.00348.x. [DOI] [PubMed] [Google Scholar]
- Kubera M, Lin AH, Kenis G, Bosmans E, van Bockstaele D, Maes M. Anti-Inflammatory effects of antidepressants through suppression of the interferon-gamma/interleukin-10 production ratio. J Clin Psychopharmacol. 2001;21:199–206. doi: 10.1097/00004714-200104000-00012. [DOI] [PubMed] [Google Scholar]
- Leon-Ponte M, Ahern GP, O’Connell PJ. Serotonin provides an accessory signal to enhance T-cell activation by signaling through the 5-HT7 receptor. Blood. 2007;109:3139–46. doi: 10.1182/blood-2006-10-052787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesche WJ, Grossman NS. Periodontal disease as a specific, albeit chronic, infection: diagnosis and treatment. Clin Microbiol Rev. 2001;14:727–52. doi: 10.1128/CMR.14.4.727-752.2001. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lore K, Sonnerborg A, Spetz AL, Andersson U, Andersson J. Immunocytochemical detection of cytokines and chemokines in Langerhans cells and in vitro derived dendritic cells. J Immunol Methods. 1998;214:97–111. doi: 10.1016/s0022-1759(98)00040-4. [DOI] [PubMed] [Google Scholar]
- Maes M, Song C, Lin AH, Bonaccorso S, Kenis G, De Jongh R, Bosmans E, Scharpe S. Negative immunoregulatory effects of antidepressants: inhibition of interferon-gamma and stimulation of interleukin-10 secretion. Neuropsychopharmacology. 1999;20:370–9. doi: 10.1016/S0893-133X(98)00088-8. [DOI] [PubMed] [Google Scholar]
- Mahajan S, Cervera A, MacLeod M, Fillatreau S, Perona-Wright G, Meek S, Smith A, MacDonald A, Gray D. The role of ICOS in the development of CD4 T cell help and the reactivation of memory T cells. Eur J Immunol. 2007;37:1796–808. doi: 10.1002/eji.200636661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdam AJ, Greenwald RJ, Levin MA, Chernova T, Malenkovich N, Ling V, Freeman GJ, Sharpe AH. ICOS is critical for CD40-mediated antibody class switching. Nature. 2001;409:102–5. doi: 10.1038/35051107. [DOI] [PubMed] [Google Scholar]
- Miller MD, Krangel MS. Biology and biochemistry of the chemokines: a family of chemotactic and inflammatory cytokines. Crit Rev Immunol. 1992;12:17–46. [PubMed] [Google Scholar]
- Mouton C, Hammond PG, Slots J, Genco RJ. Serum antibodies to oral Bacteroides asaccharolyticus (Bacteroides gingivalis): relationship to age and periondontal disease. Infect Immun. 1981;31:182–192. doi: 10.1128/iai.31.1.182-192.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell PJ, Wang X, Leon-Ponte M, Griffiths C, Pingle SC, Ahern GP. A novel form of immune signaling revealed by transmission of the inflammatory mediator serotonin between dendritic cells and T cells. Blood. 2006;107:1010–7. doi: 10.1182/blood-2005-07-2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozkaynak E, Gao W, Shemmeri N, Wang C, Gutierrez-Ramos JC, Amaral J, Qin S, Rottman JB, Coyle AJ, Hancock WW. Importance of ICOS-B7RP-1 costimulation in acute and chronic allograft rejection. Nat Immunol. 2001;2:591–6. doi: 10.1038/89731. [DOI] [PubMed] [Google Scholar]
- Rottman JB, Smith T, Tonra JR, Ganley K, Bloom T, Silva R, Pierce B, Gutierrez-Ramos JC, Ozkaynak E, Coyle AJ. The costimulatory molecule ICOS plays an important role in the immunopathogenesis of EAE. Nat Immunol. 2001;2:605–11. doi: 10.1038/89750. [DOI] [PubMed] [Google Scholar]
- Roumestan C, Michel A, Bichon F, Portet K, Detoc M, Henriquet C, Jaffuel D, Mathieu M. Anti-inflammatory properties of desipramine and fluoxetine. Respir Res. 2007;8:35–45. doi: 10.1186/1465-9921-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour GJ, Ford PJ, Cullinan MP, Leishman S, Yamazaki K. Relationship between periodontal infections and systemic disease. Clin Microbiol Infect. 2007;13(Suppl 4):3–10. doi: 10.1111/j.1469-0691.2007.01798.x. [DOI] [PubMed] [Google Scholar]
- Shiao SL, McNiff JM, Pober JS. Memory T cells and their costimulators in human allograft injury. J Immunol. 2005;175:4886–96. doi: 10.4049/jimmunol.175.8.4886. [DOI] [PubMed] [Google Scholar]
- Smith KM, Brewer JM, Webb P, Coyle AJ, Gutierrez-Ramos C, Garside P. Inducible costimulatory molecule-B7-related protein 1 interactions are important for the clonal expansion and B cell helper functions of naive, Th1, and Th2 T cells. J Immunol. 2003;170:2310–5. doi: 10.4049/jimmunol.170.5.2310. [DOI] [PubMed] [Google Scholar]
- Smith KM, Garside P, McNeil RC, Brewer JM. Analysis of costimulatory molecule expression on antigen-specific T and B cells during the induction of adjuvant-induced Th1 and Th2 type responses. Vaccine. 2006;24:3035–43. doi: 10.1016/j.vaccine.2005.10.027. [DOI] [PubMed] [Google Scholar]
- Tafuri A, Shahinian A, Bladt F, et al. ICOS is essential for effective T-helper-cell responses. Nature. 2001;409:105–9. doi: 10.1038/35051113. [DOI] [PubMed] [Google Scholar]
- Taubman MA, Valverde P, Han X, Kawai T. Immune response: the key to bone resorption in periodontal disease. J Periodontol. 2005;76:2033–41. doi: 10.1902/jop.2005.76.11-S.2033. [DOI] [PubMed] [Google Scholar]
- Teng YT, Mahamed D, Singh B. Gamma interferon positively modulates Actinobacillus actinomycetemcomitans-specific RANKL+ CD4+ Th-cell-mediated alveolar bone destruction in vivo. Infect Immun. 2005;73:3453–61. doi: 10.1128/IAI.73.6.3453-3461.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoeven GT, Van Haarst JM, De Wit HJ, Simons PJ, Hoogsteden HC, Drexhage HA. Glucocorticoids hamper the ex vivo maturation of lung dendritic cells from their low autofluorescent precursors in the human bronchoalveolar lavage: decreases in allostimulatory capacity and expression of CD80 and CD86. Clin Exp Immunol. 2000;122:232–40. doi: 10.1046/j.1365-2249.2000.01354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicari AP, Vanbervliet B, Massacrier C, et al. In vivo manipulation of dendritic cell migration and activation to elicit antitumour immunity. Novartis Found Symp. 2004;256:241–54. doi: 10.1002/0470856734.ch18. discussion 254–69. [DOI] [PubMed] [Google Scholar]
- Xia Z, DePierre JW, Nassberger L. Tricyclic antidepressants inhibit IL-6, IL-1 beta and TNF-alpha release in human blood monocytes and IL-2 and interferon-gamma in T cells. Immunopharmacology. 1996;34:27–37. doi: 10.1016/0162-3109(96)00111-7. [DOI] [PubMed] [Google Scholar]
- Yaron I, Shirazi I, Judovich R, Levartovsky D, Caspi D, Yaron M. Fluoxetine and amitriptyline inhibit nitric oxide, prostaglandin E2, and hyaluronic acid production in human synovial cells and synovial tissue cultures. Arthritis Rheum. 1999;42:2561–8. doi: 10.1002/1529-0131(199912)42:12<2561::AID-ANR8>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Yoshimura S, Bondeson J, Foxwell BM, Brennan FM, Feldmann M. Effective antigen presentation by dendritic cells is NF-kappaB dependent: coordinate regulation of MHC, co-stimulatory molecules and cytokines. Int Immunol. 2001;13:675–83. doi: 10.1093/intimm/13.5.675. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.