Abstract
Induced pluripotent stem cells have been generated from single human hairs, providing an easily accessible source of cells amenable to efficient reprogramming.
Since the first report on induced pluripotent stem (iPS) cells a little over two years ago1, research in this field has moved at a breathtaking pace, with multiple independent laboratories confirming and extending the original findings. A major effort has focused on understanding and improving the efficiency of the reprogramming protocol, which converts only a tiny fraction of the starting cell population into pluripotent cells. In this issue, Aasen et al.2 report remarkably rapid and efficient reprogramming of human epidermal cells isolated from foreskin and from single hairs plucked from the adult scalp. These findings highlight the importance of the starting cell population in reprogramming and establish an easier method for generating iPS cells.
iPS cells promise a source of patient-specific tissue for cell replacement therapies as well as in vitro models for a variety of genetic diseases. As originally described, the technology relies on retroviral delivery of just four genes (Sox2, Oct4, Klf4 and c-Myc) into mouse1 or human3 fibroblasts to generate cells with embryonic stem (ES) cell properties. Reprogramming of adult human fibroblasts remains inefficient, with only ~0.02% of the somatic cells converted to pluripotent cells in a given experiment3. Possible explanations for the low efficiency include poor viral infectivity, heterogeneous genomic integration of viral vectors, stochastic limitations on the proper levels and timing of transgene expression, and innate, cell type–specific resistance to reprogramming. Recently, Wernig et al.4 controlled for infectivity and genetic heterogeneity using a clever system in which large numbers of genetically identical ‘secondary’ mouse somatic cells were generated from primary iPS cells carrying doxycycline-inducible versions of the four reprogramming factors. When secondary embryonic fibroblasts were induced to reprogram by doxycycline addition, efficiencies increased to 2–4%—an improvement of 25- to 50-fold compared with direct infection of primary cells, but far short of 100%—indicating that infectivity and heterogeneous genomic integration account for some, but not most, of the inefficiency.
Aasen et al.2 describe a method to increase reprogramming of human cells 50- to 100-fold (reaching ~1% efficiency) compared with previous reports. This large leap in efficiency was achieved by targeting keratinocytes rather than fibroblasts. Keratinocytes form the outer layers of the skin, including the epidermis and hair follicle, making them easily accessible for cultivation using methods developed decades ago by Rheinwald and Green5. Keratinocytes can even be isolated from plucked hair, thus obviating the need for an incision into the skin, as is required to collect fibroblasts.
Aasen et al.2 find that the relatively high reprogramming efficiency of keratinocytes is not explained by differences in infectivity between fibroblasts and keratinocytes, as infection of the two cell types with a GFP-expressing reporter virus resulted in a similar percentage of GFP-positive cells. In fact, keratinocyte-derived iPS cell lines had lower numbers of viral integrants than did fibroblast-derived iPS cell lines. These results highlight the importance of the starting somatic cell type and its endogenous transcriptional network in the generation of iPS cells.
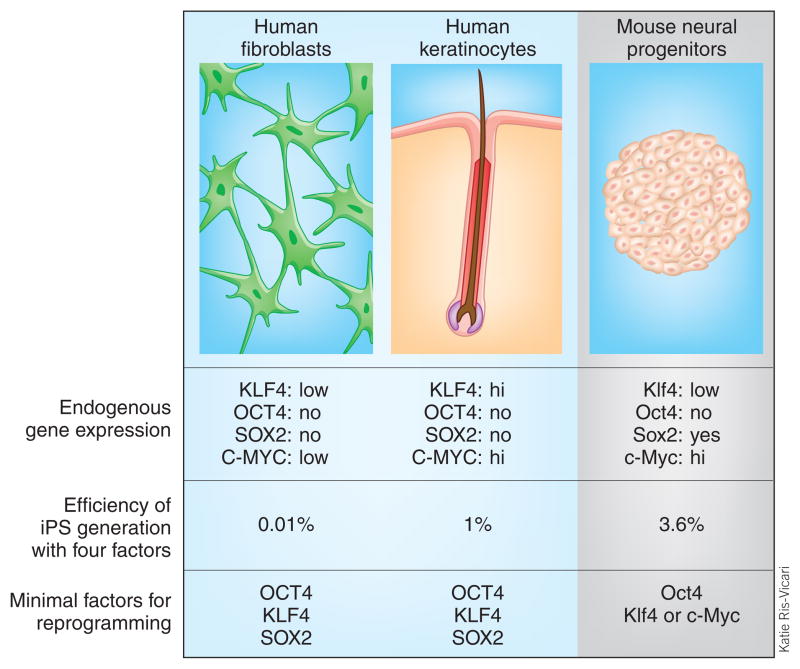
The susceptibility of a somatic cell to reprogramming may depend on its similarity to an ES cell and/or its endogenous expression of the reprogramming genes (Fig. 1). The current study and several recent reports6–8 support this view. Aasen et al.2 show that human keratinocytes, which express much higher levels of the reprogramming factors KLF4 and c-MYC, are more efficient than fibroblasts in iPS cell generation. In an analogous fashion, mouse neural progenitors, which express the reprogramming factor Sox2 as well as higher levels of c-Myc compared with fibroblasts, generate iPS cells with an efficiency of ~3.6% compared with <0.08% for fibroblasts6. Two groups6,7 showed that exogenous Sox2 is not required for mouse neural progenitor reprogramming, and one group6 produced iPS cells with just two factors, Oct4 and either Klf4 or c-Myc. Using microarrays to assay global gene expression, Aasen et al.2 also find that keratinocytes are closer to ES cells than fibroblasts are to ES cells. These results are consistent with work by Huangfu et al.8, who showed that a histone deacetylase inhibitor can drive the gene expression profile of mouse fibroblasts toward that of ES cells and improve the efficiency of iPS generation.
Figure 1.
The starting cell population used to generate iPS cells influences the efficiency and number of genes necessary for reprogramming. Cells with intrinsic baseline expression of reprogramming transcription factors require fewer factors and convert more easily.
Another possible explanation for the higher reprogramming efficiency of keratinocytes relates to the presence of rare stem cells in the starting cell population. The hair follicle from which the iPS cells formed possesses several populations of adult stem cells, including a keratinocyte, a melanocyte and a poorly defined nestin-positive stem cell population. Foreskin keratinocytes also possess a subpopulation of keratinocyte stem cells. The procedures and culture conditions used by Aasen et al.2 indicate that the iPS cells were generated from keratinocytes; however, whether keratinocyte stem cells from the hair follicle preferentially converted to iPS cells remains to be determined. Interestingly, the 1–4% reprogramming efficiency under optimized conditions reported here is similar to the percentage of keratinocyte stem cells thought to be present in hair follicle and foreskin epidermis.
In another very recent study, Maherali et al.9 also created iPS cell lines from human fibroblasts and keratinocytes. Although this group saw the increased speed of keratinocyte reprogramming described by Aasen et al.2, they did not observe differences in reprogramming efficiency between fibroblasts and keratinocytes (~0.002% for both cell types). Maherali et al.9 used a lentiviral doxycycline-inducible system, whereas Aasen et al.2 used a retrovirus. In addition, the two groups adopted different criteria for scoring the generation of iPS clones: Maherali et al.9 used maintenance of iPS cell clones after withdrawal of doxycycline, and Aasen et al.2 used morphology and expression of other ES cell–associated markers. Future comparative studies will be needed to reconcile these differences.
The work of Aasen et al.2 raises the question of whether somatic cell types other than keratinocytes possess an even higher propensity to be reprogrammed. Mouse neural precursors6,7 and hepatocytes10 are also more susceptible to reprogramming than fibroblasts, although from a practical standpoint these tissues are inferior to keratinocytes. More broadly, can any cell type be reprogrammed once the proper set of factors has been identified? Regardless, the current study places skin and hair at the center of iPS cell research.
Contributor Information
Paul Gadue, Children’s Hospital of Philadelphia, 302 ARC, 3615 Civic Center Blvd., Philadelphia, Pennsylvania 19104, USA.
George Cotsarelis, University of Pennsylvania School of Medicine M8 SCL 422 Curie Blvd., Philadelphia, Pennsylvania 19104, USA.
References
- 1.Takahashi K, Yamanaka S. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 2.Aasen T, et al. Nat Biotechnol. 2008;26:1276–1284. doi: 10.1038/nbt.1503. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi K, et al. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 4.Wernig M, et al. Nat Biotechnol. 2008;26:916–924. doi: 10.1038/nbt1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rheinwald JG, Green H. Cell. 1975;6:331–343. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- 6.Kim JB, et al. Nature. 2008;454:646–650. doi: 10.1038/nature07061. [DOI] [PubMed] [Google Scholar]
- 7.Eminli S, Utikal JS, Arnold K, Jaenisch R, Hochedlinger K. Stem Cells. 2008 July 17; doi: 10.1634/stemcells.2008-0317. published online. [DOI] [PubMed] [Google Scholar]
- 8.Huangfu D, et al. Nat Biotechnol. 2008;26:795–797. doi: 10.1038/nbt1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maherali N, et al. Cell Stem Cell. 2008;3:340–345. doi: 10.1016/j.stem.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aoi T, et al. Science. 2008;321:699–702. doi: 10.1126/science.1154884. [DOI] [PubMed] [Google Scholar]