Abstract
Idiopathic giant cell myocarditis is a rare condition with a poor prognosis. Patients with giant cell myocarditis typically die of refractory ventricular arrhythmias or progressive congestive heart failure in about 3 months. The benefit of immunosuppressive therapy varies among patients with giant cell myocarditis, and no factors that would predict which patients will respond to therapy have been identified. Mechanical circulatory support devices, from intra-aortic balloon pumps to more permanent systems, have been used for ventricular support in cases of acute heart failure.
Herein, we describe a case of giant cell myocarditis in a previously healthy 44-year-old woman who presented with cardiogenic shock. She was supported hemodynamically with the Impella Recover LP 2.5 left ventricular assist device until a permanent device could be surgically implanted. To our knowledge, this is the 1st reported case of the successful use of the Impella device for hemodynamic support in a patient with giant cell myocarditis until more definitive treatment could be instituted.
Key words: Autoimmune diseases/complications/drug therapy; disease progression; equipment design; giant cells/drug effects/pathology; heart failure/immunology/pathology; heart-assist devices; hemodynamics/physiology; myocarditis/diagnosis/epidemiology/physiopathology/therapy; treatment outcome; ventricular dysfunction, left
Idiopathic giant cell myocarditis (GCM) is a rare inflammatory condition that is rapidly fatal. It usually affects otherwise healthy adults; symptom onset typically occurs between the 3rd and 5th decades of life.1 Until 1987, when endocardial biopsy was introduced as a diagnostic method, GCM was usually diagnosed at autopsy because of the grim prognosis: in general, survival was less than 3 months from symptom onset.1–3 Several subsequent reports described the benefit of immunosuppressive therapy, and the prolonged time to heart transplantation, in patients who were diagnosed by means of endomyocardial biopsy.4 Cardiac transplantation has been the most effective treatment, although GCM can recur in the transplanted heart.1 Ventricular assist devices have been used to provide support before transplantation.
Herein, we report the case of a woman who presented in cardiogenic shock with a rapidly fulminant course. We used an Impella® Recover® LP 2.5 microaxial device (ABIOMED, Inc.; Danvers, Mass) as a bridge to left ventricular assist device (LVAD) implantation.
Case Report
In September 2009, a 44-year-old woman was admitted to a local hospital because of severe cardiogenic shock. She had been in good health until 4 days before presentation, when she experienced low-grade fever, chills, malaise, and chest tightness followed by severe shortness of breath. She had had no previous medical problems of note, and there was no family history of heart disease. She had never smoked cigarettes or abused alcohol or illicit drugs. In the emergency department, she was in severe respiratory distress, so endotracheal intubation and mechanical ventilation were instituted. She became hypotensive and required vasopressors. Her blood pressure was 75/61 mmHg, and her heart rate was 125 beats/min. Auscultation revealed decreased breath sounds on both sides and diffuse crackles bilaterally, a normal S1, a physiologic splitting of the S2, an S3 gallop best heard over the apex, and no murmurs or rubs. The carotid upstrokes were diminished, and jugular venous pressure was elevated. Chest radiography showed a normal cardiac silhouette with increased pericentral congestion and interstitial markings consistent with pulmonary edema. An electrocardiogram revealed sinus tachycardia, new right bundle branch block, new Q waves and ST elevation in the anterolateral leads, and low-voltage complexes. Multiple short runs of ventricular tachycardia were suppressed by a bolus of amiodarone followed by intravenous infusion. Laboratory test results included an elevated creatinine level of 2.2 mg/dL, blood urea nitrogen level of 49 mg/dL, erythrocyte sedimentation rate of 9 mm/hr, C-reactive protein level of 0.5 mg/L, and leukocytosis (16,000/mm3) with 78% neutrophils. Levels of cardiac markers were elevated, as follows: troponin I, 4 ng/mL; creatine kinase–MB isoenzyme, 21.6 ng/mL; and brain natriuretic peptide, 3,000 pg/mL. A 2-dimensional echocardiogram showed severely reduced global left ventricular (LV) function with an estimated ejection fraction of 0.10, normal LV cavity size, and normal right ventricular (RV) systolic function. In the cardiac catheterization laboratory, the results of emergent coronary angiography were normal. The Impella LP 2.5 was inserted through the patient's left femoral artery and was advanced across the aortic valve under fluoroscopic guidance (Fig. 1). The increased pump flow yielded a total cardiac output of 2.5 L/min. Intermittent episodes of complete heart block during the procedure required the insertion of a temporary transvenous pacemaker.
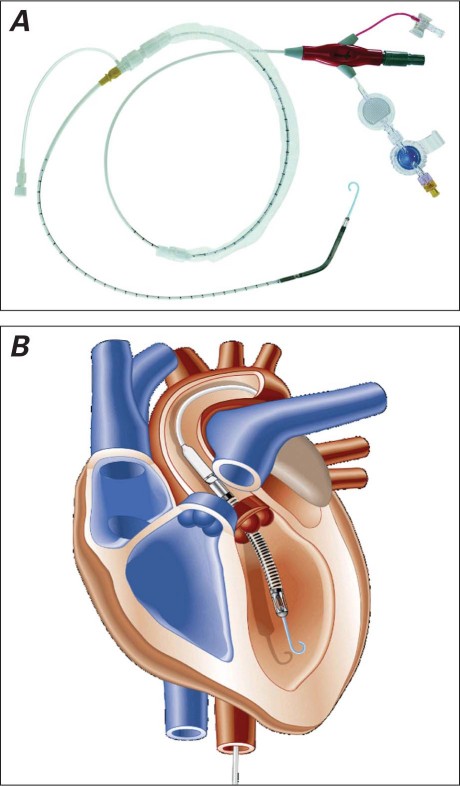
Fig. 1 A) The Impella LP 2.5 microaxial left ventricular assist device. B) Illustration shows the correct position of the Impella device across the aortic valve. (Figures courtesy of ABIOMED, Inc.; Danvers, Mass.)
The patient was then transferred to our institution. Her hemodynamic status had improved with the use of the Impella device, and she was gradually weaned from the vasopressors over 24 hours. At the time of transfer, there was no clinical or echocardiographic evidence of right-sided heart dysfunction. Swan-Ganz pressure readings showed mildly elevated pulmonary capillary wedge pressure (18 mmHg) and normal mean pulmonary arterial and right atrial pressures (15 and 8 mmHg, respectively). It was decided to proceed with implantation of a permanent LVAD, which was delayed for 2 days until consent was obtained. During that time, the patient remained hemodynamically stable, and her respiratory status improved.
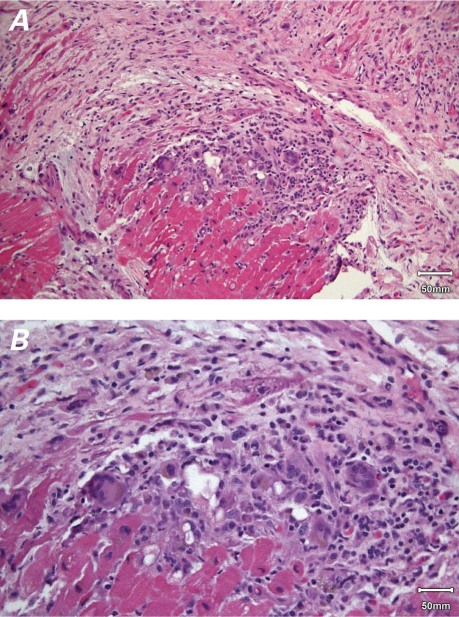
At surgery, the Impella device was removed, and the patient underwent LVAD implantation with an ABIOMED AB5000™. Her condition remained stable throughout the operation. A LV core biopsy specimen obtained during surgery was notable for marked fibrosis and diffuse loss of cardiac myocytes, a distinct mononuclear infiltrate, and numerous multinucleated giant cells. The mononuclear cells were primarily lymphocytes and histiocytes (Fig. 2). Postoperative immunosuppressive therapy consisted of methylprednisolone (1,000 mg/d) and mycophenolate mofetil (1,000 mg, twice daily). Serologic tests for cardiotropic viruses (echovirus, Coxsackievirus, cytomegalovirus, adenovirus, influenza, and parainfluenza) were negative. Levels of antinuclear, anti-DNA, anticardiolipin, and antineutrophil cytoplasmic antibodies were within normal ranges, as were levels of circulating immune complexes C3c and C4.
Fig. 2 A) Photomicrograph shows multifocal myocyte damage and replacement of myocardial fibers by fibrosis and a mixed inflammatory cell infiltrate rich in multinucleated giant cells (H & E, orig. ×50). B) Photomicrograph shows a collection of multinucleated giant cells, lymphocytes, histiocytes, and plasma cells within the myocardium. The infiltrate lacks the eosinophil component, and some of the histiocytes contain hemosiderin (H & E, orig. ×100).
Two days after LVAD placement, the patient became hypotensive with elevated right-sided heart pressure. Acute RV failure was suspected, and echocardiographic results confirmed severely depressed RV function. She was taken to the operating room for emergent RV assist device (RVAD) placement. The sudden deterioration in RV function was believed to have been caused by a hemodynamic imbalance between the failing RV and the newly supported LV; the LVAD may have increased the venous return beyond the capacity of the RV. Another possible contributing factor to RV failure was the development of acute lung injury.
Postoperatively, the patient had recurrent ventricular tachycardia and paroxysmal atrial fibrillation. No recovery of cardiac function was evident on echocardiography. Over 48 hours, the patient experienced renal failure, liver failure, acute respiratory distress syndrome, and disseminated intravascular coagulopathy. She became severely hypoxic with worsening pulmonary edema and significant bleeding from the LVAD inflow cannula. Due to the severe coagulopathy, she was a poor surgical candidate. Her clinical condition deteriorated despite all resuscitative efforts, and she died 4 days after RVAD placement.
Discussion
Giant cell myocarditis is a rare, devastating disease that usually affects otherwise healthy adults. It is characterized by rapid cardiac deterioration with ventricular arrhythmias, congestive heart failure, and associated high mortality rates. The mean survival period for untreated GCM is 3 months. The exact cause of the disease remains unknown, although a link between GCM and a variety of autoimmune disorders has been reported.1 Data from human and experimental cases suggest that GCM is mediated by T lymphocytes and may respond to treatment aimed at attenuating T-cell activity.5,6 Pathologically, GCM is characterized by widespread degeneration and necrosis of myocardial fibers with a mixed inflammatory infiltrate composed of lymphocytes and histiocytes. The presence of eosinophils is usually noted.1,7 Immunosuppressive therapy has been beneficial in some patients in terms of prolonging survival and the time to cardiac transplantation1,4; however, no factors have been identified that predict which patients will respond to therapy.
The Impella (Fig. 1) is a novel, minimally invasive ventricular-unloading system intended for percutaneous insertion in the catheterization laboratory. Currently, 2 LVAD models are available: the Impella Recover LP 2.5 and the Recover LP 5.0. The Impella 2.5 is a 12F catheter suited for percutaneous implantation, whereas the larger Impella 5.0 catheter requires surgical cutdown to the femoral artery for device insertion. The Impella has a caged blood-flow inlet that is placed retrograde into the LV to aspirate oxygenated blood. A microaxial pump then ejects the blood into the ascending aorta, establishing LV-to-aortic bypass. The device has been used to provide short-term circulatory support in patients with acute heart failure from myocardial infarction or viral myocarditis, and in cases of acute deterioration secondary to ischemic and dilated cardiomyopathies8 or after coronary artery bypass grafting. In the treatment of cardiogenic shock, the Impella has provided superior hemodynamic support in comparison with that of standard intra-aortic balloon pumps.9
The Impella LVAD has numerous advantages, including ease of insertion in the catheterization laboratory or intensive care unit without the need for cardiopulmonary bypass, survival rates similar to those associated with other circulatory support devices,10 low complication rates, and high-output ventricular support. Although a need to reposition the pump has been documented,11 no aortic valve injuries have been reported.
To our knowledge, this is the 1st report of the successful treatment of cardiogenic shock secondary to GCM with use of the Impella LP 2.5 microaxial LVAD.
Footnotes
Address for reprints: Hussam Suradi, MD, Krannert Institute of Cardiology, 1801 N. Senate Blvd., Suite E400, Indiana University School of Medicine, Indianapolis, IN 46208
E-mail: hsuradi@iupui.edu
References
- 1.Cooper LT Jr, Berry GJ, Shabetai R. Idiopathic giant-cell myocarditis–natural history and treatment. Multicenter Giant Cell Myocarditis Study Group Investigators. N Engl J Med 1997;336(26):1860–6. [DOI] [PubMed]
- 2.Desjardins V, Pelletier G, Leung TK, Waters D. Successful treatment of severe heart failure caused by idiopathic giant cell myocarditis. Can J Cardiol 1992;8(8):788–92. [PubMed]
- 3.Levy NT, Olson LJ, Weyand C, Brack A, Tazelaar HD, Edwards WD, Hammill SC. Histologic and cytokine response to immunosuppression in giant-cell myocarditis. Ann Intern Med 1998;128(8):648–50. [DOI] [PubMed]
- 4.Cooper LT Jr. Giant cell myocarditis: diagnosis and treatment. Herz 2000;25(3):291–8. [DOI] [PubMed]
- 5.Kodama M, Hanawa H, Saeki M, Hosono H, Inomata T, Suzuki K, Shibata A. Rat dilated cardiomyopathy after autoimmune giant cell myocarditis. Circ Res 1994;75(2):278–84. [DOI] [PubMed]
- 6.Kodama M, Matsumoto Y, Fujiwara M, Masani F, Izumi T, Shibata A. A novel experimental model of giant cell myocarditis induced in rats by immunization with cardiac myosin fraction. Clin Immunol Immunopathol 1990;57(2):250–62. [DOI] [PubMed]
- 7.Litovsky SH, Burke AP, Virmani R. Giant cell myocarditis: an entity distinct from sarcoidosis characterized by multiphasic myocyte destruction by cytotoxic T cells and histiocytic giant cells. Mod Pathol 1996;9(12):1126–34. [PubMed]
- 8.LaRocca GM, Shimbo D, Rodriguez CJ, Stewart A, Naka Y, Weinberger J, et al. The Impella Recover LP 5.0 left ventricular assist device: a bridge to coronary artery bypass grafting and cardiac transplantation. J Am Soc Echocardiogr 2006;19 (4):468.e5-7. [DOI] [PubMed]
- 9.Seyfarth M, Sibbing D, Bauer I, Frohlich G, Bott-Flugel L, Byrne R, et al. A randomized clinical trial to evaluate the safety and efficacy of a percutaneous left ventricular assist device versus intra-aortic balloon pumping for treatment of cardiogenic shock caused by myocardial infarction. J Am Coll Cardiol 2008;52(19):1584–8. [DOI] [PubMed]
- 10.Siegenthaler MP, Brehm K, Strecker T, Hanke T, Notzold A, Olschewski M, et al. The Impella Recover microaxial left ventricular assist device reduces mortality for postcardiotomy failure: a three-center experience. J Thorac Cardiovasc Surg 2004;127(3):812–22. [DOI] [PubMed]
- 11.Henriques JP, Remmelink M, Baan J Jr, van der Schaaf RJ, Vis MM, Koch KT, et al. Safety and feasibility of elective high-risk percutaneous coronary intervention procedures with left ventricular support of the Impella Recover LP 2.5. Am J Cardiol 2006;97(7):990–2. [DOI] [PubMed]