Abstract
Bone morphogenetic signaling (BMP) is a key pathway during neurogenesis and depends on many downstream intermediators to carry out its signaling. One such signaling pathway utilizes neurotrophin receptor-interacting MAGE protein (NRAGE), a member of the melanoma-associated antigen (MAGE) family, to upregulate p38 mitogen activated protein kinase (p38MAPK) in response to cellular stress and activate caspases which are critical in leading cells to death. NRAGE consists of two conserved MAGE homology domains separated by a unique hexapeptide repeat domain. Although we have previously implicated NRAGE in inducing apoptosis in neural progenitors and P19 cells, a model system for neural progenitors, its domains have yet to be explored in determining which one may be responsible for setting up the signaling for apoptosis. Here, we overexpressed a series of deletion mutations in P19 cells to show that only those with at least half of the repeat domain, activated p38MAPK and underwent apoptosis offering intriguing incite into NRAGE’s contribution in BMP apoptotic signaling.
Keywords: NRAGE, BMP, MAGE, p38MAPK, Caspase, Apoptosis
Introduction
Neurotrophin receptor-interacting MAGE homolog (NRAGE) was originally identified in a yeast two-hybrid screen searching for proteins that interacted with the intracellular domain of the apoptosis related low affinity nerve growth factor p75 neurotrophin receptor (p75NTR) [1]. Concurrent with our cloning of NRAGE from rat screening, two similar proteins, MAGE-D1 [2] and Dlxin-1 [3] cloned from human and mouse screens respectively, were orthologs of NRAGE with 87% identity to the human MAGE-D1 [1]. MAGE gene family members encode tumor specific antigens that act as anti-tumoral immune targets [4] recognized by autologous cytotoxic T lymphocytes which can mediate rejection responses [5]. Moreover, NRAGE has been recently shown to suppress metastasis of melanoma and pancreatic cancer in vitro and in vivo [6]. NRAGE has also been demonstrated to regulate p53 transcriptional activity and inhibit cell proliferation [7].
NRAGE is a 775 amino acid protein with a predicted weight of about 87 kDa and co-immunoprecipitates with p75NTR. We mapped the interaction to the juxtamembrane region, exclusive of the presumptive death domain within p75 and demonstrated that NRAGE facilitated nerve growth factor (NGF)-dependent apoptosis in sympathetic neuron precursor cells [1]. Subsequently, work from the Barker laboratory suggested that part of NRAGE’s apoptotic activity was mediated though a Jun N-terminal kinase (JNK)-dependent mitochondrial pathway [1, 8, 9].
Recently, we demonstrated that NRAGE functions independently of p75NTR in modulating neural apoptosis and glial differentiation of BMP sensitive cortical precursors [10, 11]—the same set of BMP sensitive neural progenitors that may underlie the genesis of brain tumor stem cells [12] and references within. This mechanism was independent of p75 and canonical (SMAD-dependent) BMP signaling and instead utilized the non-canonical SMAD-independent pathway by forming a complex with TAK1, TAB 1, and XIAP facilitating p38 map kinase (p38 MAPK) activation [10]. This pathway was also confirmed by us in BMP7-treated mIMCD-3 cells, an accepted model for the kidney ureteric bud cellular environment, in which activation of p38MAPK was inhibited using an NRAGE morpholino [13]. Jordan and colleagues initially demonstrated that the binding of NRAGE to the C-terminal RING domain of IAPs induces cleavage of XIAP, increases caspase-mediated cell death, and presumably increases p38 activation or that NRAGE can function independently to promote caspase activation [14].
NRAGE is comprised of two MAGE homology domains (MHD) separated by 25 hexapeptide repeats. The MHD at the C-terminus is common to all MAGE genes and is highly conserved in many multicellular organisms. It is the only domain present in Necdin which may play a role in Prader-Willi syndrome, a neurogenetic disorder. A second MHD (MHD2) at the N-terminus is common to some MAGE-D family members with no significant homology to other proteins. The repeat domain, located between the MAGE homology domains, is not present in other proteins although is conserved in NRAGE homologs in mouse, rat, and human [15]. Definitive functions of the MHD2 and the repeat domain have not been published. Here, we report the structure–function relationship of this pro-apoptotic cancer related protein. By creating a series of NRAGE deletion mutations, we were able to better dissect the functional domains of NRAGE that are responsible for BMP-responsive apoptosis. We found that the MHD2 was not necessary for BMP-induced p38MAPK activation or caspase-mediated apoptosis and that apoptosis was ameliorated when the unique hexapeptide repeat region was eliminated. These studies show that it is not the classic MHD that could make a potential therapeutic target but the repeat region that holds clinical and pharmaceutical potential in regulating cell viability and needs to be explored further.
Results
The hexapeptide repeat domain is necessary to induce cell death
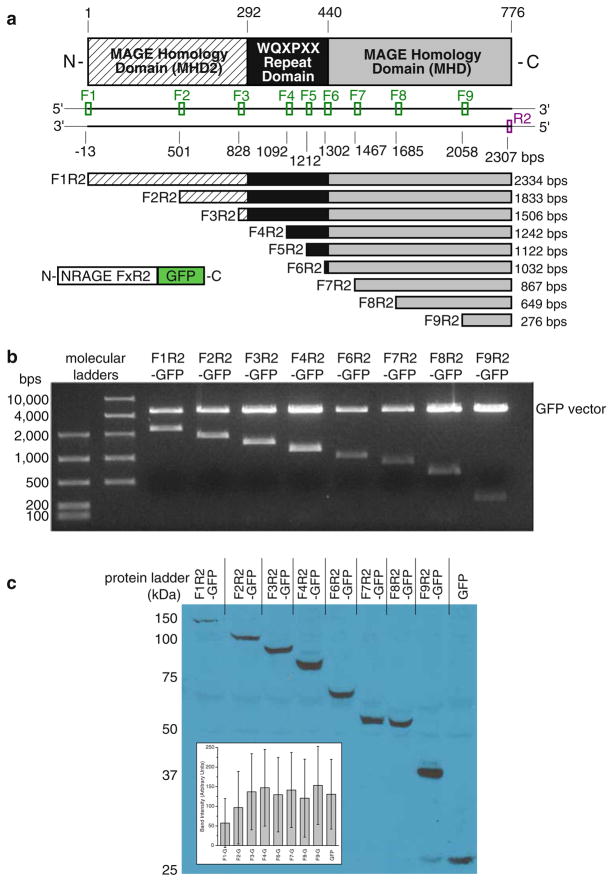
Although we previously showed that overexpressed full-length NRAGE can induce apoptosis in P19 cells [16], we wished to elucidate the domains of NRAGE to further examine their effects on apoptosis. As NRAGE expression is correlated with changes in mitotic activity and cell viability and numerous proteins have been shown to interact with NRAGE to modulate these functions, we created a series of NRAGE deletion mutations in order to screen for their ability to induce apoptosis and proliferation. As depicted in Fig. 1a, our NRAGE deletion mutations leave the C-terminus intact and incrementally delete from the N-terminus and were created in frame with green fluorescent protein (see Methods). Nucleic acid digests (Fig. 1b) and protein distributions in HEK293 (Fig. 1c), P19, 293T, and N2A cells (data not shown) produce a cascade of decreasing construct sizes.
Fig. 1.
NRAGE deletion mutations shown by schematic (a) where “Fx” is the respective forward primer, restriction enzyme digest of nucleic acid (b), and protein distribution normalized to even protein concentration in HEK293 total cell lyses (c). (F5R2 was not accomplished.)
We sought to provoke changes in apoptosis and transfected the NRAGE-EGFP constructs into P19 cells. Since NRAGE is a unique member of the MAGE superfamily because it contains the repeat domain, we theorized that this region would be critical in BMP induced apoptosis. Although addition of BMP4 can accelerate the timeline of apoptosis with NRAGE overexpression, we elected to wait (~48 h) to assay for apoptosis in order to demonstrate the true behavior of NRAGE without any BMP supplementation. As shown in Fig. 2 from staining of three independent transfections, similar to over-expression of full-length NRAGE [10], NRAGE F1R2-EGFP and deletion mutations F2R2 through F4R2, showed roughly an average of 40% of GFP cells to be Annexin V-PE positive 48 h after transfection (~60% survival) when expressed in P19 cells (Fig. 2a). When comparing this group to the negative controls, untransfected and GFP vector (no fusion) transfected P19 cells, the difference was very significant (P < 0.0005) and was only slightly less significant when F6R2 was included (P < 0.005) before becoming insignificant with F7R2 included. This result was similar to results previously reported by Kendall for full-length NRAGE in P19 cells and murine cortical progenitors [10] and was surprising, as the highly conserved MHD2 domain did not appear to be necessary to alter cell viability (F3R2 and F4R2, Fig. 2a). Meanwhile, F7R2, F8R2, and F9R2 had a much lower incidence of Annexin V-PE staining and was not statistically significant when compared to negative controls. This suggests that at least the repeat domain of NRAGE contributes to the effect of apoptosis in P19 cells. Conversely, at 48 h post-transfection with the NRAGE-GFP series of constructs, P19 cells were assessed for incorporation of bromodeoxyuridine (BrdU) as a measure of DNA synthesis and proliferation and analyzed by flow cytometry. There was suppressed proliferation of cells with over-expression of full-length NRAGE (F1R2), F2R2, F3R2, F4R2, F6R2, and F7R2 (P < 0.0005), whereas F8R2 and F9R2 were more alike with untransfected and GFP vector transfected cells (P = 0.1867; Fig. 2b).
Fig. 2.
Apoptosis (a) and proliferation (b) of P19 cells as assessed by Annexin V-PE and BrdU incorporation, respectively, from NRAGE-EGFP deletion mutation transfections (** P <0.0005 and * P < 0.005 to negative controls)
The MHD is necessary to activate the p38MAPK pathway
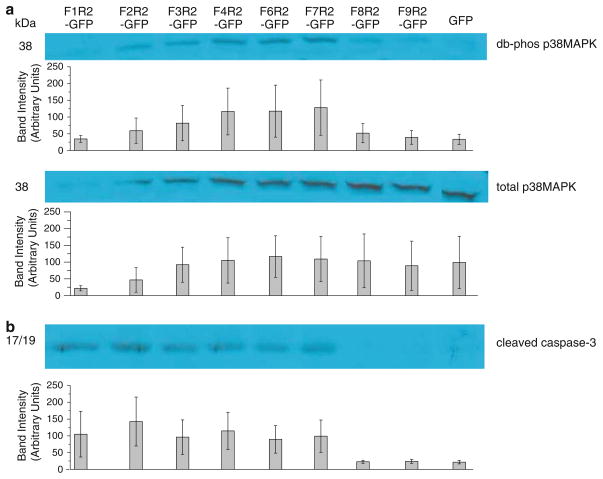
NRAGE can control progenitor viability by both the JNK [1, 8] and the p38MAPK pathway [10]. Therefore, it was possible that the death we observed was not, as we predicted, mediated through the non-canonical BMP signaling pathway but by some other pathway leading to caspase activation. Western analysis of P19 total cell lysates transfected with F1R2- through F9R2-EGFP were conducted and probed for activated (cleaved) caspase-3 and activated (double-phosphorylated) p38MAPK. Detection for double-phosphorylated and total p38MAPK was very low for cells transfected with F1R2-EGFP (Fig. 3a), which was consistent with previous transfections where full-length NRAGE-EGFP overexpression is considerably diminished compared to smaller constructs (for example, Fig. 1c). Cleaved caspase-3 was detected in P19 total cell lyses for F1R2 through F7R2 where most of the MHD is present. Here, the possibility of a diminished transfection efficiency for full-length F1R2-EGFP may still be enough to set off a caspase cascade as effective as smaller constructs F2R2 through F7R2 (Fig. 3b). Collectively, these results suggest that the MHD is necessary for death to occur by this pathway, correlating well with our results for apoptosis and proliferation. Taken together with the results of the repeat domain being necessary for cell viability, it is possible that one series of events involving the repeat domain may trigger another series of events involving the MHD leading to the demise of cells.
Fig. 3.
Apoptotic indicators double phosphorylated p38MAPK (a) and activated cleaved caspase-3 (b) protein levels per western blotting in P19 total cell lyses after being overexpressed with the NRAGE deletion mutations
Discussion
NRAGE is a member of the MAGE family of proteins which are expressed not only in melanoma, but also in other malignant tumors [17]. Some antigens coded by the MAGE genes are potentially useful for cancer-specific immunotherapy renewing their interest to clinicians and basic scientists alike. NRAGE is ubiquitously expressed throughout development [11] and induces apoptosis in a variety of cell types [6, 10, 13] suggesting its importance as an adaptor protein. Herein we report on the structure–function relationship of NRAGE in mediating apoptosis in P19 cells. NRAGE-EGFP overexpression of F1R2 through F4R2 (containing at least half of the repeat domain) in P19 cells showed the most death by apoptosis and F7R2 through F9R2 (no repeat domain) showed the least with F6R2 being inconclusive for either group. F6R2 does contain a very small portion of the repeat domain (6 amino acids or 1 hexapeptide repeat; Fig. 1a) which could ascribe to its uncertainty. Results from inclusion of BrdU with the same transfection regime were lowest for NRAGE F1R2-through F7R2-EGFP which mostly reinforced that there is an inverse relationship between proliferation and apoptosis.
We found that overexpression of NRAGE in which most of the MHD was present drew consistent levels of cleaved caspase-3 (Fig. 3b) making it reasonable to surmise that the MHD is required for caspase-3 activation. Caspase-3 is one of the key executioners of apoptosis, as it is either partially or totally responsible for the proteolytic cleavage of many key proteins [18]. XIAP inhibits active caspase-3 by directly blocking downstream events such as further activation of caspases [19, 20]. We have shown that NRAGE interacts with XIAP with and without BMP4 treatment and may serve to prevent XIAP from carrying out its inhibitory behavior of caspases [10].
When NRAGE was overexpressed in P19 cells, all of the constructs which contained all or part of the repeat domain (F2R2 through F6R2) and most of the MHD (F7R2) yielded detectable levels of activated p38MAPK excluding full-length NRAGE (Fig. 3a). Typically, we experienced low EGFP fluorescence for full-length NRAGE-GFP transfections which could easily translate into a cellular signal too low to trigger p38MAPK phosphorylation explaining a lack of detectable activated and total p38 levels. NRAGE functions as an adaptor protein for the XIAP-TAB 1-TAK1 complex that allows TAB 1 to activate TAK1. In turn, TAK1 activates p38MAPK [21, 22] and phosphorylated p38MAPK correlates with cellular apoptosis in neural progenitor and P19 cells [10]. Conversely, a decrease in activated p38MAPK results in decreased apoptosis in E11.5 mouse kidney explants [13]. Taken together, overexpression of NRAGE deletion mutations containing at least the repeat domain upregulate activation of caspase-3 and p38MAPK correlating with apoptosis in P19 cells. In order to determine this, deletion mutations will need to be studied in which the N-terminus is intact and the C-terminus truncated. These mutations would be similar to NRAGE clones identified in a yeast two-hybrid screening for interaction with XIAP in which 32D cells stably expressing anti-apoptotic Bcl-2 were infected with NRAGE retrovirus and underwent accelerated cell death [14].
The next logical step would be to determine binding between the N-terminus NRAGE deletion mutations and BMP non-canonical members XIAP, TAB 1, and TAK1 to further implicate specific domains instrumental in apoptotic signaling. However, because protein expression for full-length NRAGE-EGFP and even F2R2-EGFP are low (inset, Fig. 1c), detection for interactions by western blotting may be difficult as they are for downstream effectors such as activated p38MAPK (Fig. 3a). Establishing stable cells lines overexpressing each NRAGE-EGFP would also seem sensible except that overexpression of some of these constructs induces cell loss. Therefore, consideration will be needed in selecting an approach. For example, as with the apoptosis assay where only GFP + NRAGE deletion mutations were assessed, fluorescence resonance energy transfer (FRET) between donor (EGFP) tagged NRAGE constructs and acceptor tagged non-canonical members could be used on cells that are positive for donor and acceptor fluorescence. FRET would also help determine if interactions are cytoplasmic or nuclear. Additionally, an indication of FRET signals would suggest direct interactions between proteins whereas western blotting does not discern between direct or indirect relationships.
Methods
Cell culture
P19, HEK293, 293T, and N2A cells were purchased from American Type Culture Collection (ATCC) and maintained per ATCC specifications.
NRAGE cloning
An adult brain was dissected from a female wild-type ICR mouse, freed of meninges, washed twice with sterile PBS, minced, and homogenized. RNA was isolated using Trizol reagent (Invitrogen) and cDNA was reverse transcribed using iScript reaction mix (Bio-Rad) following manufacturers’ instructions. NRAGE cDNA was verified to accession # NM_019791 (National Center for Biotechnology Information) and fragments were PCR’d using High Fidelity AccuPrime Taq DNA polymerase (Invitrogen). The domains of NRAGE were identified by the protein sequence and whenever possible native methioninones were used as the starting codon for each construct. Forward primers (F1–F9) were designed so that they would contain a Kozak consensus start site to facilitate translation [23, 24]. Reverse primer R2 was designed to contain a Sal I restriction enzyme cut site. Primer sequences and PCR cycles are given in Tables 1 and 2, respectively. Each NRAGE PCR product was cloned into a pCR-Blunt II-TOPO plasmid vector (Invitrogen), excised using Sac I and Sal I restriction enzymes (New England BioLabs), and cloned in frame into pEGFP-N3 plasmid vector (Clontech) so that the N-terminus of the EGFP tag was fused to the C-terminus of each NRAGE deletion mutation (EGFP downstream of NRAGE DNA) and denoted as NRAGE FxR2-EGFP where “Fx” is the respective forward primer. F1R2 encodes the complete NRAGE coding sequence while each subsequent mutation takes off an incrementally larger portion of the N terminal sequence (see schematic in Fig. 1). All constructs were verified by sequencing and by exhibiting EGFP fluorescence emission as viewed from an Axiovert 200 microscope (Carl Zeiss) with fluorescent filters 470/40 BP, 495 FT, 525/50 BP (Carl Zeiss).
Table 1.
NRAGE PCR primer sequences
| Mutation | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| F1R2 | GAGACGCTCGAGATGGCTCAGA AACCG | GACAATTTAG TCGACCCAGAAGAAGCC |
| F2R2 | AGTGCCCTCGAGATGCCAAACAACCAG |
|
| F3R2 | GTGACCCTCGAGATGGACCCACCTGGAGCA | |
| F4R2 | TGGCCACTCGAGATGGCCTGGCAGAGTACA | |
| F5R2 | CCTGACCTCGAGATGCCTCCTGACTGG | |
| F6R2 | GACTGGCTCGAGATGGGACCCTCACCTAA | |
| F7R2 | AAGCGCCTCGAGATGGTGAGGGATATCATC | |
| F8R2 | GGCATTCTCGAGATGGATGGCAACCGTGCC | |
| F9R2 | ATTGAGCTCGAGATGGGAATTGGAGATGAG |
Table 2.
NRAGE PCR cycles
| Mutation | Incubation | 30 cycles
|
Hold | ||
|---|---|---|---|---|---|
| Denature | Anneal | Extend | |||
| F1R2 | 1 min., 94 °C | 30 sec., 94 °C | 30 sec., 54 °C | 2 min. 30 sec., 68 °C | 4 °C |
| F2R2 |
|
|
|
2 min., 68 °C |
|
| F3R2 | 1 min. 50 sec., 68 °C | ||||
| F4R2 | 1 min. 50 sec., 68 °C | ||||
| F5R2 | 1 min. 20 sec., 68 °C | ||||
| F6R2 | 1 min. 20 sec., 68 °C | ||||
| F7R2 | 1 min., 68 °C | ||||
| F8R2 | 1 min., 68 °C | ||||
| F9R2 | 30 sec., 68 °C | ||||
Transfections
Cells were seeded in 6-well plates and transfected at 70–80% confluency with 8 μg NRAGE-EGFP:10 μl Lipofectamine 2000 (Invitrogen) in serum-free media following manufacturer’s protocol.
Western blots
Dishes of transfected cells were placed on ice, washed with ice cold PBS, and incubated with ice cold RIPA buffer [150 mM NaCl, 10 mM Tris pH 7.2, 0.1% SDS, 1% Triton X-100, 1% deoxycholate, 5 mM EDTA, and protease inhibitor cocktail (Sigma, P8340)] for 30 min. Cells were then triturated and aspirated into 1.5 ml centrifuge tubes, rocked for an additional 30 min at 4°C, and centrifuged at 10,000g for 10 min at 4°C. Protein concentrations were determined for even protein loading using BCA protein assay kit (Pierce), run on 8 or 10% SDS-PAGE, and transferred to nitrocellulose membrane (Amersham Biosciences). Membranes were washed in TBS 1% Tween-20 (TBST) and blocked in TBST with 5% BSA (for phosphospecific detection) or 5% non-fat dried milk for 1–2 h at room temperature. Primary antibodies used were rabbit anti-GFP antibody (Santa Cruz), rabbit anti-p38 antibody (Cell Signaling), rabbit anti-double phosphorylated p38 antibody (Cell Signaling), rabbit anti-cleaved caspase-3 antibody (Cell Signaling) applied with rocking overnight at 4°C. Secondary antibody was HRP conjugated goat anti-rabbit IgG (Bio-Rad) and SuperSignal chemiluminescent substrate (Pierce) was used to detect HRP signal on HyBlot CL (Denville Scientific) or Hyperfilm (Amersham) autoradiography film.
Apoptosis assay
Cells were trypsinized and spun at 1,200 rpm for 3 min to pellet. Cells were counted and diluted to 1 million per ml in antibody binding buffer included in the apoptosis detection kit (BD Biosciences). For controls, 100 μl of cells were placed in 5 ml culture tubes with 5 μl of either Annexin V-PE or 7AAD or both and incubated in the dark for at least 20 min. 250 μl of sample cells were incubated with 15 μl of each apoptosis marker for at least 20 min in the dark. Upon completion of the incubation period, cells were diluted in 400 μl (control) or 750 μl (sample) of binding buffer and analyzed by flow cytometry using the Becton-Dickinson FASCCalibur flow cytometer and Cell Quest software version 3.3. GFP + cells were gated and the incorporation of Annexin V-PE and 7AAD by the cells read. Gates were determined through the use of untransfected and unstained cells GFP and PE were excited by the Argon 488 nm laser and GFP read on the FL1 channel and PE on the FL2 channel.
Proliferation assay
P19 cells were transfected as described above then on the morning of day 2 (24 h post transfection) pulsed with 10 μg/ml BrdU for 2 h. Cells were detached from the plate using 10 mM EDTA for 2 min at room temperature. 1 ml of media was added and the cells triturated off the plate into 15 ml conical tubes. Cells were spun at 400 rpm for 10 min to pellet. Media was removed and cells resuspended in 0.5% BSA in PBS (wash buffer) at a concentration of 1 million per 100 μl as recommended by BD Biosciences. Cells were pelleted again and fixed in ice cold 70% ethanol for 10 min. The fixation solution was diluted with 500 μl wash buffer and cells pelleted. Cells were then treated with 100 μl of 2 M HCl for 10 min to release the DNA from histones. Centrifugation at 400 rpm was used to pellet the cells and clear the acid; residual acid was neutralized with 0.2 M sodium borate for 3 min. Cells were washed again, pelletted, and resuspended in wash buffer plus 0.5% goat serum. A 1:50 dilution of BrdU-Alexa647 and GFP-Alexa488 antibodies (Molecular Probes) were added and incubated for 20 min at room temperature in the dark. The cells were then washed twice to remove residual antibody, and analyzed by flow cytometry.
Statistics
Apoptosis and proliferation assays were each performed 3 times on P19 cells with graphs depicting averages and standard deviations created by Origin (Microcal) software. P values were calculated by single factor ANOVA performed in Excel (Microsoft).
Acknowledgments
This work was supported by National Institutes of Health (NIH) grant R01NS055304 awarded to JMV and NIH Center of Biomedical Research Excellence (COBRE) P20RR018789 in Stem Cell Biology and Regenerative Medicine to Maine Medical Center (Portland, Maine). J.A.R. was funded by the Integrative Graduate Education Research Training (IGERT) Functional Genomics Ph.D. program through a fellowship from National Science Foundation grant 0221625 awarded to the University of Maine (Orono, Maine), Maine Medical Center Research Institute (Scarborough, Maine), and the Jackson Laboratory (Bar Harbor, Maine).
Contributor Information
Jennifer A. Rochira, IGERT Functional Genomics Ph.D. Program, University of Maine, 267A Engineering Science and Research Building/Barrows Hall, Orono, ME 04469, USA. Maine Medical Center Research Institute, Center for Molecular Medicine, 81 Research Drive, Scarborough, ME 04074, USA
Rebecca A. Cowling, Department of Biochemistry, Microbiology, and Molecular Biology, University of Maine, 5735 Hitchner Hall, Orono, ME 04469, USA. Maine Medical Center Research Institute, Center for Molecular Medicine, 81 Research Drive, Scarborough, ME 04074, USA
Joshua S. Himmelfarb, Maine Medical Center Research Institute, Center for Molecular Medicine, 81 Research Drive, Scarborough, ME 04074, USA
Tamara L. Adams, Maine Medical Center Research Institute, Center for Molecular Medicine, 81 Research Drive, Scarborough, ME 04074, USA
Joseph M. Verdi, Email: verdij@mmc.org, Maine Medical Center Research Institute, Center for Molecular Medicine, 81 Research Drive, Scarborough, ME 04074, USA
References
- 1.Salehi AH, Roux PP, Kubu CJ, et al. NRAGE, A Novel MAGE Protein, Interacts with the p75 neurotrophin receptor and facilitates nerve growth factor dependent apoptosis. Neuron. 2000;27:279–288. doi: 10.1016/s0896-6273(00)00036-2. [DOI] [PubMed] [Google Scholar]
- 2.Pold M, Zhou J, Chen GL, Hall JM, Vescio RA, Berenson JR. Identification of a new, unorthodox member of the MAGE gene family. Genomics. 1999;59:161–167. doi: 10.1006/geno.1999.5870. [DOI] [PubMed] [Google Scholar]
- 3.Masuda Y, Sasaki A, Shibuya H, Ueno N, Ikeda K, Watanabe K. Dlxin-1, a novel protein that binds Dlx5 and regulates its transcriptional function. J Biol Chem. 2001;276:5331–5338. doi: 10.1074/jbc.M008590200. [DOI] [PubMed] [Google Scholar]
- 4.Xiao J, Chen HS. Biological functions of melanoma-associated antigens. World J Gastroenterol. 2004;10:1849–1853. doi: 10.3748/wjg.v10.i13.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boon T, Cerottini JC, Van den Eynde B, van der Bruggen P, Van Pel A. Tumor antigens recognized by T lymphocytes. Annu Rev Immunol. 1994;12:337–365. doi: 10.1146/annurev.iy.12.040194.002005. [DOI] [PubMed] [Google Scholar]
- 6.Chu C, Xue B, Tu C, et al. NRAGE suppresses metastasis of melanoma and pancreatic cancer in vitro and in vivo. Cancer Lett. 2007;250:268–275. doi: 10.1016/j.canlet.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 7.Wen C, Xue B, Qin W, et al. hNRAGE, a human neurotrophin receptor interacting MAGE homologue, regulates p53 transcriptional activity and inhibits cell proliferation. FEBS Lett. 2004;564:171–176. doi: 10.1016/S0014-5793(04)00353-9. [DOI] [PubMed] [Google Scholar]
- 8.Salehi AH, Morris SJ, Ho WC, et al. AEG3482 is an antiapoptotic compound that inhibits Jun kinase activity and cell death through induced expression of heat shock protein 70. Chem Biol. 2006;13:213–223. doi: 10.1016/j.chembiol.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 9.Salehi AH, Xanthoudakis S, Barker PA. NRAGE, a p75 neurotrophin receptor-interacting protein, induces caspase activation and cell death through a JNK-dependent mitochondrial pathway. J Biol Chem. 2002;277:48043–48050. doi: 10.1074/jbc.M205324200. [DOI] [PubMed] [Google Scholar]
- 10.Kendall SE, Battelli C, Irwin S, et al. NRAGE mediates p38 activation and neural progenitor apoptosis via the bone morphogenetic protein signaling cascade. Mol Cell Biol. 2005;26:7711–7724. doi: 10.1128/MCB.25.17.7711-7724.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kendall SE, Goldhawk DE, Kubu C, Barker PA, Verdi JM. Expression analysis of a novel p75NTR signaling protein, which regulates cell cycle progression and apoptosis. Mech Dev. 2002;117:187–200. doi: 10.1016/s0925-4773(02)00204-6. [DOI] [PubMed] [Google Scholar]
- 12.Nakano I, Saiqusa K, Kornblum HI. BMPing off glioma stem cells. Cancer Cell. 2008;13:3–4. doi: 10.1016/j.ccr.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 13.Nikopoulos GN, Martins JF, Adams TL, et al. NRAGE: a potential rheostat during branching morphogenesis. Mech Dev. 2009;126:337–349. doi: 10.1016/j.mod.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jordan BWM, Dinev D, LeMellay V, et al. Neurotrophin receptor-interacting mage homologue is an inducible inhibitor of apoptosis protein-interacting protein that augments cell death. J Biol Chem. 2001;276:39985–39989. doi: 10.1074/jbc.C100171200. [DOI] [PubMed] [Google Scholar]
- 15.Barker PA, Salehi A. The MAGE proteins: emerging roles in cell cycle progression, apoptosis, and neurogenetic disease. J Neurosci Res. 2002;67:705–712. doi: 10.1002/jnr.10160. [DOI] [PubMed] [Google Scholar]
- 16.McBurney MW. P19 embryonal carcinoma cells. Int J Dev Biol. 1993;37:135–140. [PubMed] [Google Scholar]
- 17.Tahara K, Mori M, Sadanaga N, Sakamoto Y, Kitano S, Makuuchi M. Expression of the MAGE gene family in human hepatocellular carcinoma. Cancer. 1999;85:1234–1240. [PubMed] [Google Scholar]
- 18.Fernandes-Alnemri T, Litwack G, Alnemri ES. CPP32, a novel human apoptotic protein with homology to Caenorhabditis elegans cell death protein Ced-3 and mammalian interleukin-1 beta-converting enzyme. J Biol Chem. 1994;269:30761–30764. [PubMed] [Google Scholar]
- 19.Deveraux QL, Roy N, Stennicke HR, et al. IAPs block apoptotic events induced by caspase-8 and cytochrome c by direct inhibition of distinct caspases. EMBO J. 1998;17:2215–2223. doi: 10.1093/emboj/17.8.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deveraux QL, Takahashi R, Salvesen GS, Reed JC. X-linked IAP is a direct inhibitor of cell-death proteases. Nature. 1997;388:300–304. doi: 10.1038/40901. [DOI] [PubMed] [Google Scholar]
- 21.Kaminska B, Wesolowska A, Danilkiewicz M. TGF beta signalling and its role in tumour pathogenesis. Acta Biochim Pol. 2005;52:329–337. [PubMed] [Google Scholar]
- 22.Kimura N, Matsuo R, Shibuya H, Nakashima K, Taga T. BMP2-induced apoptosis is mediated by activation of the TAK1-p38 kinase pathway that is negatively regulated by Smad6. J Biol Chem. 2000;275:17647–17652. doi: 10.1074/jbc.M908622199. [DOI] [PubMed] [Google Scholar]
- 23.Kozak M. Selection of initiation sites by eucaryotic ribosomes: effect of inserting AUG triplets upstream from the coding sequence for preproinsulin. Nucleic Acids Res. 1984;12:3873–3893. doi: 10.1093/nar/12.9.3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984;12:857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]