Abstract
Malignant metastases to the heart and pericardium, which occur far more often than do primary cardiac neoplasms, typically lead to fatal outcomes. The phyllodes tumor is a rare, predominantly benign fibroepithelial breast neoplasm with variable malignancy potential. Herein, we describe the case of a 35-year-old woman who, 3 years after undergoing a simple mastectomy for a rapidly enlarging breast neoplasm, presented with cardiogenic shock and was found to have a large right ventricular tumor that obstructed the right ventricular outflow tract. Despite successful resection of the ventricular mass and a right atrial mass of organized thrombus, the patient died 8 days postoperatively of multiorgan failure due to severe right ventricular dysfunction. Histopathologic analysis determined that the right ventricular mass was a malignant, metastatic phyllodes tumor. To our knowledge, this is only the 2nd reported case of a phyllodes tumor that metastasized to the heart and presented as an intracavitary mass with cardiogenic shock. In addition to discussing our patient's case, we review the pertinent medical literature.
Key words: Breast neoplasms/pathology, cardiac surgical procedures, electrocardiography, fatal outcome, heart neoplasm metastasis, heart ventricles, neoplasm invasiveness, neoplasms/secondary/surgery, phyllodes tumor/pathology/secondary/surgery, recurrence
WEB SITE FEATURE
Malignant cardiac and pericardial metastases, which are diagnosed more frequently than are primary cardiac neoplasms, typically have a poor prognosis.1,2 Previously, these metastases were discovered only at autopsy in approximately one ninth of all patients with malignancies,3,4 but advances in imaging techniques have since improved diagnostic capabilities.
Phyllodes is a rare tumor that occurs almost exclusively in the female breast.5 In comparison with the very similar and more common fibroadenoma, phyllodes tumors have a more cellular stromal element that can outgrow the epithelium. The course of phyllodes tumors is unpredictable: clinically, they tend to behave in a benign manner, but they can recur locally or become malignant with or without metastasis.6 It is extremely rare that a phyllodes tumor will metastasize to the heart and present as an intracardiac mass. Herein, we report such a case.
Case Report
In April 2010, a 35-year-old woman presented at our emergency department with a 15-day history of dyspnea, fatigue, ascites, and pitting pedal edema, all of which had acutely worsened during the previous 3 days. She had undergone a simple mastectomy for a left-breast tumor 3 years earlier, and the histologic specimen was apparently benign. Within 5 months of that surgery, she underwent total excision of a 7 × 5-cm lump, again from the left breast. Histopathologic evaluation at that time suggested a phyllodes tumor with no evident malignancy, and a nuclear bone scan showed no evidence of skeletal metastasis. Afterwards, she was asymptomatic until the onset and progression of the current symptoms.
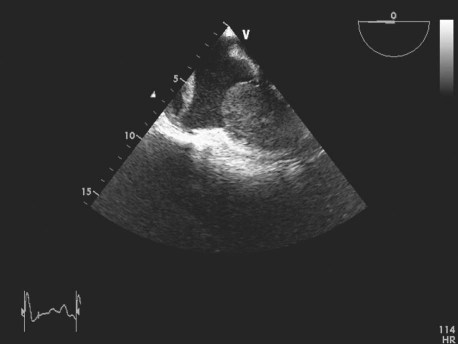
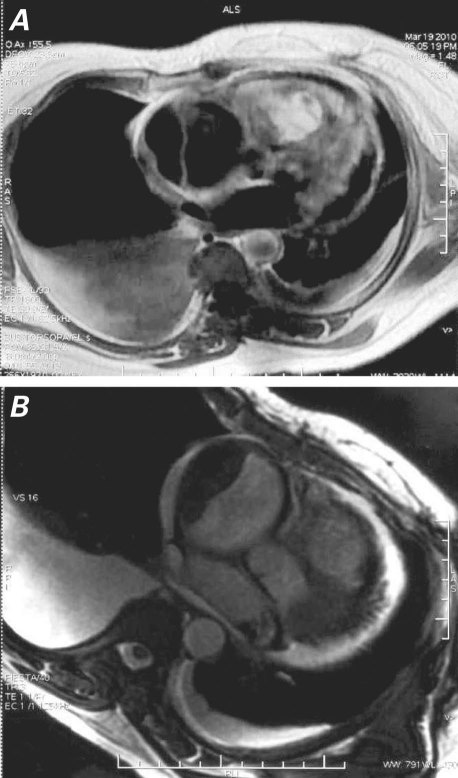
Upon admission to our hospital, she was dyspneic at rest and hypotensive. Physical examination revealed elevated jugular venous pressure, indications of congestive heart failure, and no evident lymphadenopathy. Results of electrocardiography and chest radiography were unremarkable. Transesophageal echocardiography showed a 9 × 7 × 6-cm mass, of homogeneous echogenicity, that occupied nearly all of the right ventricular (RV) cavity and almost completely obstructed the RV outflow tract (RVOT). Another mass was attached to the right atrial free wall. The first mass appeared to infiltrate the RV endocardium, because no clear space could be seen between the mass and the endocardium (Fig. 1). Severe RV dysfunction was also apparent. The left ventricle and left-sided cardiac valves were normal, and there was no evidence of pericardial effusion or pericardial metastasis. Cardiovascular magnetic resonance (CMR), performed for better tissue characterization, showed RVOT obstruction, a hyperintense intracardiac mass that occupied almost the entire RV, and a noncontrast-enhancing mass attached to the right atrial free wall (Fig. 2). Cardiac computed tomography (CT) disclosed similar findings. Results of abdominal ultrasonography were normal, with no evidence of metastasis or a primary tumor.
Fig. 1 Transesophageal echocardiography shows a giant, homogeneous intracardiac mass occupying nearly all the right ventricle and another mass attached to the right atrial free wall.
Real-time motion image is available at www.texasheart.org/journal.
Fig. 2 Magnetic resonance images show A) an intracavitary mass that occupies almost the entire right ventricle and B) a nonenhancing right atrial mass, possibly of organized thrombus.
The possibility of metastasis from the previous phyllodes tumor was considered, as were other differential diagnoses, such as primary cardiac tumor. Because of the severe mechanical RVOT obstruction with persistent hypotension, the patient was scheduled for urgent surgery.
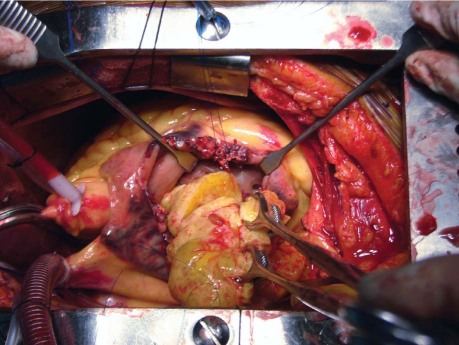
Median sternotomy was performed, cardiopulmonary bypass was started, the right atrium was opened, and a firm, solitary, 8 × 5 × 4-cm mass was excised from the RV (Fig. 3). This mass adhered to and infiltrated the RV wall; the tricuspid and pulmonary valves were not involved. A mass of organized thrombus that adhered to the right atrial free wall was also excised. The patient was weaned from cardiopulmonary bypass with heavy inotropic support. Postoperative echocardiography showed no residual mass, a dilated RV with severe dysfunction, and mild tricuspid regurgitation. The benefit of a RV assist device was considered; however, no such device was available. The patient's RV function did not improve after surgery. On the 8th postoperative day, she died of multiorgan failure secondary to prolonged hypotension.
Fig. 3 Intraoperative photograph shows the removal of the large mass from the right ventricular cavity.
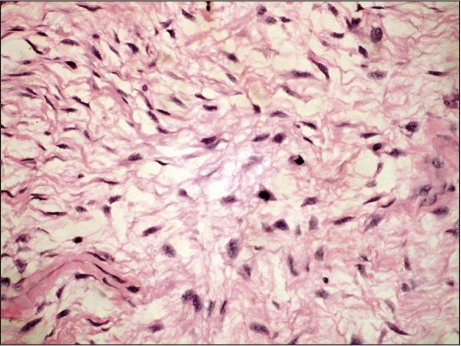
Macroscopically, the specimen from the RV consisted of irregular grayish tissue with a smooth outer surface, measuring 7 × 5 × 4 cm. The cut surface was mucoid and greenish, with brownish hemorrhagic areas. Histopathologic evaluation revealed a mesenchymal tumor composed of spindle cells that were present in a myxoid background. The tumor cells had hyperchromatic nuclei, inconspicuous nucleoli, foci of necrosis and hemorrhage, and increased mitotic activity (4–6 mitotic figures per high-power field) (Fig. 4). Microscopic examination of the right atrial mass showed features of layered, organized thrombus. All findings suggested a malignant, metastatic phyllodes tumor.
Fig. 4 Photomicrograph of a right ventricular mass specimen shows spindled-to-stellate cells embedded in a myxoid background and displaying hyperchromatic nuclei and inconspicuous nucleoli (H & E, orig. ×100).
Discussion
Breast cancers, lung cancers, melanoma, leukemia, and lymphoma are the malignancies that most commonly metastasize to the heart and pericardium. Cardiac metastasis usually occurs in the context of wider metastatic involvement, and the prognosis is typically poor.7 In 1984, Schoen and co-authors8 proposed 4 pathways through which tumors might involve the heart and pericardium: retrograde lymphatic extension, hematogenous spread, direct contiguous extension, or through the bloodstream. Even with advances in imaging techniques in recent years, malignant cardiac metastases are sometimes not discovered until autopsy.9
Phyllodes is an uncommon fibroepithelial neoplasm of the female breast, accounting for perhaps 0.5% of all breast tumors.10 This tumor is usually benign, but it can recur locally in a benign, borderline malignant, or malignant state.6,11 A small number of apparently or initially benign phyllodes tumors will metastasize.12
Upon histopathologic analysis, malignant phyllodes tumors typically exhibit nuclear atypia, a higher degree of stromal cellularity and epithelial overgrowth than the similar and more common fibroadenoma, high mitotic activity, and infiltrative borders.6 In the early 1980s, 16% to 30% of phyllodes tumors were thought to be histologically malignant13; however, it has since been determined that tumor grade can be accurately evaluated only by histologic evaluation of the entire excised specimen, and not by core biopsy alone. Phyllodes tumors are heterogeneous, and each may contain benign, borderline malignant, and malignant regions.14
In a comprehensive review, Kessinger and colleagues15 analyzed distant spread in 67 patients who had metastatic phyllodes tumors. The most common sites of metastasis were the lungs (in 66% of the patients), bones (28%), heart (9%), and liver (6%). Metastasis has been detected from the time of initial therapy to more than a decade later.15
Secondary heart tumors that display partial or total intracavitary growth, as in our patient, are very rare. Myojin and colleagues16 reported what we believe is the only case similar to ours: a female patient underwent emergency surgery because of a large RV mass (phyllodes metastasis) that extended into the pulmonary artery. The mass was resected as completely as possible, but the patient died 15 days later of multiorgan failure.
In patients with malignancies and metastases, imaging should be performed to rule out intracardiac or pericardial involvement. It is particularly important to evaluate the lung tissue and pleura around the mediastinum, as well as the vessels that enter the heart. Echocardiography is the noninvasive imaging method used most frequently. Cardiac CT and CMR provide a large field of view that enables a broad evaluation of cardiothoracic disease. The excellent contrast resolution of CMR enables differentiation between tumor and myocardium, and CMR better distinguishes between tumor, thrombus, and blood flow artifact than does CT.17 In the imaging of cardiac tumors, 3-dimensional (3D) transthoracic echocardiography (TTE) can acquire full volumes, live 3D images, and smaller, magnified, or pyramidal data at high resolution. Whereas 2D TTE and transesophageal echocardiography can underestimate mass volume, 3D TTE reveals copious information about a tumor's type, site, surface features, and anatomic orientation.18 Positron emission tomography is another helpful imaging method.18 Because phyllodes tumors and fibroadenomas are so similar in presentation and on imaging, phyllodes tumors may not be definitively diagnosed preoperatively with the use of 2D echocardiography.
In the treatment of phyllodes tumors, the effectiveness of radiation therapy, chemotherapy, and axillary node excision is unclear. The ideal surgical treatment is a matter of controversy. Local control of phyllodes tumors can be achieved by means of surgical excision with at least a 1-cm clear margin.14 The decision to perform breast-conserving surgery or mastectomy (simple, modified, or radical) greatly depends upon the size and grade of the tumor and the size of the patient's breast.14,19 It is likely that initially inadequate surgical margins contributed to the recurrence and cardiac metastasis of the ultimately fatal phyllodes tumor in our patient. In instances of apparently benign phyllodes tumor, we recommend adequate surgical excision along with imaging—before and afterwards—by multiple techniques.
Supplementary Material
Footnotes
Address for reprints: Naveen Garg, DM, FACC, Department of Cardiology, Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow 226014, India
E-mail: navgarg@sgpgi.ac.in
References
- 1.Lam KY, Dickens P, Chan AC. Tumors of the heart. A 20-year experience with a review of 12,485 consecutive autopsies. Arch Pathol Lab Med 1993;117(10):1027–31. [PubMed]
- 2.Mukai K, Shinkai T, Tominaga K, Shimosato Y. The incidence of secondary tumors of the heart and pericardium: a 10-year study. Jpn J Clin Oncol 1988;18(3):195–201. [PubMed]
- 3.Abraham KP, Reddy V, Gattuso P. Neoplasms metastatic to the heart: review of 3314 consecutive autopsies. Am J Cardiovasc Pathol 1990;3(3):195–8. [PubMed]
- 4.Klatt EC, Heitz DR. Cardiac metastases. Cancer 1990;65(6): 1456–9. [DOI] [PubMed]
- 5.Hoover HC. Cystosarcomas of the breast. In: Raaf JH, editor. Soft tissue sarcomas: diagnosis and treatment. St. Louis: Mosby; 1993. p. 113–21.
- 6.Jones AM, Mitter R, Poulsom R, Gillett C, Hanby AM, Tomlinson IP, et al. mRNA expression profiling of phyllodes tumours of the breast: identification of genes important in the development of borderline and malignant phyllodes tumours. J Pathol 2008;216(4):408–17. [DOI] [PubMed]
- 7.Diaz ML, Villanueva A, Bastarrika G, Zudaire B, del Barrio LG, Noguera JJ. Non-electrocardiogram-gated multidetector-row computed tomography findings of cardiac pathology in oncologic patients. Curr Probl Diagn Radiol 2009;38(5):206–17. [DOI] [PubMed]
- 8.Schoen FJ, Berger BM, Guerina NG. Cardiac effects of noncardiac neoplasms. Cardiol Clin 1984;2(4):657–70. [PubMed]
- 9.Al-Mamgani A, Baartman L, Baaijens M, de Pree I, Incrocci L, Levendag PC. Cardiac metastases. Int J Clin Oncol 2008; 13(4):369–72. [DOI] [PubMed]
- 10.Rowell MD, Perry RR, Hsiu JG, Barranco SC. Phyllodes tumors. Am J Surg 1993;165(3):376–9. [DOI] [PubMed]
- 11.Roa JC, Tapia O, Carrasco P, Contreras E, Araya JC, Munoz S, Roa I. Prognostic factors of phyllodes tumor of the breast. Pathol Int 2006;56(6):309–14. [DOI] [PubMed]
- 12.Khan SA, Badve S. Phyllodes tumors of the breast. Curr Treat Options Oncol 2001;2(2):139–47. [DOI] [PubMed]
- 13.Rosenfeld JC, DeLaurentis DA, Lerner H. Cystosarcoma phyllodes. Diagnosis and management. Cancer Clin Trials 1981;4(2):187–93. [PubMed]
- 14.Guillot E, Couturaud B, Reyal F, Curnier A, Ravinet J, Lae M, et al. Management of phyllodes breast tumors. Breast J 2011;17(2):129–37. [DOI] [PubMed]
- 15.Kessinger A, Foley JF, Lemon HM, Miller DM. Metastatic cystosarcoma phyllodes: a case report and review of the literature. J Surg Oncol 1972;4(2):131–47. [DOI] [PubMed]
- 16.Myojin K, Murakami T, Ishii K, Kunihara T. An emergent operation for metastatic cardiac tumor of malignant cystosarcoma phyllodes [in Japanese]. Jpn J Thorac Cardiovasc Surg 1998;46(2):202–6. [DOI] [PubMed]
- 17.Chiles C, Woodard PK, Gutierrez FR, Link KM. Metastatic involvement of the heart and pericardium: CT and MR imaging. Radiographics 2001;21(2):439–49. [DOI] [PubMed]
- 18.Leja MJ, Shah DJ, Reardon MJ. Primary cardiac tumors. Tex Heart Inst J 2011;38(3):261–2. [PMC free article] [PubMed]
- 19.Sotheran W, Domjan J, Jeffrey M, Wise MH, Perry PM. Phyllodes tumours of the breast–a retrospective study from 1982–2000 of 50 cases in Portsmouth. Ann R Coll Surg Engl 2005;87(5):339–44. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.