Abstract
Objective
To identify patient-care practices related to an increased prevalence of hepatitis C virus (HCV) infection among chronic hemodialysis patients.
Design
Survey
Setting
Chronic hemodialysis facilities in the United States
Participants
An equal probability two-stage cluster sampling was used to select 87 facilities from all Medicare-approved providers treating 30–150 patients; 53 facilities and 2933/3680 eligible patients agreed to participate.
Methods
Patients were tested for HCV antibody and HCV RNA. Data on patient-care practices were collected using direct observation.
Results
Overall prevalence of HCV infection was 9.9% (95% confidence interval [CI], 8.2–11.6); only 2/294 HCV-positive patients were detected solely by HCV RNA. After adjusting for non-dialysis-related HCV risk factors, patient-care practices independently associated with higher prevalence of HCV infection included reusing priming receptacles without disinfection (odds ratio [OR] 2.3; 95% CI, 1.4–3.9), handling blood specimens adjacent to medications and clean supplies (OR 2.2; 95% CI, 1.3–3.6), and using mobile carts to deliver injectable medications (OR 1.7; 95% CI, 1.0–2.8). Independently-related facility covariates were ≥10% patient HCV prevalence (OR 3.0; 95% CI, 1.8–5.2), patient-to-staff ratio ≥7-to-1 (OR 2.4; 95% CI, 1.4–4.1), and treatment duration ≥2 years (OR 2.4; 95% CI, 1.3–4.4).
Conclusions
This study provides the first epidemiologic evidence of associations between specific patient-care practices and higher HCV infection prevalence among hemodialysis patients. Staff should review practices to ensure that hemodialysis-specific infection control practices are being implemented, especially handling clean and contaminated items in separate areas, reusing items only if disinfected, and prohibiting mobile medication/clean supply carts within treatment areas.
Hepatitis C virus (HCV) infection is associated with a high rate of chronic liver disease and cirrhosis,1 and chronic hemodialysis patients in the United States have a higher prevalence of this infection than the general adult population.2,3 HCV-infected renal failure patients are at increased risk for death whether they remain on hemodialysis or undergo kidney transplantation,4,5 and current HCV therapy is of limited utility in these patients due to lack of tolerability and suboptimal response rates.6 Therefore, it is of major concern that HCV infections continue to be transmitted between chronic hemodialysis patients within their treatment facilities.7–9
To promote patient-care practices that prevent transmission of HCV and other bloodborne pathogens in chronic hemodialysis facilities, updated recommendations were published by the Centers for Disease Control and Prevention (CDC) in 2001.10 In addition to Standard Precautions, the more stringent precautions that had been recommended for all hemodialysis patients since 1977 were reiterated.11 There is controversy regarding these recommendations because there were no epidemiological data that demonstrated relationships between HCV infection and specific patient-care practices in hemodialysis facilities. We conducted this study to determine the practices and characteristics associated with an increased prevalence of HCV infection among chronic hemodialysis patients in the United States.
Methods
Study population and data collection
Facilities were selected for participation from a list of Medicare-approved providers using equal probability two-stage cluster sampling.12 Dialysis facilities eligible for the study were located within the continental United States, had an outpatient census of 30 to 150 patients on 31 December 1998, were Medicare-certified for ≥3 years, and located in cities with ≥3 other eligible facilities within a 34-mile radius to facilitate on-site visits. Patient criteria for eligibility included age ≥18 years and dialysis at the study facility for ≥30 days. All participants provided written informed consent. Institutional Review Board approval was obtained from the University of North Carolina at Chapel Hill and the CDC.
From July 2000 to November 2001, trained research staff visited each facility to enroll patients and interview them about their medical history and risk factors for HCV. A plasma sample was collected prior to heparin infusion from each enrolled patient and chart reviews were conducted. Information on the characteristics and practices of each facility was obtained from interviews with nurse managers and reviews of procedure manuals. Patient-care practices, disinfection procedures, and handling of clean and contaminated items were directly observed by research staff for an average of 2–4 hours per center, and recorded on a standardized form. Multiple observations of key practices were carried out; five separate observations of those used to connect and disconnect patients from their dialysis machines, including decontaminating the dialysis stations; three of preparing, distributing and administering injections and infusions; five of the contents of staffs’ pockets, and 80 of hand hygiene and glove changes by staff moving between patient stations and between contaminated and clean areas.
Laboratory testing
Anti-HCV and HCV RNA detection
All plasma specimens were tested for anti-HCV by enzyme immunoassay (EIA) (ORTHO® HCV Version 3.0 ELISA, Ortho-Clinical Diagnostic Systems, Raritan NJ), and repeatedly reactive specimens by a supplemental anti-HCV strip immunoblot assay (RIBA® HCV 3.0 SIA, Chiron Corporation, Emeryville CA). RIBA-indeterminate and RIBA-positive samples were tested for HCV RNA using Amplicor® HCV (Roche Molecular Systems, Branchburg, NJ). EIA-negative and RIBA-negative specimens were tested for HCV RNA in 16-member mini-pools using the discriminatory HCV transcription-mediated amplification (dHCV TMA) component of a combined HIV-1/HCV assay for blood screening (Procleix™, Chiron Corporation) now FDA approved as HCV Aptima Assay (Gen-Probe Inc., San Diego, CA).13 Reactive pools were resolved down to the individual member, and single positives confirmed by Amplicor HCV and nested RT-PCR. HCV-positivity was defined as anti-HCV positive by RIBA or HCV RNA positive.
Nucleic acid sequencing and genotyping
A 1590 base pair (bp) nucleotide fragment encompassing part of the 5′ untranslated region (5′ UTR), the core, and envelope (E1 and part of the E2) regions of the HCV genome was amplified by first round reverse transcriptase (RT)-PCR from all HCV RNA-positive samples. For genotyping, first round PCR products were re-amplified and the sequence of each fragment compared with published sequences using Multiple comparison and Evolution programs (Accelrys GCG, version 11.1.2-UNIX, Accelrys Software Inc., San Diego, CA).14
Characterization of HCV quasispecies
We cloned and sequenced the HCV hypervariable region 1 (HVR1), as previously described,15 from all genotyped patients in three facilities to determine if HCV-infected patients identified by a cross-sectional study could be linked to one another on the basis of the relatedness of their virus isolates. Approximately 32 clones were selected from each specimen, their HVR1 sequences aligned, and the number of viral variants determined. Preliminary pairwise analysis was conducted using a multiple sequence alignment program and nucleotide distances were calculated using the model of Kimura 2-parameter distance (Accelrys GCG, version 11.1.2-UNIX).14 Phylogenetic analysis was performed using DNADIST, NEIGHBOR, SEQBOOT and DRAWTREE programs in the PHYLIP Phylogeny Inference Package (version 3.66, written by J. Felsenstein, University of Washington, Seattle, WA). Bootstrap analysis was done to evaluate the reliability of the phylogenetic tree analysis.16
Statistical analysis
All statistics, unless otherwise stated, were adjusted for correlation resulting from the two-stage cluster sampling design by use of sampling weights derived from the sampling design, and robust variance estimates and generalized estimating equations generated by SUDAAN 8 (RTI International, Cary NC). Sampling weights were adjusted for facility non-response and post-sampling changes to the sampling frame. To assess the generalizability of the results, study facility and patient characteristics were compared to Medicare-approved chronic hemodialysis facilities operating in the year 2000 with a census of at least 30 patients and chronic hemodialysis patients from the 2001 United States Renal Data System, respectively.12,17
The potential association between HCV-positivity and patient characteristics unrelated to their dialysis was evaluated by univariate and multivariate analyses using the entire study population and a subpopulation of patients considered at risk for hemodialysis-associated HCV infection (defined below). Patient characteristics with p<0.05 in univariate analysis were entered into multivariate models. The analysis of the potential association between HCV-positivity and hemodialysis-related characteristics was restricted to the subpopulation of patients considered at risk for hemodialysis-associated HCV infection in their respective study facilities. These patients had spent ≥80% of their hemodialysis career at the study facility, were not known to be HCV-positive at the time of dialysis initiation at the study facility, attended a study facility with at least one HCV-positive patient, and reported no history of injection drug use (IDU). This subpopulation comprised 70% of the original study population. Hemodialysis facility-specific and patient care-related characteristics with p<0.25 in univariate analysis were entered into multivariate models. Each patient-care practice was assessed in a separate multivariate model in order to minimize the possibility of having highly correlated practice variables in the same model. Each model was adjusted for covariates, i.e., other hemodialysis-related characteristics and patient risk factors unrelated to dialysis that had been identified as significant in their respective univariate analyses. Crude and adjusted odds ratios with 95% confidence intervals were calculated. Factors with p<0.05 in the final models were considered significant.
Results
Study population
Of 87 facilities invited to participate, 53 (61%) agreed. The 53 study facilities had an average weekly patient census of 77.5, and most (83.0%) were free-standing and for-profit. These characteristics were not significantly different from all Medicare-approved chronic hemodialysis facilities with a census of at least 30 patients. Of the 4093 hemodialysis patients attending the 53 study facilities, 3680 (90%) were eligible and 2933 (79.7%) participated. The age, gender and racial/ethnic characteristics of the study participants (Table 1) were similar to all hemodialysis patients in the ESRD database.
Table 1.
Prevalence of Hepatitis C Virus (HCV) Infection among Chronic Hemodialysis Patients by Patient Characteristics and Risk Histories.
| Characteristic or Lifetime History | No. (%) | % HCV Positive* | Unadjusted Odds Ratio (95% Confidence Interval)* | Adjusted Odds Ratio (95% Confidence Interval)† |
|---|---|---|---|---|
| All Participants | 2,933 (100) | 9.9 | ||
| Age (years) | ||||
| <60 | 1,288 (43.9) | 17.0 | 4.7 (3.2–6.9) | 2.0 (1.4–2.8) |
| ≥60 | 1,645 (56.1) | 4.2 | 1.0 | 1.0 |
| Gender | ||||
| Male | 1605 (55.1) | 11.6 | 1.6 (1.2–2.2) | 0.7 (0.5–1.01) |
| Female | 1307 (44.9) | 7.4 | 1.0 | 1.0 |
| Race/Ethnicity | ||||
| White, non-Hispanic | 1,231 (42.6) | 4.7 | 1.0 | 1.0 |
| Black, non-Hispanic | 1,264 (43.7) | 16.9 | 4.1 (3.0–5.5) | 2.7 (2.1–3.6) |
| Hispanic | 267 (9.2) | 4.7 | 1.0 (0.5–2.2) | 1.0 (0.5–1.9) |
| Other, non-Hispanic | 131 (4.5) | 4.1 | 0.9 (0.3–2.4) | 0.4 (0.1–2.3) |
| Years on hemodialysis | ||||
| ≥2 | 1634 (55.8) | 11.7 | 1.6 (1.2–2.1) | 1.3 (0.9–1.8) |
| <2 | 1297 (44.3) | 7.7 | 1.0 | 1.0 |
| Number facilities attended | ||||
| 1 (study facility) | 2206 (75.8) | 8.4 | 1.0 | 1.0 |
| 2 | 559 (19.2) | 13.0 | 1.6 (1.2–2.2) | 0.9 (0.4–1.9) |
| ≥3 | 144 (5.0) | 19.7 | 2.7 (1.4–5.1) | 2.3 (1.2–4.4) |
| Blood transfusion before 1992 | ||||
| Yes | 592 (20.2) | 14.4 | 1.8 (1.4–2.2) | 2.3 (1.2–4.4) |
| No | 2341 (79.8) | 8.8 | 1.0 | 1.0 |
| Kidney transplant before 1992 | ||||
| Yes | 72 (2.5) | 22.2 | 2.7 (1.2–5.9) | 2.7 (1.7–4.2) |
| No | 2861 (97.6) | 9.6 | 1.0 | |
| Injecting drug use | ||||
| Yes | 125 (4.3) | 84.5 | Infinite | Not applicable |
| No | 2781 (95.7) | 6.5 | 1.0 | |
| Intranasal drug use | ||||
| Yes | 267 (9.2) | 40.1 | 9.3 (7.1–12.2) | 1.6 (0.9–2.5) |
| No | 2652 (90.9) | 6.7 | 1.0 | 1.0 |
| Number opposite sex partners | ||||
| ≥25 | 376 (14.5) | 23.0 | 3.5 (2.4–5.2) | 1.9 (1.2–2.9) |
| <25 | 2224 (85.5) | 7.8 | 1.0 | 1.0 |
Univariate analysis adjusted for complex sampling design.
Calculated using multivariate regression, including adjustment for complex sampling design, after excluding participants with a history of injecting drug use.
Prevalence of HCV infection
All 2933 patients were tested for anti-HCV and HCV RNA. HCV-positivity was confirmed in 294 for an overall prevalence of 9.9% (95% CI, 8.2%–11.6%); 273 (92.9%) of these were HCV RNA-positive. HCV prevalence ranged from 0 to 29.4% (median 7.8%, 95% CI, 6.1%–11.1%) among individual facilities, and did not differ significantly by geographic region.
Of the 294 confirmed HCV-positive patients, 292 (99.3%) were detected with anti-HCV. An additional 51 samples were EIA–positive, but RIBA- and HCV RNA-negative; these were judged falsely positive for anti-HCV.
Patient risk factors and HCV infection
IDU was reported by 4.3% of all study participants of whom 84.5% were HCV-positive compared with 6.5% among those who reported never injecting drugs (Table 1). After excluding participants who reported IDU, risk factors independently associated with an increased prevalence of HCV infection included age <60 years, non-Hispanic black race/ethnicity, having completed >8 years of education, and histories of blood transfusion before 1992, kidney transplantation before 1992, ≥25 lifetime heterosexual partners, and attending ≥3 dialysis facilities (Table 1). In the subpopulation of 2056 patients classified as at risk of hemodialysis-associated HCV infection, older age, non-Hispanic black race/ethnicity, and history of blood transfusion were also independently associated with an increased prevalence of HCV infection.
Hemodialysis-related factors and HCV infection
The characteristics and patient-care practices at the study facilities of the patients at risk of hemodialysis-associated HCV infection were similar to those of the entire study population. Sixty-four (3.1%) of the 2056 at-risk patients met the criteria for presumed hemodialysis-associated HCV infection. By univariate analysis, the prevalence of hemodialysis-associated HCV infection increased with increasing years treated at the study facility, a higher patient-to-staff ratio, and increasing proportion of HCV-positive patients treated at the study facility (Table 2). There was no relationship between HCV prevalence and reuse of dialyzers or type of facility ownership.
Table 2.
Prevalence of Hemodialysis-Associated Hepatitis C Virus (HCV) Infection among Patients by Selected Characteristics of Their Treatment and Their Study Facilities.
| Patients At Risk For Hemodialysis-Associated HCV Infection* | ||||
|---|---|---|---|---|
| Study Facility Characteristics | No. (%) of Total Patients | No. (%) | % HCV Positive† | Unadjusted Odds Ratio (95% Confidence Interval)† |
| Total Patients | 2933 (100) | 2056 (100) | 3.1 | |
| Years dialyzed at facility | ||||
| <1 | 826 (28.2) | 605 (29.5) | 1.4 | 1.0 |
| 1–<2 | 663 (22.7) | 480 (23.4) | 2.0 | 1.4 (0.6–3.4) |
| 2–<5 | 996 (34.0) | 692 (33.7) | 3.9 | 2.9 (1.3–6.7) ‡ |
| ≥5 | 442 (15.1) | 275 (13.4) | 6.0 | 4.6 (1.8–11.4) ‡ |
| Received dialysis as inpatient | ||||
| Yes | 2584 (90.4) | 1792 (88.9) | 3.3 | 1.8 (0.6–5.4)§ |
| No | 273 (9.6) | 223 (11.1) | 1.8 | 1.0 |
| Dialyzed at other facilities while traveling | ||||
| Yes | 696 (23.9) | 486 (23.7) | 2.2 | 0.7 (0.4–1.2)§ |
| No | 2213 (76.1) | 1567 (76.3) | 3.4 | 1.0 |
| Patient census | ||||
| 30 – 59 | 616 (21.0) | 386 (18.8) | 3.4 | 1.0 |
| 60 – 89 | 815 (27.8) | 588 (28.6) | 2.3 | 0.7 (0.2–2.3) |
| 90 – 119 | 784 (26.7) | 574 (27.9) | 3.2 | 0.9 (0.4–2.2) |
| ≥120 | 718 (24.5) | 508 (24.7) | 3.8 | 1.1 (0.4–3.3) |
| Patient to staff ratio | ||||
| <5 to 1 | 208 (7.1) | 128 (6.2) | 1.9 | 1.0 |
| 5 – 6 to 1 | 1600 (54.6) | 1100 (53.5) | 2.7 | 1.4 (0.5–3.8) |
| ≥7 to 1 | 1125 (38.4) | 828 (40.3) | 4.2 | 2.3 (1.1–4.7) § |
| Prevalence of HCV-positive patients¶ | ||||
| 0 – <5 | 768 (26.2) | 571 (27.8) | 1.2 | 1.0 |
| 5 – <10 | 738 (25.2) | 540 (26.3) | 1.1 | 0.9 (0.4–2.5) |
| 10 – <15 | 884 (30.1) | 634 (30.8) | 5.0 | 4.4 (1.8–10.5) ‡ |
| 15 – <19 | 123 (4.2) | 60 (2.9) | 3.3 | 2.8 (1.2–6.5) ‡ |
| ≥>20 | 420 (14.3) | 251 (12.2) | 9.2 | 8.5 (3.6–19.8) ‡ |
Defined as patients who had spent ≥80% of their hemodialysis career at the study facility, were not known to be HCV-positive at the time of admission to the study facility, attended a study facility with at least one HCV-positive patient, and reported no history of injecting drug use.
Univariate analysis adjusted for complex sampling design
P<0.05
P≥0.05<0.25
Includes all HCV-positive patients treated at the facility regardless of source.
Patient-care practices associated (p<0.25) with increased prevalence of hemodialysis-associated HCV infection by univariate analysis included using tape from rolls carried in staff pockets for multiple patients; using mobile medication carts to distribute medications, inconsistent cleaning of machine monitors between patients; reusing priming receptacles without decontaminating between patients; and handling blood specimens in or adjacent to areas used for medications or clean supplies, regardless of the presence or absence of the “clean” items at the time of observation (Table 3).
Table 3.
Prevalence of Hemodialysis-Associated Hepatitis C Virus (HCV) Infection among Patients by the Observed Patient-Care Practices in Their Study Treatment Facilities with Recommended Practices Given for Reference.
| Patient-Care Practices | Patients At Risk For Hemodialysis-Associated HCV Infection* | ||||
|---|---|---|---|---|---|
| Recommended† | Observed in Study Facility | No. (%) of Total Patients | No. (%) | % HCV Positive‡ | Unadjusted Odds Ratio (95% Confidence Interval)‡ |
| Avoid sharing equipment and supplies between patients. Items taken into the dialysis station should be disposed of, dedicated for use only on a single patient, or cleaned and disinfected before taken to a clean area or used on another patient. Do not carry medication vials, syringes, alcohol swabs, or supplies in pockets. |
Items reused for multiple patients without cleaning and disinfection (e.g., fistula clamps) | ||||
| Yes | 329 (11.2) | 216 (10.5) | 3.8 | 1.3 (0.3–5.0) | |
| No | 2604 (88.8) | 1840 (89.5) | 3.0 | 1.0 | |
| Unused clean supplies at dialysis station not discarded between patients (e.g., vacutainers) | |||||
| Yes | 374 (12.8) | 276 (13.4) | 3.6 | 1.2 (0.4–3.5) | |
| No | 2559 (87.3) | 1780 (86.6) | 3.0 | 1.0 | |
| Supplies carried in staff pockets (e.g., rolls of adhesive tape) | |||||
| Yes | 2163 (74.7) | 1528 (75.2) | 3.9 | 2.4 (0.9–6.4) ¶ | |
| No | 734 (25.3) | 505 (24.8) | 1.6 | 1.0 | |
| Medication preparation and distribution. When multiple dose medication vials are used, prepare individual patient doses in a clean (centralized) area away from dialysis stations and deliver separately to each patient. Do not use common medication carts to deliver medications to patients. |
Medications prepared in centralized area outside treatment area | ||||
| Yes | 1544 (53.9) | 1137 (56.8) | 2.7 | 1.0 | |
| No | 1323 (46.2) | 866 (43.2) | 3.8 | 1.5 (0.7–2.9) | |
| Mobile medication cart used to distribute injectable medications in treatment area | |||||
| Yes | 1373 (46.8) | 943 (45.9) | 3.9 | 1.6 (0.8–3.2) ¶ | |
| No | 1560 (53.2) | 1113 (54.1) | 2.5 | 1.0 | |
| Disinfection of patient care station. Clean and disinfect the dialysis station between patients. Give special attention to cleaning control panels on the dialysis machines. Discard all fluid and clean and disinfect all surfaces and containers associated with the prime waste. |
Frequency dialysis machine monitor knobs decontaminated between patients | ||||
| ≤70% of time | 688 (23.5) | 467 (22.7) | 5.3 | 2.1 (1.2–4.0)§ | |
| >70% of time | 2245 (76.5) | 1589 (77.3) | 2.6 | 1.0 | |
| Priming receptacle reused between patients without decontamination | |||||
| Yes | 1254 (42.8) | 860 (41.8) | 4.8 | 2.8 (1.4–5.6)§ | |
| No | 1679 (57.3) | 1196 (58.2) | 1.8 | 1.0 | |
| Separation of clean and dirty areas. Clean areas should be clearly separated from contaminated areas where used supplies and equipment are handled. Do not handle and store medications or clean supplies in the same or adjacent area to where used equipment or blood samples are handled. |
Blood specimens handled in same area or adjacent to medications and clean supplies | ||||
| Yes, when medications were being prepared or other clean supplies were present | 313 (10.7) | 192 (9.3) | 6.3 | 2.4 (1.02–5.7)§ | |
| Yes, although no clean items were present at the same time | 237 (8.1) | 167 (8.1) | 4.7 | 1.7 (0.8–3.9)¶ | |
| No | 2018 (68.8) | 1448 (70.4) | 2.7 | 1.0 | |
| Hand washing and glove changing. Wear disposable gloves when caring for the patient or touching the patient’s equipment at the dialysis station; remove gloves and wash hands between each patient or station |
Frequency of hand washing only** | ||||
| <33% | 2215 (75.5) | 1595 (77.6) | 3.3 | 1.4 (0.5–3.5) | |
| ≥33% | 718 (24.5) | 461 (22.4) | 2.5 | 1.0 | |
| Frequency of glove changing only** | |||||
| <41% | 2085 (71.1) | 1454 (70.7) | 3.2 | 1.1 (0.4–2.7) | |
| ≥41% | 848 (28.9) | 602 (29.3) | 3.0 | 1.0 | |
| Frequency of hand washing and glove changing** | |||||
| <17% | 2103 (71.7) | 1493 (72.6) | 3.4 | 1.5 (0.6–4.0) | |
| ≥17% | 830 (28.3) | 563 (27.4) | 2.3 | 1.0 | |
Defined as patients who had spent ≥80% of their hemodialysis career at the study facility, were not known to be HCV-positive at the time of admission to the study facility, attended a study facility with at least one HCV-positive patient, and reported no history of injection drug use.
See reference14.
Univariate analysis adjusted for complex sampling design.
P<0.05
P≥0.05<0.25
Between patient care stations, between a patient care station and a clean area (e.g., nurse’s station), and between a contaminated area (e.g., where blood specimens or used items were handled) and a patient care station.
By multivariate analysis, these same patient care practices were independently associated with an increased prevalence of hemodialysis-associated HCV infection except for using tape from rolls carried in pockets and inconsistent cleaning of monitors (Table 4). The facility-related covariates that were consistently associated with an increased prevalence of infection included treatment at the facility for ≥2 years, patient-to-staff ratio ≥7 to 1, and an HCV patient prevalence ≥10% (Table 4); age <60 years and non-Hispanic black race/ethnicity were the two significant patient covariates (data not shown).
Table 4.
Multivariate Analysis of Patient-Care Practices Associated with Increased Prevalence of Hemodialysis-Associated Hepatitis C Virus Infection, Adjusted for Facility Characteristics and Non-Dialysis-Related HCV Risk Factors
| Observed Patient-Care Practices | Adjusted Odds Ratio (95% Confidence Interval) * |
|---|---|
| Priming receptacles reused between patients without cleaning or disinfection | 2.3 (1.4–3.9) |
| Blood specimens handled in or adjacent to an area where medications were being prepared or other clean supplies were present | 2.2 (1.3–3.8) |
| Blood specimens handled in or adjacent to a designated clean area, although clean items not present at the time of observation | 2.2 (1.3–3.6) |
| Mobile medication carts used in treatment areas to distribute injectable medications | 1.7 (1.0–2.8) |
| Dialysis machine monitor knobs decontaminated between patients ≤70% of time | 1.2 (0.7–2.1) |
| Adhesive tape carried in staff pockets | 1.5 (0.8–2.9) |
| Facility-related covariates† | |
| ≥10% HCV patient prevalence | 3.0 (1.8–5.2) |
| Patient to staff ratio ≥7 to 1 | 2.4 (1.4–4.1) |
| ≥2 years treatment duration | 2.4 (1.3–4.4) |
For each category, the adjusted odds ratio was calculated relative to the referent group (patients treated in facilities in which the practices were not observed or which did not have the characteristics) assigned an odds ratio of 1.0.
Included in all models, but for purposes of example adjusted odds ratios and 95% confidence intervals shown only from one model. These measures were similar across all models.
Relatedness of HCV isolates among patients
Genotype was determined for 255 (97.7%) of the 261 HCV RNA-positive samples; 59.2% were 1a, 31.4% 1b, 1.6% 2a, 5.5% 2b, 0.9% 2c, 0.9% 3a, and 0.5% 4a. Forty-one facilities had two or more patients infected with the same HCV sub-genotype; of these, 11 (20.8%) had at least one pair of patients whose virus isolates shared ≥96% nucleotide identity in the HCV core-E1 region.
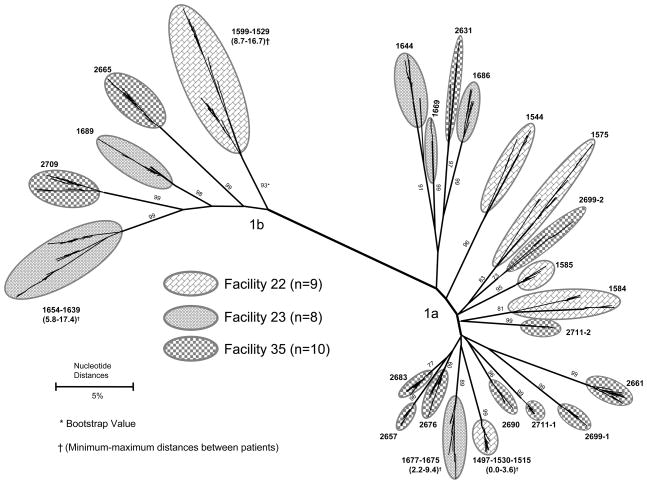
Samples from all genotyped patients in three of the 11 facilities were selected for quasispecies characterization. The unweighted HCV RNA prevalence in the three facilities (identified as numbers 22, 23 and 35) was 11.3% (11/97), 10.5% (8/76) and 29.4% (15/51), respectively. In facility 22, three (1497, 1530, and 1515) of seven 1a-infected patients shared identical HVR1 quasispecies (bootstrap value 99%); and in facility 23, two (1677 and 1675) of five 1a-infected patients had 97.8% homology in HVR1 (bootstrap value 89%) (Figure 1). These patients had received dialysis on the same or adjacent shifts relative to the patients infected with related isolates.
Figure 1.
Phylogenetic analysis of HVR1 quasispecies from 27 HCV-infected chronic hemodialysis patients attending three facilities
Among the 1b-infected patients in facilities 22 and 23, the highest degree of HVR1 quasispecies relatedness was 94.2% (bootstrap value 99%), which was found between the two patients in facility 23. In facility 35, no phylogenetic clustering among quasispecies was observed from the 1a- or 1b-infected patients (Figure 1). HCV was classified as hemodialysis-associated in three of these patients.
Discussion
The most important finding of this study was the identification of specific patient-care practices related to an increased prevalence of hemodialysis-associated HCV infection, indicating that HCV transmission in these settings can be reduced or prevented by modifying these practices. Reusing priming receptacles between patients without decontamination, handling blood specimens in the same or adjacent areas designated for medications or clean supplies, and using mobile carts within treatment areas to deliver injectable medications provide opportunities for cross-contamination of blood in settings where multiple patients require vascular access for prolonged periods. Patients’ tubing can become contaminated from being draped over priming receptacles that were not disinfected after previous patients’ treatments, and entry points of medication vials and intravenous solutions can be contaminated by used vacutainers and other injection-related supplies when they are handled in the same or adjacent areas. Unapparent blood contamination of medication vials and diluents also can occur when injectables are prepared and distributed from mobile carts in proximity to patient stations.
HCV in plasma remains viable after drying and environmental exposure to room temperature for at least 16 hours,18 therefore, blood contaminated surfaces and objects can serve as sources for HCV transmission,19–22 Similar characteristics for hepatitis B virus were demonstrated >20 years ago,23 although HCV circulates at lower titers and has a shorter environmental survival time.18
Higher patient prevalences of HCV, lower staff-to-patient ratios, and failure by staff to change gloves between patients have been shown to be linearly related to increases in incidence of HCV infection.24 Inadequate staffing may result in less adherence to appropriate infection control practices thereby increasing the likelihood of blood contamination of the environment;25 higher patient HCV prevalences may increase the probability that such blood contamination contains HCV. 26–29 The non-association with glove changing and hand hygiene in this study may be related to the extremely low frequency with which both of these activities were routinely performed;30 a median 11.1% (range, 3%–43%) of the time by staff when they moved between patient stations or from contaminated to clean areas.
The inherent difficulty in quantifying facility-related factors that increase the risk for unapparent environmental blood contamination may explain why many outbreak investigations did not find associations with specific practices but did report multiple breaches in infection control practices and generally inadequate hygienic standards in the involved facilities.7,9,31 These circumstances could explain others’ findings of increased risk of HCV infection associated with receiving dialysis on the same machine following an HCV-positive patient or on the same shift with HCV-positive patients, and being connected to the dialysis machine by the same staff member who had connected an HCV-positive patient.25,27,32,33
Determining the relatedness of virus isolates between HCV-infected hemodialysis patients in the same facility has been useful for establishing patient-to-patient transmission in studies of incident infections.27,31 We were able to demonstrate a genetic link between potential source patients and patients classified with hemodialysis-associated HCV infection in a few instances; the failure to do so in others is likely related to the cross-sectional nature of the study. The turnover in chronic hemodialysis patient populations is high, and transmission may have occurred from an HCV-infected patient who died or transferred before this study was conducted.
The other major finding of this study was confirmation that HCV antibody assays are sufficiently sensitive for routine screening of hemodialysis patients in the United States.10,34 Only 0.08% (two) of >2600 anti-HCV negative patients tested positive for HCV RNA. Higher rates of HCV RNA detection in anti-HCV negative dialysis patients have been reported by a few studies, and may have represented patients in the early viremic phase of acute infection before antibody seroconversion.35,36 This pattern also occurs infrequently in patients with chronic HCV infection as observed in our study, and has been reported even in immune competent individuals.34,37 Our study also demonstrated the importance of more specific testing to confirm anti-HCV immunoassay-positive results as 11% of the EIA positive results were falsely-positive.
This study had several limitations. The data collection was performed during 2000 and 2001, the study design was cross-sectional, and it was difficult to distinguish the impact of the various practices. However, patient-to-patient transmission of HCV infection in hemodialysis centers has continued during the past decade and the characteristics of the more recent outbreaks are no different than those that occurred in the past.7,9,25,27 The study population was representative of the national database; the HCV infection prevalence consistent with that reported from national surveillance in the same year as the study, and risk factors conventionally associated with HCV identified. The quality of patient-care practices at each facility was measured using direct observation, considered the gold standard since self-reported data often do not correlate with actual practice.38 Given the low frequencies with which recommended patient-care practices were performed, the Hawthorne effect (which occurs when individuals change their behavior when they believe someone is watching them) was probably minimal and if present, biased our results towards the null. Further, the at-risk population for hemodialysis-associated infection was conservatively defined which could have resulted in an underestimate of hemodialysis-associated infections by classifying the source of some HCV-positive patients as not facility-related.
Our study is the first to identify specific patient-care practices associated with higher HCV prevalence among chronic hemodialysis patients. Other prevalence studies did not collect such information, relied on facilities’ self-reports regarding the extent to which “sharing” of medication vials or supplies was carried out, or interpreted “Universal” now called Standard Precautions as “adequate” practices for chronic hemodialysis facilities.26,28,31,39 Because exposure to blood and potentially contaminated items can be routinely anticipated in chronic hemodialysis facilities, recommendations for precautions in these settings include routinely wearing gloves when caring for patients or touching patients’ equipment; restricting the use of common supplies, instruments, and medications for multiple patients; and prohibiting the use of mobile carts within treatment areas to store or distribute medications and clean supplies.10,40 A staff member should be designated in every facility to review practices to ensure they are consistent with these recommendations and applied routinely to all patients. Staffing needs also should be reviewed to ensure they are not a barrier to implementing best practices. The recommended practices, and the strategies for overcoming barriers to their implementation, should be included in continuing education of all staff members.
Acknowledgments
We thank Heather Wurtzel for help in designing the data collection instrument and enrolling facilities and Teresa Womack RN, Allys Anselmi RN, Suzanne Moore RN, and Mary Kirkland forhelp in data collection.
This research was supported by a cooperative agreement and fellowship (Gayle Shimokura) from the Centers for Disease Control and Prevention (CDC) through the Association of Schools of Public Health (Grant Number U36/CCU300430-18 and Fellowship code S-16/16-CID97-001); short-term training opportunities from the CDC through the Association of Teachers of Preventive Medicine and the Research Participation Program administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and CDC (Gayle Shimokura); unconditional salary support from Ortho Diagnostics and GlaxoSmithKline (Gayle Shimokura) and by a General Clinical Research Center Grant (Number RR00046, for David Weber) and a National Institutes of Health grant (Number RO1-HL-076902, for Drs. Busch and Tobler).
Role of the Sponsor. The CDC assisted in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation and review of the manuscript.
Footnotes
Preliminary data were presented at meetings of the American Association of Blood Banks, October 2003 and the Society for Hospital Epidemiology of America, April 2005.
Authors report no conflicts of interest.
Reference List
- 1.Davis GL, Alter MJ, El-Serag H, Poynard T, Jennings LW. Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology. 2010;138:513–21. 521. doi: 10.1053/j.gastro.2009.09.067. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705–714. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 3.Finelli L, Miller JT, Tokars JI, Alter MJ, Arduino MJ. National Surveillance of Dialysis-Associated Diseases in the United States, 2002. Semin Dial. 2005;18:52–61. doi: 10.1111/j.1525-139X.2005.18108.x. [DOI] [PubMed] [Google Scholar]
- 4.Fabrizi F, Takkouche B, Lunghi G, Dixit V, Messa P, Martin P. The impact of hepatitis C virus infection on survival in dialysis patients: meta-analysis of observational studies. J Viral Hepat. 2007;14:697–703. doi: 10.1111/j.1365-2893.2007.00868.x. [DOI] [PubMed] [Google Scholar]
- 5.Maluf DG, Fisher RA, King AL, et al. Hepatitis C virus infection and kidney transplantation: predictors of patient and graft survival. Transplantation. 2007;83:853–857. doi: 10.1097/01.tp.0000259725.96694.0a. [DOI] [PubMed] [Google Scholar]
- 6.Terrault NA, Adey DB. The kidney transplant recipient with hepatitis C infection: pre- and posttransplantation treatment. Clin J Am Soc Nephrol. 2007;2:563–575. doi: 10.2215/CJN.02930806. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Hepatitis C virus transmission at an outpatient hemodialysis unit --- New York, 2001–2008. Morbidity & Mortality Weekly Report. 2009;58:189–194. [PubMed] [Google Scholar]
- 8.Saab S, Martin P, Brezina M, Gitnick G, Yee HF., Jr Serum alanine aminotransferase in hepatitis C screening of patients on hemodialysis. Am J Kidney Dis. 2001;37:308–315. doi: 10.1053/ajkd.2001.21294. [DOI] [PubMed] [Google Scholar]
- 9.Thompson ND, Novak RT, Datta D, et al. Hepatitis C virus transmission in hemodialysis units: importance of infection control practices and aseptic technique. Infect Control Hosp Epidemiol. 2009;30:900–903. doi: 10.1086/605472. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. Recommendations for preventing transmission of infections among chronic hemodialysis patients. MMWR Recommendations and Reports. 2001;50:1–45. [PubMed] [Google Scholar]
- 11.Centers for Disease Control. HEW Publication No [CDC] 78–8358 (Viral Hepatitis Investigations and Control Series) 1977. Hepatitis--control measures for hepatitis B in dialysis centers. [Google Scholar]
- 12.Centers for Medicare and Medicaid Services. ESRD Provider File. 2000. [Google Scholar]
- 13.Page-Shafer K, Pappalardo BL, Tobler LH, et al. Testing strategy to identify cases of acute hepatitis C virus (HCV) infection and to project HCV incidence rates. J Clin Microbiol. 2008;46:499–506. doi: 10.1128/JCM.01229-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cody SH, Nainan OV, Garfein RS, et al. Hepatitis C virus transmission from an anesthesiologist to a patient. Arch Intern Med. 2002;162:345–350. doi: 10.1001/archinte.162.3.345. [DOI] [PubMed] [Google Scholar]
- 16.Felsenstein J. Estimating effective population size from samples of sequences: a bootstrap Monte Carlo integration method. Genet Res. 1992;60:209–220. doi: 10.1017/s0016672300030962. [DOI] [PubMed] [Google Scholar]
- 17., editor. U.S.Renal Data System. USRDS 2001 annual data report: atlas of end-stage renal disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2001. [Google Scholar]
- 18.Kamili S, Krawczynski K, McCaustland K, Li X, Alter MJ. Infectivity of hepatitis C virus in plasma after drying and storing at room temperature. Infect Control Hosp Epidemiol. 2007;28:519–524. doi: 10.1086/513727. [DOI] [PubMed] [Google Scholar]
- 19.Carducci A, Verani M, Casini B, et al. Detection and potential indicators of the presence of hepatitis C virus on surfaces in hospital settings. Lett Appl Microbiol. 2002;34:189–193. doi: 10.1046/j.1472-765x.2002.01066.x. [DOI] [PubMed] [Google Scholar]
- 20.Froio N, Nicastri E, Comandini UV, et al. Contamination by hepatitis B and C viruses in the dialysis setting. Am J Kidney Dis. 2003;42:546–550. doi: 10.1016/s0272-6386(03)00787-x. [DOI] [PubMed] [Google Scholar]
- 21.Girou E, Chevaliez S, Challine D, et al. Determinant roles of environmental contamination and noncompliance with standard precautions in the risk of hepatitis C virus transmission in a hemodialysis unit. Clin Infect Dis. 2008;47:627–633. doi: 10.1086/590564. [DOI] [PubMed] [Google Scholar]
- 22.Simjanovska LJ, Porcu K, Amitov V, Efremov GD, Polenakovic M. Reverse transcriptase/polymerase chain reaction analyses of hemodialysis ultrafiltrates and sera of hepatitis C virus positive patients. Int J Artif Organs. 2004;27:35–39. doi: 10.1177/039139880402700108. [DOI] [PubMed] [Google Scholar]
- 23.Bond WW, Favero MS, Petersen NJ, Gravelle CR, Ebert JW, Maynard JE. Survival of hepatitis B virus after drying and storage for one week. Lancet. 1981;1:550–551. doi: 10.1016/s0140-6736(81)92877-4. [DOI] [PubMed] [Google Scholar]
- 24.Laporte F, Tap G, Jaafar A, et al. Mathematical modeling of hepatitis C virus transmission in hemodialysis. Am J Infect Control. 2009;37:403–407. doi: 10.1016/j.ajic.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 25.Savey A, Simon F, Izopet J, Lepoutre A, Fabry J, Desenclos JC. A large nosocomial outbreak of hepatitis C virus infections at a hemodialysis center. Infect Control Hosp Epidemiol. 2005;26:752–760. doi: 10.1086/502613. [DOI] [PubMed] [Google Scholar]
- 26.Fissell RB, Bragg-Gresham JL, Woods JD, et al. Patterns of hepatitis C prevalence and seroconversion in hemodialysis units from three continents: the DOPPS. Kidney Int. 2004;65:2335–2342. doi: 10.1111/j.1523-1755.2004.00649.x. [DOI] [PubMed] [Google Scholar]
- 27.Izopet J, Sandres-Saune K, Kamar N, et al. Incidence of HCV infection in French hemodialysis units: a prospective study. J Med Virol. 2005;77:70–76. doi: 10.1002/jmv.20415. [DOI] [PubMed] [Google Scholar]
- 28.Petrosillo N, Gilli P, Serraino D, et al. Prevalence of infected patients and understaffing have a role in hepatitis C virus transmission in dialysis. Am J Kidney Dis. 2001;37:1004–1010. doi: 10.1016/s0272-6386(05)80017-4. [DOI] [PubMed] [Google Scholar]
- 29.Sivapalasingam S, Malak SF, Sullivan JF, Lorch J, Sepkowitz KA. High prevalence of hepatitis C infection among patients receiving hemodialysis at an urban dialysis center. Infect Control Hosp Epidemiol. 2002;23:319–324. doi: 10.1086/502058. [DOI] [PubMed] [Google Scholar]
- 30.Shimokura G, Weber DJ, Miller WC, Wurtzel H, Alter MJ. Factors associated with personal protection equipment use and hand hygiene among hemodialysis staff. Am J Infect Control. 2006;34:100–107. doi: 10.1016/j.ajic.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 31.Fabrizi F, Messa P, Martin P. Transmission of hepatitis C virus infection in hemodialysis: current concepts. Int J Artif Organs. 2008;31:1004–1016. doi: 10.1177/039139880803101204. [DOI] [PubMed] [Google Scholar]
- 32.Delarocque-Astagneau E, Baffoy N, Thiers V, et al. Outbreak of hepatitis C virus infection in a hemodialysis unit: potential transmission by the hemodialysis machine? Infect Control Hosp Epidemiol. 2002;23:328–334. doi: 10.1086/502060. [DOI] [PubMed] [Google Scholar]
- 33.Tu AW, Buxton JA, Whitlock M, et al. Prevalence and incidence of hepatitis C virus in hemodialysis patients in British Columbia: Follow-up after a possible breach in hemodialysis machines. Can J Infect Dis Med Microbiol. 2009;20:e19–e23. doi: 10.1155/2009/641941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Centers for Disease Control and Prevention. Guidelines for laboratory testing and result reporting of antibody to hepatitis C virus. MMWR Recommendations and Reports. 2003;52:1–13. 15. [PubMed] [Google Scholar]
- 35.Ghafur A, Raza M, Labbett W, et al. Travel-associated acquisition of hepatitis C virus infection in patients receiving haemodialysis. Nephrol Dial Transplant. 2007;22:2640–2644. doi: 10.1093/ndt/gfm202. [DOI] [PubMed] [Google Scholar]
- 36.Glynn SA, Wright DJ, Kleinman SH, et al. Dynamics of viremia in early hepatitis C virus infection. Transfusion. 2005;45:994–1002. doi: 10.1111/j.1537-2995.2005.04390.x. [DOI] [PubMed] [Google Scholar]
- 37.Orton SL, Stramer SL, Dodd RY, Alter MJ. Risk factors for HCV infection among blood donors confirmed to be positive for the presence of HCV RNA and not reactive for the presence of anti-HCV. Transfusion. 2004;44:275–281. doi: 10.1111/j.1537-2995.2004.00623.x. [DOI] [PubMed] [Google Scholar]
- 38.O’Boyle CA, Henly SJ, Larson E. Understanding adherence to hand hygiene recommendations: the theory of planned behavior. Am J Infect Control. 2001;29:352–360. doi: 10.1067/mic.2001.18405. [DOI] [PubMed] [Google Scholar]
- 39.Siegel JD, Rhinehart E, Jackson M, Chiarello L and the Healthcare Infection Control Practices Advisory Committee. Guideline for isolation precautions: preventing transmission of infectious agents in healthcare settings. [Last accessed 03/20/10 A.D.];2007 doi: 10.1016/j.ajic.2007.10.007. http://www.cdc.gov/ncidod/dhqp/pdf/isolation2007. [DOI] [PMC free article] [PubMed]
- 40.Centers for Disease Control and Prevention. Infection control requirements for dialysis facilities and clarification regarding guidance on parenteral medication vials. Morbidity & Mortality Weekly Report. 2008;57:875–876. [PubMed] [Google Scholar]