Abstract
We studied prospectively whether atherosclerotic progression in apolipoprotein-E knockout mice could be noninvasively and accurately measured by use of high-resolution ultrasonographic biomicroscopy. We examined the correlation between the ultrasonographic characterization of ascending aortic atherosclerotic plaque and plasma C-reactive protein, interleukin-1, and interleukin-6 levels in these mice.
In 4 age groups (8, 16, 24, and 32 wk) of 8 male knockout mice each (atherosclerotic groups) and age-matched male C57BL/6 mice (control groups), we used ultrasonographic biomicroscopy to measure maximal plaque thickness or intima-media thickness in the ascending aorta. We compared the findings with corresponding histologic measurements, and we measured plasma C-reactive protein, interleukin-1, and interleukin-6 levels in each group.
Mean atherosclerotic thicknesses and C-reactive protein and interleukin levels were significantly higher in each atherosclerotic group than in the control groups (all P < 0.05). Ultrasonographically measured atherosclerotic thickness correlated well with histologic measurements of the same vascular regions (r = 0.81, P < 0.001). C-reactive protein levels increased concomitantly with age in the knockout mice, and ultrasonographically measured atherosclerotic thickness correlated with those levels (r = 0.626, P < 0.001). However, there was no correlation between plasma interleukin levels and atherosclerotic severity as measured by ultrasonographic biomicroscopy.
In the apolipoprotein-E knockout mice, we found that measurements of intima-media or maximal plaque thickness by ultrasonographic biomicroscopy noninvasively and accurately detected atherosclerotic progression, that plasma C-reactive protein levels correlated with atherosclerosis, and that elevated plasma C-reactive protein levels correlated with atherosclerotic severity.
Key words: Apolipoproteins E/deficiency; arteriosclerosis/diagnosis/physiopathology/ultrasonography; C-reactive protein/physiology; diagnostic imaging/methods; disease models, animal; inflammation/etiology/physiopathology; interleukins/physiology; mice, inbred C57BL; mice, knockout; microscopy; ultrasonography/instrumentation/methods
Atherosclerosis is the leading cause of cardiovascular morbidity and death in the world. Numerous murine models of atherosclerosis are available today. The great success yielded by the apolipoprotein-E knockout (apoE−/−) mouse model is due to the ready availability of the mice, the relative ease of their breeding and colony maintenance, their spontaneously elevated cholesterol levels on a regular chow diet, and their rapid development of atherosclerotic lesions with histopathologic progression, similar to that in human beings.1 Noninvasively and accurately measuring the characteristics of vessels in mice has become increasingly important in the field of vascular biology.2–4 Ultrasonographic biomicroscopy (UBM) with high-resolution scanning enables the noninvasive, real-time evaluation of murine atherosclerotic lesions in vivo.5 The Vevo® 770 (VisualSonics Inc., a division of SonoSite Inc.; Toronto, Canada), one of the newest UBM imaging systems, enables imaging at a relatively low frequency of 30 MHz and a high resolution of 40 μm. We used this system to study prospectively the progression of atherosclerotic plaque in the ascending aortas of apoE−/− mice. To investigate the feasibility of UBM in evaluating atherosclerotic progression in apoE−/− mice in vivo and without dietary manipulation, we compared UBM and histologic measurements of intima-media thickness (IMT) or maximal plaque thickness. We used C57BL/6 mice as nonatherosclerotic control animals.
Proinflammatory cytokines of the interleukin (IL) category are considered to have major roles in the chronic vascular inflammation that is typical of atherosclerosis.6 The serum levels of several of these cytokines have correlated positively with coronary artery disease and its sequelae. Analysis of local vascular inflammation and the cytokines expressed in atherosclerotic plaques has revealed a balance between proinflammatory and anti-inflammatory cytokines, and this balance is crucial in lesion development. In addition, large studies have shown that C-reactive protein (CRP) is a marker of choice for monitoring cardiovascular risk, because it is an even stronger predictor of atherosclerosis than plasma low-density-lipoprotein concentration.7,8 Accordingly, we studied how IMT or maximal plaque thickness measured by UBM correlates with plasma levels of the inflammatory markers CRP, IL-1, and IL-6 in apoE−/− mice.
Materials and Methods
We divided 32 male apoE−/− mice equally into 4 age groups (8, 16, 24, and 32 wk) and used 32 age-matched male C57BL/6 mice as nonatherosclerotic control animals. The mice were purchased from the Animal Center of Beijing University (Beijing, PRC). All were fed chow without dietary manipulation and were cared for in accordance with national guidelines. All procedures conformed with our institution's animal-study guidelines.
Ultrasonographic Biomicroscopy
Ultrasonographic biomicroscopy was performed in all of the mice (Fig. 1A). Before examination, each mouse was given a 0.10- to 0.12-mL/10-g intraperitoneal injection of 0.5% pentobarbital sodium (45 mg/kg, in 0.2 mL of phosphate-buffered saline) as anesthesia, which resulted in a heart rate of 300 to 400 beats/min. The neck hair of each mouse was carefully shaved, and warm ultrasound transmission gel was liberally applied to ensure optimal image quality. Baseline ultrasonographic images of the aortic root and ascending aorta were obtained with the Vevo 770 system's 30-MHz scan head at a 12.7-mm focus and a high resolution of 40 μm. Electrocardiography with a lead II configuration was used for monitoring. All of the mice survived the imaging process.
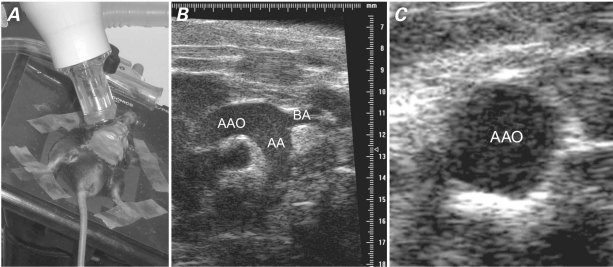
Fig. 1 Ultrasonographic biomicroscopy. A) Photograph shows image acquisition with use of a 30-MHz scan head. B) Long-axis and C) short-axis views of a mouse's ascending aorta (AAO), including the brachiocephalic artery (BA) branch, aortic arch (AA), and proximal descending aorta.
Right parasternal long-axis images captured the ascending aorta, aortic arch, and neck vessels in 1 plane in systole (Fig. 1B). Use of the aortic valve ring and the brachiocephalic artery branch as anatomic landmarks enabled precise location of the measurement site during longitudinal studies. Parasternal short-axis imaging (also in systole) yielded a cross-sectional view of the same arterial site immediately proximal to the branch of the brachiocephalic artery (Fig. 1C). Adjusting the distance between the transducer and the arterial site readily enabled determination of IMT or maximal plaque thickness. A 10-s cine loop was stored digitally for offline examination on a VisualSonics image analysis system. All measurements were performed 3 times at the same vascular site, and all images were analyzed by an operator who was unaware of the classifications of the mice. During inspection of the cine loop, an optimal freeze-frame image of the ascending aorta was taken manually in order to view and measure maximal plaque thickness (if atherosclerotic lesions in the ascending aorta were seen) or maximal IMT (if lesions in the ascending aorta were not seen).
Measurements of Intima-Media and Maximal Plaque Thickness
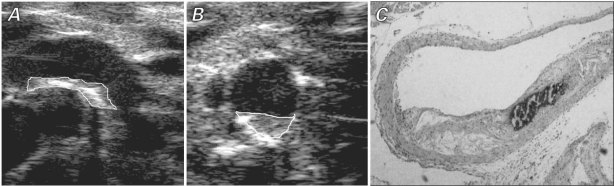
The measurements were performed according to previously validated protocols in human beings: the vascular luminal–intimal interface was the internal measurement site, and the medial–adventitial interface was the external border.9 Localized elevated lesions that had a point of inflection on the surface of the intima-media complex were defined as plaques. The plaques were included when maximal IMT was measured. As with measurement of IMT, maximal plaque thickness was measured at the thickest point at the border of the vascular lumen and the adventitial layer. It was averaged from 3 lesion sites around the thickest part of a plaque, approximately 100 μm apart from each other (Figs. 2A and 2B).
Fig. 2 A) Long-axis and B) short-axis views by ultrasonographic biomicroscopy and C) corresponding histologic photomicrograph (H & E, orig. ×40) show the ascending aorta of a 32-week-old apolipoprotein-E knockout mouse. The ultrasonographic images show plaque in the minor curvature of the ascending aorta; the white lines indicate the border of the plaque.
Intra- and Interobserver Variability
Intra- and interobserver coefficients of variation for the atherosclerotic thickness measurements were analyzed first by 1 operator on 2 different occasions, for validation of intraobserver variability; and then by a different operator, for evaluation of interobserver variability.
C-Reactive Protein and Interleukin Measurements
To determine whether plasma CRP, IL-1, and IL-6 levels were elevated in the apoE−/− mice, each level was quantified and compared with levels in the control mice. All measurements were performed independently by 2 researchers who used standard, commercially available enzyme-linked immunosorbent assay (ELISA) kits for mice. The plasma specimens were mixed thoroughly before assay.
Histologic Measurements
After the UBM data were collected, the mice were killed. The right atrium was cut open for exsanguination. The left ventricular apex was punctured with a butterfly needle, and the mouse was perfused with phosphate-buffered saline and subsequently perfusion-fixed with use of 4% paraformaldehyde. The ascending aorta with neck vessels was removed, fixed in 4% paraformaldehyde overnight, and embedded in paraffin for histologic study. Starting at the aortic root (at 100 μm above the aortic valve ring—or, for morphologic reasons, at the site of maximal plaque thickness), 5-μm serial cross-sections were cut at 50-μm intervals along the ascending aorta, and the sections were stained with hematoxylin and eosin (Fig. 2C). The IMT or maximal plaque thickness was measured by use of a Motic Med 6.0 automated image-analysis system (Motic China Group Co., Ltd.; Xiamen, PRC).
Statistical Analysis
All analyses were performed with the use of SPSS 13.0 software (IBM Corporation; Somers, NY). Resulting values are expressed as mean ± SD. For normally distributed data, unpaired 1-way analysis of variance was used to test vascular wall thicknesses in the 4 different age groups of mice. Unpaired t tests were used when comparing each apoE−/− group and control group of the same age. The Pearson test was used to correlate UBM and histologic values. A P value < 0.05 was considered statistically significant. Intra- and interobserver variability were calculated using coefficient of variation according to the formula SD (x − y)/mean (x, y) × 100.
Results
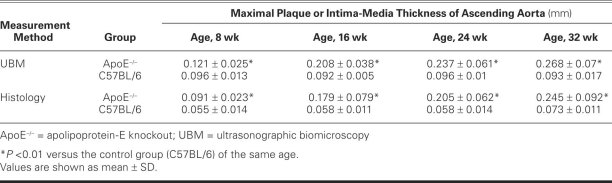
In our UBM study, IMT thickness was significantly higher in the 8-week-old apoE−/− mice than in the age-matched control group (mean thickness, 0.121 ± 0.025 vs 0.096 ± 0.013 mm, P < 0.01), and indeed higher than in the control mice of any age group (Table I). However, in the 8-week-old apoE−/− mice, no clear plaque appeared. With age, IMT gradually increased and the plaque appeared. Accordingly, when atherosclerotic plaque in the ascending aorta was seen in the apoE−/− mice, we measured maximal plaque thickness; and because we found no plaque in the control mice, we measured IMT.
TABLE I. Maximal Plaque or Intima-Media Thickness Measured by UBM and Histology in 4 Age Groups of ApoE−/− (Atherosclerotic) and C57BL/6 (Control Group) Mice
Age-Dependent Progression of Intima-Media or Plaque Thickness
In the apoE−/− mice, mean maximal plaque thickness or mean IMT of the ascending aorta increased significantly with age (UBM, P < 0.01; histology, P < 0.001). There was no corresponding change in the IMT of the control mice (P = NS). The mean atherosclerotic thickness in the ascending aorta in each apoE−/− group was higher than that in each age-matched control group (all P < 0.01).
Intra- and interobserver variability was 5.2% and 7.1%, respectively, for the measurements of thickness.
Atherosclerotic Thickness Correlation between Histology and Ultrasonography
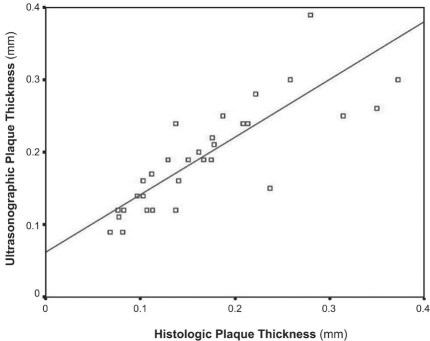
By histologic measurement, the mean atherosclerotic thickness in the apoE−/− mice was 0.091 ± 0.023, 0.179 ± 0.079, 0.205 ± 0.062, and 0.245 ± 0.092 mm at 8, 16, 24, and 32 weeks of age, respectively. Correlation between the UBM and histologic measurements was good (r = 0.81, P < 0.001) (Fig. 3).
Fig. 3 Diagram shows a significant correlation between ultrasonographic and histologic measurements of atherosclerotic thickness in apolipoprotein-E knockout mice of different ages (r = 0.81, P < 0.001).
Assayed C-Reactive Protein and Interleukin Levels
Results of ELISA showed no significant change in plasma CRP, IL-1, or IL-6 levels by age in the control mice. Levels of these inflammatory markers were significantly higher in the apoE−/− mice than in the control mice, and the CRP level increased significantly by age in the apoE−/− mice (Table II).
TABLE II. Inflammatory Marker Levels by ELISA in ApoE−/− Mice and C57BL/6 Mice of Different Ages
Correlation of Plasma Inflammatory Markers and Atherosclerotic Thickness as Measured by Ultrasonography
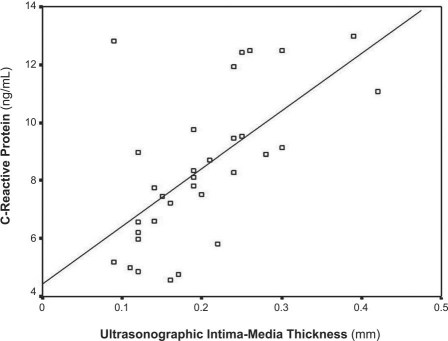
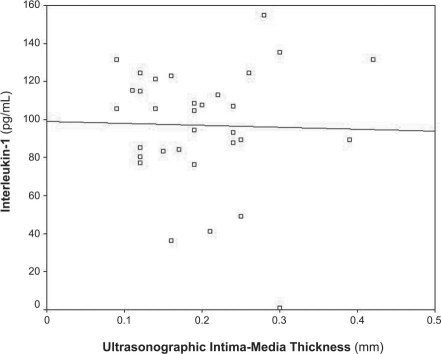
Figure 4 shows that the plasma CRP level in the apoE−/− mice correlated well with atherosclerotic thickness as measured by UBM (r = 0.626, P < 0.001). Figures 5 and 6 show no correlation between the level of IL-1 (r = 0.026) or IL-6 (r = 0.104) and atherosclerotic thickness in the apoE−/− mice (both P > 0.05).
Fig. 4 Diagram shows a significant correlation between plasma C-reactive protein levels and atherosclerotic thickness as measured by ultrasonographic biomicroscopy in apolipoprotein-E knockout mice of different ages (r = 0.626, P < 0.001).
Fig. 5 Diagram shows no correlation between plasma interleukin-1 levels and atherosclerotic thickness as measured by ultrasonographic biomicroscopy in apolipoprotein-E knockout mice of different ages (r = 0.026, P >0.05).
Fig. 6 Diagram shows no correlation between plasma interleukin-6 levels and atherosclerotic thickness as measured by ultrasonographic biomicroscopy in apolipoprotein-E knockout mice of different ages (r = 0.104, P >0.05).
Discussion
Intima-media thickness is measured in arterial walls, usually by ultrasonography, to detect the presence and to track the progression of atherosclerotic disease. In human beings, IMT is typically measured in the common carotid arteries because of their superficial location. Intima-media thickness is an established surrogate marker for human atherosclerosis and is associated with cardiovascular outcome.10 In mice, the common carotid artery is relatively free from atherosclerosis, and plaque formation commonly starts at the aortic root and ascending aorta.11 Accordingly, we chose the ascending aorta in which to follow lesion progression in mice, and we measured vascular wall thickness in systole. Using UBM, we were able to follow the progression of atherosclerosis in the successively older groups of apoE−/− mice by measuring the maximal plaque thickness or IMT in the ascending aorta. Our study showed that UBM measurements of atherosclerotic thickness correlated highly with the histologic data: the respective atherosclerotic thickness values increased linearly with age in the apoE−/− mice. However, UBM is limited because it cannot determine the contents of lipids and fibrosis in plaque tissue, a characterization of plaque vulnerability that is important in clinical studies.
Inflammation is thought to play a major role in the initiation and progression of atherosclerosis and in the development of its clinical sequelae.12 The role of inflammation and CRP in the development of atherosclerosis and cardiovascular disease became of special interest after CRP and other inflammatory markers were identified as cardiovascular disease risk factors.13 C-reactive protein, a classical plasma protein marker that is notably elevated in the acute phase of inflammation, infection, and tissue damage, has been widely used in monitoring and in differential diagnosis.14,15 Upon binding to various ligands that are exposed on damaged tissue or bacteria for the development of phagocytosis (opsonization) and activation of the complement pathway, CRP exerts both pro- and anti-inflammatory functions.16 Emerging evidence indicates that high levels of CRP may be atherogenic.17 Our study showed that plasma CRP level increased significantly with age in the apoE−/− mice and was well correlated with maximal plaque thickness and IMT—indicating a close relationship between plasma CRP level and atherosclerosis in apoE−/− mice. In our apoE−/− mice, elevated CRP levels correlated with the severity of atherosclerosis. In future studies, we plan to investigate whether the elevation of CRP in plasma is a cause of atherosclerosis, a consequence of it, or both.
Interleukin-1 is a prototypic proinflammatory cytokine that exerts biological functions mediated through the IL-1 receptor (IL-1R). After binding to the IL-1R, IL-1 induces the production of a broad spectrum of cytokines and chemokines, as well as the expression of adhesion molecules on endothelial cells, leading to the recruitment of inflammatory cells. Chi and colleagues18 showed that the absence of the IL-1R markedly reduced the progression of atherosclerosis in apoE knockout mice. The activity of IL-1 is counterregulated by its endogenous receptor antagonist (IL-1ra),19,20 and a report showed that the IL-1ra is expressed in endothelial cells and atherosclerotic lesions.21 The balance between IL-1 and the IL-1ra has significant effects on host responses to inflammation. Our study showed that apoE−/− mice had significantly higher plasma IL-1 levels than did the nonatherosclerotic control animals; however, the plasma IL-1 level did not increase with age in the apoE−/− mice. This indicates that a relationship between IL-1 and atherosclerosis exists, but how and when IL-1 acts in the progression of atherosclerosis is not yet fully understood. The action may relate to the balance between IL-1 and the IL-1ra.
The pro- and anti-inflammatory activities of IL-6 might have a biphasic effect on the atherosclerotic process.22–24 Chow-fed apoE−/− mice that were IL-6-deficient (IL-6−/−) showed a tendency toward decreased fatty-streak formation when compared with apoE−/− mice, despite a significant increase in plasma non-high-density-lipoprotein cholesterol in the IL-6−/−/apoE−/− mice.25 In male IL-6−/−/apoE−/− double-knockout mice that were fed a normal chow diet for 1 year, a lifetime deficiency of IL-6 in the male apoE−/− model resulted in increased atherosclerotic lesion formation.26 There is a probable balance between the pro- and anti-inflammatory properties of IL-6 that is crucial in lesion development. Although our study showed that apoE−/− mice had significantly higher plasma IL-6 levels than did the control mice, we found no correlation between IL-6 level and atherosclerotic thickness.
Conclusion
In summary, we found that UBM is a feasible, noninvasive, uncomplicated method that enables accurate, real-time imaging of atherosclerotic progression in mice. In apoE−/− mice, plasma CRP levels correlated with atherosclerosis, and elevated levels of plasma CRP correlated with the severity of atherosclerosis. Further investigation is warranted to determine whether elevated plasma CRP is a cause of atherosclerosis, a consequence of it, or both.
Acknowledgments
We are indebted to the medical and technical staff members of the ultrasound departments of Beijing An Zhen Hospital and the Key Laboratory of Remodeling-Related Cardiovascular Diseases, Capital Medical University, Ministry of Education, Beijing, PRC.
Footnotes
Address for reprints: Ya Yang, MD, Ultrasound Department, Beijing Anzhen Hospital, Capital Medical University, Beijing 100029, PRC
E-mail: Yangya99@hotmail.com
This work was supported by the Natural Science Foundation of China (grant no. 30672000) and Beijing Natural Science Foundation (grant no. 7102044).
References
- 1.Nakashima Y, Plump AS, Raines EW, Breslow JL, Ross R. ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler Thromb 1994;14(1):133–40. [DOI] [PubMed]
- 2.Balaban RS, Hampshire VA. Challenges in small animal noninvasive imaging. ILAR J 2001;42(3):248–62. [DOI] [PubMed]
- 3.Hoit BD. New approaches to phenotypic analysis in adult mice. J Mol Cell Cardiol 2001;33(1):27–35. [DOI] [PubMed]
- 4.Foster FS, Pavlin CJ, Harasiewicz KA, Christopher DA, Turnbull DH. Advances in ultrasound biomicroscopy. Ultrasound Med Biol 2000;26(1):1–27. [DOI] [PubMed]
- 5.Wikstrom J, Gronros J, Bergstrom G, Gan LM. Functional and morphologic imaging of coronary atherosclerosis in living mice using high-resolution color Doppler echocardiography and ultrasound biomicroscopy. J Am Coll Cardiol 2005;46 (4):720–7. [DOI] [PubMed]
- 6.Mangge H, Hubmann H, Pilz S, Schauenstein K, Renner W, Marz W. Beyond cholesterol–inflammatory cytokines, the key mediators in atherosclerosis. Clin Chem Lab Med 2004; 42(5):467–74. [DOI] [PubMed]
- 7.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO 3rd, Criqui M, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 2003;107(3):499–511. [DOI] [PubMed]
- 8.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med 2002;347(20):1557–65. [DOI] [PubMed]
- 9.Pignoli P, Tremoli E, Poli A, Oreste P, Paoletti R. Intimal plus medial thickness of the arterial wall: a direct measurement with ultrasound imaging. Circulation 1986;74(6):1399–406. [DOI] [PubMed]
- 10.Bots ML, Hoes AW, Koudstaal PJ, Hofman A, Grobbee DE. Common carotid intima-media thickness and risk of stroke and myocardial infarction: the Rotterdam Study. Circulation 1997;96(5):1432–7. [DOI] [PubMed]
- 11.Gan LM, Gronros J, Hagg U, Wikstrom J, Theodoropoulos C, Friberg P, Fritsche-Danielson R. Non-invasive real-time imaging of atherosclerosis in mice using ultrasound biomicroscopy. Atherosclerosis 2007;190(2):313–20. [DOI] [PubMed]
- 12.Ross R. Atherosclerosis–an inflammatory disease. N Engl J Med 1999;340(2):115–26. [DOI] [PubMed]
- 13.Ridker PM; JUPITER Study Group. Rosuvastatin in the primary prevention of cardiovascular disease among patients with low levels of low-density lipoprotein cholesterol and elevated high-sensitivity C-reactive protein: rationale and design of the JUPITER trial. Circulation 2003;108(19):2292–7. [DOI] [PubMed]
- 14.Pepys MB, Baltz ML. Acute phase proteins with special reference to C-reactive protein and related proteins (pentaxins) and serum amyloid A protein. Adv Immunol 1983;34:141–212. [DOI] [PubMed]
- 15.Black S, Kushner I, Samols D. C-reactive protein. J Biol Chem 2004;279(47):48487–90. [DOI] [PubMed]
- 16.Pepys MB, Hirschfield GM. C-reactive protein: a critical update [published erratum appears in J Clin Invest 2003;112(2): 299]. J Clin Invest 2003;111(12):1805–12. [DOI] [PMC free article] [PubMed]
- 17.Labarrere CA, Zaloga GP. C-reactive protein: from innocent bystander to pivotal mediator of atherosclerosis. Am J Med 2004;117(7):499–507. [DOI] [PubMed]
- 18.Chi H, Messas E, Levine RA, Graves DT, Amar S. Interleukin-1 receptor signaling mediates atherosclerosis associated with bacterial exposure and/or a high-fat diet in a murine apolipoprotein E heterozygote model: pharmacotherapeutic implications. Circulation 2004;110(12):1678–85. [DOI] [PubMed]
- 19.Kishimoto T. The biology of interleukin-6. Blood 1989;74(1): 1–10. [PubMed]
- 20.Ohsuzu F. The roles of cytokines, inflammation and immunity in vascular diseases. J Atheroscler Thromb 2004;11(6):313–21. [DOI] [PubMed]
- 21.Dinarello CA. Interleukin-1 and interleukin-1 antagonism. Blood 1991;77(8):1627–52. [PubMed]
- 22.Dewberry R, Holden H, Crossman D, Francis S. Interleukin-1 receptor antagonist expression in human endothelial cells and atherosclerosis. Arterioscler Thromb Vasc Biol 2000; 20(11):2394–400. [DOI] [PubMed]
- 23.Tilg H, Dinarello CA, Mier JW. IL-6 and APPs: anti-inflammatory and immunosuppressive mediators. Immunol Today 1997;18(9):428–32. [DOI] [PubMed]
- 24.Xing Z, Gauldie J, Cox G, Baumann H, Jordana M, Lei XF, Achong MK. IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J Clin Invest 1998;101(2):311–20. [DOI] [PMC free article] [PubMed]
- 25.Elhage R, Clamens S, Besnard S, Mallat Z, Tedgui A, Arnal J, et al. Involvement of interleukin-6 in atherosclerosis but not in the prevention of fatty streak formation by 17beta-estradiol in apolipoprotein E-deficient mice. Atherosclerosis 2001;156 (2):315–20. [DOI] [PubMed]
- 26.Schieffer B, Selle T, Hilfiker A, Hilfiker-Kleiner D, Grote K, Tietge UJ, et al. Impact of interleukin-6 on plaque development and morphology in experimental atherosclerosis. Circulation 2004;110(22):3493–500. [DOI] [PubMed]