Abstract
In this study, we reviewed a 15-year experience with the treatment of a severe sequela of cardiac surgery: post-sternotomy mediastinitis. We compared the outcomes of conventional treatment with those of negative-pressure wound therapy, focusing on mortality rate, sternal reinfection, and length of hospital stay.
We reviewed data on 157 consecutive patients who were treated at our institution from 1995 through 2010 for post-sternotomy mediastinitis after cardiac surgery. Of these patients, 74 had undergone extensive wound débridement followed by negative-pressure wound therapy, and 83 had undergone conventional treatment, including primary wound reopening, débridement, closed-chest irrigation without rewiring, topical application of granulated sugar for recurrent cases, and final plastic reconstruction with pectoral muscle flap in most cases.
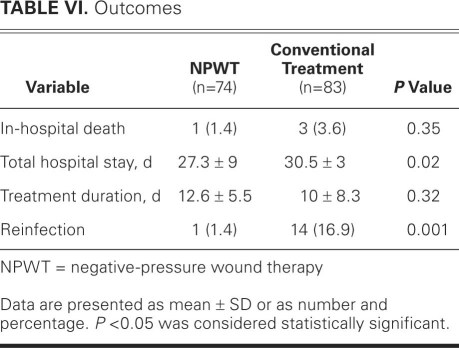
The 2 study groups were homogeneous in terms of preoperative data and operative variables (the primary cardiac surgery was predominantly coronary artery bypass grafting). Negative-pressure wound therapy was associated with lower early mortality rates (1.4% vs 3.6%; P = 0.35) and significantly lower reinfection rates (1.4% vs 16.9%; P = 0.001). Significantly shorter hospital stays were also observed with negative pressure in comparison with conventional treatment (mean durations, 27.3 ± 9 vs 30.5 ± 3 d; P = 0.02), consequent to the accelerated process of wound healing with negative-pressure therapy.
Lower mortality and reinfection rates and shorter hospital stays can result from using negative pressure rather than conventional treatment. Therefore, negative-pressure wound therapy is advisable as first-choice therapy for deep sternal wound infection after cardiac surgery.
Key words: Atmospheric pressure, bacterial infections, cardiac surgical procedures/adverse effects, length of stay, mediastinitis/prevention & control/therapy, postoperative complications/prevention & control/therapy, surgical wound infection/etiology/mortality/prevention & control/surgery, survival rate, vacuum curettage/methods, wound healing/physiology/therapy
Mediastinitis, or deep sternal infection, is a prominent cause of postoperative morbidity and death in cardiac surgery patients. The reported incidence of mediastinitis is between 1% and 3%, and its sequelae are associated with mortality rates of 10% to 25%.1–3 Various wound-healing approaches have been introduced for the treatment of this devastating infection. Conventional forms of treatment usually involve surgical revision with open dressings or closed irrigation, or reconstruction with vascularized soft-tissue flaps such as omentum or pectoral muscle.4,5 These conventional methods have reduced the mortality rate of mediastinitis, but without overwhelmingly satisfactory results.4,6 Negative-pressure wound therapy was introduced by Argenta and Morykwas.7 It has been widely used for managing different types of infected open wounds of the chest, abdomen, head, and neck, and the results appear to be efficacious and reproducible. Negative-pressure wound therapy increases dermal perfusion8–11 and granulation-tissue formation,5,8,12,13 reduces edema and interstitial tissue fluid,8 reverses tissue expansion,14 and reduces bacterial colonization.14–16 Studies have validated the effectiveness of negative-pressure wound therapy in the treatment of sternal wound infections after cardiac surgery.17–20 However, most of the published data are rather recent, and the patient populations have frequently been small. Therefore, larger studies to evaluate the performance of negative-pressure wound therapy are required.19,21
The purpose of this study was to compare the mortality rates, sternal reinfection rates, and lengths of hospital stay in 157 patients with post-sternotomy mediastinitis, treated with either negative-pressure wound therapy (74 patients) or conventional methods (83 patients).
Patients and Methods
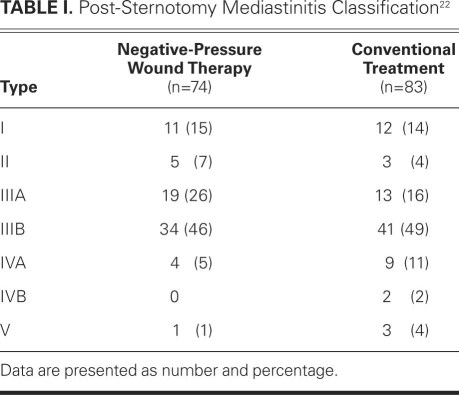
We reviewed the cases of 157 patients with post-sternotomy mediastinitis who were treated at our institution from 1995 through 2010. Post-sternotomy mediastinitis was defined according to the guidelines of the Centers for Disease Control and Prevention (CDC).8 Diagnosis required at least one of the following criteria: 1) an organism was isolated from cultures of mediastinal tissue or fluid obtained during surgery or needle aspiration; 2) evidence of mediastinitis was seen during operation; or 3) chest pain, sternal instability, or fever (>38 °C) was present in addition to purulent discharge from the mediastinum or an organism that was isolated from blood cultures or cultures of drainage from the mediastinal area. However, we excluded patients who had signs of infection but negative substernal tissue cultures. To be included in the study, patients must have undergone surgical revision with removal of the sternal wires. Patients with sterile dehiscence or superficial sternal wound infections were not included. The included patients were also classified according to the criteria of preoperative risk factors and time of presentation, as proposed by El Oakley and Wright22: type I mediastinitis is defined as mediastinitis that occurs within 2 weeks after operation in the absence of risk factors; type II occurs between 2 and 6 postoperative weeks without risk factors; type IIIA is mediastinitis type I in the presence of one or more risk factors; type IIIB is mediastinitis type II with one or more risk factors; type IVA corresponds to type I, II, or III after 1 failed therapeutic attempt; type IVB occurs after more than 1 failed therapeutic attempt; and type V is mediastinitis that first presents more than 6 weeks after surgery. All mediastinitis types were found in our study population (Table I).
TABLE I. Post-Sternotomy Mediastinitis Classification22
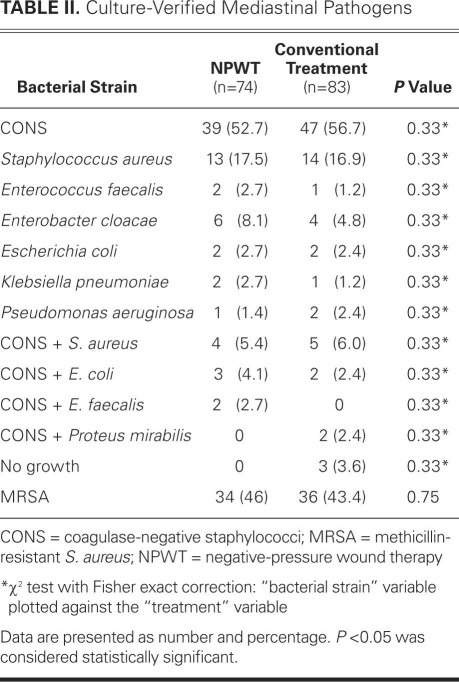
Our institution's standard perioperative antibiotic regimen was the same for both study groups. One dose of intravenous amoxicillin and clavulanic acid (2.2 g) was administered 30 minutes before surgery, 1 dose during surgery, and 6 more doses every 8 hours. When post-sternotomy mediastinitis was diagnosed early in the postoperative period, an initial 2-day washout from antibiotics was performed in order to collect blood and wound cultures, and then intravenous vancomycin was started. When the diagnosis was made after completion of the perioperative regimen, intravenous vancomycin was usually administered and continued until the results of the tissue cultures became available. Thereafter, the antibiotic therapy was adjusted according to bacterial sensitivity and strain. Table II lists all of the pathogens identified in our patients.
TABLE II. Culture-Verified Mediastinal Pathogens
From 1995 through 2001, all 83 patients with post-sternotomy mediastinitis underwent conventional treatment. The primary treatment was surgical reopening of the wound: all sternal wires and soft-tissue sutures were removed, samples of mediastinal fluids were collected for culture, and the pericardial sac was washed with antibiotic and povidone-iodine solutions. A tube for mediastinal irrigation and 2 drains (one in the pericardium and one in the anterior mediastinum) were placed. The sternum was not approximated, and only the skin and subcutaneous layers were fully closed, with nonabsorbable interrupted stitches. Mediastinal irrigation at 100 mL/hr was performed, alternating a 5% povidone-iodine solution with a 0.1% vancomycin solution every 6 hours. On the 7th postoperative day, the irrigation was stopped, and swabs for culture were taken from the drains. If cultures were negative, wound healing was satisfactory, and fever and leukocytosis had disappeared, the drains were removed and the patient was discharged from the hospital. If wound drainage or bacterial growth from cultures was observed, mediastinal irrigation was restarted and continued for 7 days, after which the drains were removed.
If the infection recurred, surgical débridement was performed again. The wound was left open, and daily dressing was performed with topical application of granulated sugar23,24 (3 patients underwent associated hyperbaric therapy). When wounds had negative cultures and appeared to be actively healing by 2nd intention, the first 35 patients were offered reconstruction with pectoral muscle flap.
Since 2002, we used negative-pressure wound therapy for deep sternal infection in all 74 patients after surgical débridement and removal of all sternal wires. Three layers of paraffin gauze dressing were placed on the heart, and sterile polyurethane foam dressing was trimmed to fit between the sternal edges. Often (usually for overweight or obese patients), a 2nd foam dressing was placed over the first to completely include the subcutaneous layer of the wound. A transparent adhesive drape was attached to the foam and 6 to 8 cm beyond, all around the margins of the wound. A drainage tube was placed on the drape to enable drainage, and the application of vacuum was with a V.A.C.® Therapy system (KCI Concepts, Inc.; San Antonio, Texas).
All patients were extubated immediately after the procedure and were returned to their ward room. All were treated with a continuous pressure level of −125 mmHg. The polyurethane foam dressings were changed 3 times weekly under aseptic conditions, without anesthesia. Gentle cleaning of necrotic tissue was also performed. The negative-pressure wound therapy was continued for at least 10 days, then discontinued if fever was absent, leukocyte levels were within normal range, C-reactive protein level was decreased, and the wound was macroscopically infection-free. Wounds were surgically reconstructed in the first 35 patients with use of pectoral muscle flap, and the sternum was rewired in the next 39 patients. During negative-pressure wound therapy, as during conventional treatment, intravenous antibiotic therapy began after microbiological tests were conducted. Regardless of the time needed for wound healing, antibiotic therapy was continued for at least 3 weeks.
Statistical Analysis
Continuous variables (expressed as mean ± SD) were compared between the 2 treatment groups by means of the unpaired t test. Discrete variables (expressed as numbers and percentages) were compared by means of the c2 test. A value of P < 0.05 was considered statistically significant. The SPSS statistical software package version 13.0 (IBM Corporation; Somers, NY) was used for statistical analysis.
Results
Preoperative Data
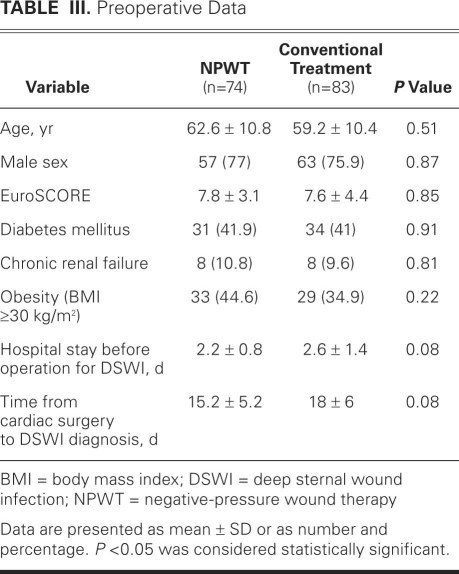
When the patients who were treated conventionally (n=83) were compared with those who underwent negative-pressure therapy (n=74), no significant differences were found regarding age, male sex, additive European System for Cardiac Operative Risk Evaluation (EuroSCORE), presence of diabetes mellitus, chronic renal failure, obesity (body mass index ≥30 kg/m2), or time from cardiac surgery to diagnosis of mediastinitis (Table III). The patients were also grouped according to risk of deep sternal wound infection: low risk included type I, II, or III and high risk included type IV or V. The difference between the 2 groups was not significant. There were 5 high-risk patients in the negative-pressure group and 14 in the conventional-treatment group (P = 0.08).
TABLE III. Preoperative Data
Intraoperative Data
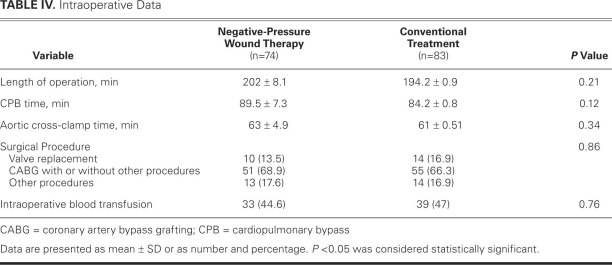
In both groups, no significant differences were found regarding the type of surgical procedure, the need for intraoperative blood transfusion, length of operation, cardiopulmonary bypass time, or aortic cross-clamping time (Table IV).
TABLE IV. Intraoperative Data
Postoperative Data
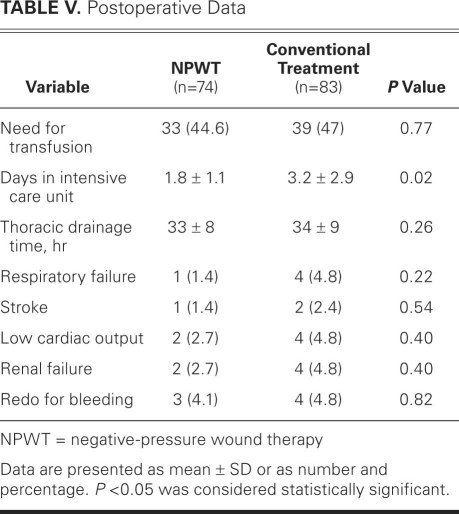
Postoperative length of stay in the intensive care unit differed between the 2 groups. Negative-pressure wound therapy was associated with a significantly shorter mean length of stay of 1.8 days, versus 3.2 days in the conventional-treatment group (Table V). No significant differences were found in other postoperative variables. When the length of intensive-care stay was compared between patients with low-risk deep sternal wound infection (types I–III) and patients at higher risk (types IV and V), a significant difference was observed (2 ± 1.2 vs 6.6 ± 4 d; P = 0.001). This difference remained significant in the conventionally treated group (P < 0.001) but not in the negative-pressure group (2.4 ± 0.9 vs 1.7 ± 1 d; P = 0.19).
TABLE V. Postoperative Data
Outcomes
The in-hospital mortality rate in the negative-pressure wound therapy group was less than half that in the conventionally treated group: 1 patient (1.4%) died in the negative-pressure group, after stroke and pneumonia (after satisfactory wound-healing and after reconstruction with pectoral muscle flap), compared with 3 patients (3.6%) in the conventionally treated group. However, this difference was not statistically significant (Table VI). The negative-pressure patient who died had presented with type IVA deep sternal wound infection. All deaths in the conventionally treated group were due to multiorgan failure caused by severe sepsis (1 patient each had type IIIB, type IVA, and type V mediastinitis). The mean hospital stay after the operation for mediastinitis was significantly lower in the negative-pressure group than in the conventionally treated group (P = 0.02), as was the rate of reinfection (P = 0.001). No local complications, such as bleeding from the wound or right ventricular laceration, were observed at any time in the negative-pressure group.
TABLE VI. Outcomes
After wound healing, secondary closure was achieved by plastic reconstruction with use of pectoralis muscle flap in the first 35 patients and by rewiring of the sternum in the next 39 patients. No differences in outcomes or postoperative complications were found between these 2 reconstruction techniques.
Discussion
The aim of this study was to evaluate the clinical outcomes of post-cardiac surgery mediastinitis, by comparing clinical characteristics and outcomes of patients who underwent negative-pressure wound therapy with those of patients who received conventional treatment. Until 2002, our patients with deep sternal wound infection were treated with surgical revision, with or without multiple open-dressing changes, followed by wound reconstruction or healing by 2nd intention. Previous authors14 have reported a mortality rate as high as 45% from this approach.
An important step forward in the treatment of sternal wound infection occurred when continuous saline and antibiotic irrigation was introduced by Bryant and colleagues in 1969.25 This technique results in a stable sternum; however, studies have reported disappointingly high rates of treatment failure9,10 and death.25
Another established method for treating post-sternotomy mediastinitis is the use of vascularized soft-tissue flaps. The use of pectoral muscle flaps, initially described by Jurkiewicz and colleagues,5 has produced varying results.12,13,15 Other authors have advocated using omentum flaps (first described by Lee and coworkers4) for closing mediastinal defects. Reconstruction with soft-tissue flaps has a relatively low mortality rate according to some reports,12,16 but the technique can be associated with flap-related morbidity.26
A new era in the treatment of infected surgical wounds began in 1997, when Argenta and Morykwas7 introduced a new technique that involved applying negative pressure on wounds. In 1999, Obdeijn and colleagues27 used this technique to treat post-sternotomy mediastinitis. The technique has yielded excellent results in terms of definitive recovery from infection and shorter healing times than those associated with classical treatment.20 The use of this method has spread rapidly, although the mechanisms responsible for its effectiveness have only partially been identified. In 2005, in an experimental study in pigs, Wackenfors and colleagues28 showed a correlation between the intensity of negative pressure and blood flow at the microvascular level. They found that values of negative pressure within the range of −100 to −75 mmHg ensured maximal increase in blood flow in the muscular microvasculature around the wound. The uniform negative pressure applied to the wound leads to arteriolar dilation and thus increases microcirculation, thereby optimizing the wound environment. By continuous suction, excess fluid and tissue edema are decreased, thereby reducing bacterial colonization. These favorable effects on the wound promote granulation and tissue proliferation and ultimately accelerate wound healing. Accordingly, definitive surgical repair (such as primary closure or plastic reconstructive surgery with muscle flaps) can now be accomplished earlier and more safely than before.
Since 2002, we have used negative-pressure wound therapy in our institution to treat deep sternal wound infections, involving surgical débridement, removal of all sternal wires, irrigation of the site, and application of negative pressure (−125 mmHg) through a porous wound filler.
As previously reported,2,23,24 we have 30 years of experience with deep sternal infection treatment, encompassing more than 200 patients with post-sternotomy mediastinitis who were treated conventionally; the mortality rate was 15%. When the outcomes of our last 83 conventionally treated patients were compared with those of the first 74 consecutive patients who underwent negative-pressure wound therapy, we found a clinically relevant (albeit not statistically significant) reduction in hospital death and significant decreases in hospital-stay duration and reinfection rate. Although a time effect could be hypothesized because the 2 groups were not treated concurrently, neither the perioperative protocols nor the indication criteria for the management of deep sternal wound infection changed during the study period. Therefore, any bias would be minimal. Doss and coworkers29 similarly reported shorter lengths of stay and treatment durations after administering negative-pressure wound therapy, as did Mokhtari and colleagues,30 who also calculated greater cost-effectiveness in treatment by negative pressure.
In conclusion, negative-pressure wound therapy has so far proved capable of significantly improving the prognosis of post-sternotomy mediastinitis, a dreaded sequela of cardiac surgery. In particular, the technique reduces the risk of reinfection and the time needed for wound healing, and minimizes further complications. All these benefits justify the use of negative-pressure wound therapy as the first-choice approach for post-sternotomy mediastinitis.
Footnotes
Address for reprints: Alessandro Della Corte, MD, PhD, Department of Cardiovascular Surgery & Transplant, V. Monaldi Hospital, Via L. Bianchi, 80131 Naples, Italy
E-mail: aledellacorte@libero.it
References
- 1.Loop FD, Lytle BW, Cosgrove DM, Mahfood S, McHenry MC, Goormastic M, et al. J. Maxwell Chamberlain memorial paper. Sternal wound complications after isolated coronary artery bypass grafting: early and late mortality, morbidity, and cost of care. Ann Thorac Surg 1990;49(2):179–87. [DOI] [PubMed]
- 2.De Feo M, Renzulli A, Ismeno G, Gregorio R, Della Corte A, Utili R, Cotrufo M. Variables predicting adverse outcome in patients with deep sternal wound infection. Ann Thorac Surg 2001;71(1):324–31. [DOI] [PubMed]
- 3.Francel TJ, Kouchoukos NT. A rational approach to wound difficulties after sternotomy: reconstruction and long-term results. Ann Thorac Surg 2001;72(4):1419–29. [DOI] [PubMed]
- 4.Lee AB Jr, Schimert G, Shaktin S, Seigel JH. Total excision of the sternum and thoracic pedicle transposition of the greater omentum; useful strategems in managing severe mediastinal infection following open heart surgery. Surgery 1976;80(4): 433–6. [PubMed]
- 5.Jurkiewicz MJ, Bostwick J 3rd, Hester TR, Bishop JB, Craver J. Infected median sternotomy wound. Successful treatment by muscle flaps. Ann Surg 1980;191(6):738–44. [DOI] [PMC free article] [PubMed]
- 6.Shumacker HB Jr, Mandelbaum I. Continuous antibiotic irrigation in the treatment of infection. Arch Surg 1963;86:384–7. [DOI] [PubMed]
- 7.Argenta LC, Morykwas MJ. Vacuum-assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg 1997;38(6):563–77. [PubMed]
- 8.Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections, 1988 [published erratum appears in Am J Infect Control 1988;16(4):177]. Am J Infect Control 1988;16(3):128–40. [DOI] [PubMed]
- 9.Catarino PA, Chamberlain MH, Wright NC, Black E, Campbell K, Robson D, Pillai RG. High-pressure suction drainage via a polyurethane foam in the management of poststernotomy mediastinitis. Ann Thorac Surg 2000;70(6):1891–5. [DOI] [PubMed]
- 10.Rand RP, Cochran RP, Aziz S, Hofer BO, Allen MD, Verrier ED, Kunzelman KS. Prospective trial of catheter irrigation and muscle flaps for sternal wound infection. Ann Thorac Surg 1998;65(4):1046–9. [DOI] [PubMed]
- 11.Grossi EA, Culliford AT, Krieger KH, Kloth D, Press R, Baumann FG, Spencer FC. A survey of 77 major infectious complications of median sternotomy: a review of 7,949 consecutive operative procedures. Ann Thorac Surg 1985;40(3):214–23. [DOI] [PubMed]
- 12.Jones G, Jurkiewicz MJ, Bostwick J, Wood R, Bried JT, Culbertson J, et al. Management of the infected median sternotomy wound with muscle flaps. The Emory 20-year experience. Ann Surg 1997;225(6):766–78. [DOI] [PMC free article] [PubMed]
- 13.Wettstein R, Erni D, Berdat P, Rothenfluh D, Banic A. Radical sternectomy and primary musculocutaneous flap reconstruction to control sternal osteitis. J Thorac Cardiovasc Surg 2002;123(6):1185–90. [DOI] [PubMed]
- 14.Sarr MG, Gott VL, Townsend TR. Mediastinal infection after cardiac surgery. Ann Thorac Surg 1984;38(4):415–23. [DOI] [PubMed]
- 15.Klesius AA, Dzemali O, Simon A, Kleine P, Abdel-Rahman U, Herzog C, et al. Successful treatment of deep sternal infections following open heart surgery by bilateral pectoralis major flaps. Eur J Cardiothorac Surg 2004;25(2):218–23. [DOI] [PubMed]
- 16.Milano CA, Georgiade G, Muhlbaier LH, Smith PK, Wolfe WG. Comparison of omental and pectoralis flaps for poststernotomy mediastinitis. Ann Thorac Surg 1999;67(2):377–81. [DOI] [PubMed]
- 17.Argenta LC, Morykwas MJ, Marks MW, DeFranzo AJ, Molnar JA, David LR. Vacuum-assisted closure: state of clinic art. Plast Reconstr Surg 2006;117(7 Suppl):127S–142S. [DOI] [PubMed]
- 18.Fuchs U, Zittermann A, Stuettgen B, Groening A, Minami K, Koerfer R. Clinical outcome of patients with deep sternal wound infection managed by vacuum-assisted closure compared to conventional therapy with open packing: a retrospective analysis. Ann Thorac Surg 2005;79(2):526–31. [DOI] [PubMed]
- 19.Fleck TM, Fleck M, Moidl R, Czerny M, Koller R, Giovanoli P, et al. The vacuum-assisted closure system for the treatment of deep sternal wound infections after cardiac surgery. Ann Thorac Surg 2002;74(5):1596–600. [DOI] [PubMed]
- 20.Sjogren J, Gustafsson R, Nilsson J, Malmsjo M, Ingemansson R. Clinical outcome after poststernotomy mediastinitis: vacuum-assisted closure versus conventional treatment. Ann Thorac Surg 2005;79(6):2049–55. [DOI] [PubMed]
- 21.Cowan KN, Teague L, Sue SC, Mahoney JL. Vacuum-assisted wound closure of deep sternal infections in high-risk patients after cardiac surgery. Ann Thorac Surg 2005;80(6):2205–12. [DOI] [PubMed]
- 22.El Oakley RM, Wright JE. Postoperative mediastinitis: classification and management. Ann Thorac Surg 1996;61(3): 1030–6. [DOI] [PubMed]
- 23.De Feo M, Gregorio R, Renzulli A, Ismeno G, Romano GP, Cotrufo M. Treatment of recurrent postoperative mediastinitis with granulated sugar. J Cardiovasc Surg (Torino) 2000;41 (5):715–9. [PubMed]
- 24.De Feo M, De Santo LS, Romano G, Renzulli A, Della Corte A, Utili R, Cotrufo M. Treatment of recurrent staphylococcal mediastinitis: still a controversial issue. Ann Thorac Surg 2003;75(2):538–42. [DOI] [PubMed]
- 25.Bryant LR, Spencer FC, Trinkle JK. Treatment of median sternotomy infection by mediastinal irrigation with an antibiotic solution. Ann Surg 1969;169(6):914–20. [DOI] [PMC free article] [PubMed]
- 26.Ringelman PR, Vander Kolk CA, Cameron D, Baumgartner WA, Manson PN. Long-term results of flap reconstruction in median sternotomy wound infections. Plast Reconstr Surg 1994;93(6):1208–16. [PubMed]
- 27.Obdeijn MC, de Lange MY, Lichtendahl DH, de Boer WJ. Vacuum-assisted closure in the treatment of poststernotomy mediastinitis. Ann Thorac Surg 1999;68(6):2358–60. [DOI] [PubMed]
- 28.Wackenfors A, Gustafsson R, Sjogren J, Algotsson L, Ingemansson R, Malmsjo M. Blood flow responses in the peristernal thoracic wall during vacuum-assisted closure therapy. Ann Thorac Surg 2005;79(5):1724–31. [DOI] [PubMed]
- 29.Doss M, Martens S, Wood JP, Wolff JD, Baier C, Moritz A. Vacuum-assisted suction drainage versus conventional treatment in the management of poststernotomy osteomyelitis. Eur J Cardiothorac Surg 2002;22(6):934–8. [DOI] [PubMed]
- 30.Mokhtari A, Petzina R, Gustafsson L, Sjogren J, Malmsjo M, Ingemansson R. Sternal stability at different negative pressures during vacuum-assisted closure therapy. Ann Thorac Surg 2006;82(3):1063–7. [DOI] [PubMed]