Abstract
Rapid growth in nanomaterial manufacturing is raising concerns about potential adverse effects on the environment. Nanoparticle contact with intact organisms in the wild may lead to different biological responses than those observed in laboratory cell-based toxicity assays. In nature, the scale and chemistry of nanoparticles coupled with the surface properties, texture, and behaviors of the organisms will influence biologically significant exposure and ultimate toxicity. We used larval and adult Drosophila melanogaster to study the effects of carbon nanomaterial exposure under several different scenarios. Dietary uptake of fullerene C60, carbon black (CB), or single-walled or multiwalled nanotubes (SWNTs, MWNTs) delivered through the food to the larval stage had no detectable effect on egg to adult survivorship, despite evidence that the nanomaterials are taken up and become sequestered in tissue. However, when these same nanocarbons were exposed in dry form to adults, some materials (CB, SWNTs) adhered extensively to fly surfaces, overwhelmed natural grooming mechanisms, and led to impaired locomotor function and mortality. Others (C60, MWNT arrays) adhered weakly, could be removed by grooming, and did not reduce locomotor function or survivorship. Evidence is presented that these differences are primarily due to differences in nanomaterial superstructure, or aggregation state, and that the combination of adhesion and grooming can lead to active fly borne nanoparticle transport.
Introduction
The scientific study of nanomaterial behavior in the natural environment is in the very early stages (1–5) with many basic principles yet to be discovered. The diversity of engineered nanomaterials coupled with the diversity of living systems makes this a rich new field for scientific inquiry. Many engineered nanomaterials have chemical compositions that are already common in the environment (e.g., elemental carbon, metal oxides) but differ from natural material through size and shape. Scale is of critical importance in biological function, and we can expect a host of unique interactions between living organisms and engineered nanoparticles that have not been present in the natural environment during our evolutionary history.
Nanotoxicology studies often employ cellular assays to identify and isolate fundamental biochemical toxicity pathways. Whole animal toxicology studies compliment cell studies by introducing new issues of function, scale, and bioavailability of nanomaterials to sensitive target cells and subcellular structures (e.g. refs 6 and 7). The fruit fly, Drosophila melanogaster, is a superb model for study of genetics and cell biology with distinct advantages for the study of toxicology (8, 9). Few studies have reported on nanoparticle interactions with Drosophila (1, 10) or Drosophila-derived biomolecules (11, 12). Here, we employed the Drosophila model to investigate nanoparticle interactions at different hierarchical scales of organization on intact whole animals at the egg, larval, and adult stages. We focus on one of the most important classes of nanomaterials, carbons (nC60; single-walled nanotubes, SWNTs; multiwalled nanotubes, MWNTs; carbon black, CB), which show a wide variation in size, shape, and secondary (aggregate) structure and have been the subject of conflicting reports in the nanotoxicology literature (13, 14). The study employs two methods of exposure: ingestion of nanomaterial aggregates suspended in the larval environment, which is a gelatinous nutrient phase, and physical contact of adults with dry nanomaterial powders. These contacting methods are relevant to environmental exposures of terrestrial organisms that may encounter nanomaterials deposited in soils or on surfaces. Because the adult exposure produced a novel effect on climbing ability, an additional assay to quantify effects on adult locomotor function was also included to broaden the functional significance of the NP toxicity studies.
Materials and Methods
Materials
Carbon nanomaterials were acquired from commercial sources: arc-synthesized SWNTs (70% purity, Ni:Y catalyst, CSI, Riverside, CA); MWNTs (MER, Tuscon, AZ) in the form of spherical aggregates (>90% purity, iron catalyst) and as vertically aligned arrays (95% purity, iron catalyst); C60 fullerene (99.5% purity, SES Research, Houston, TX); carbon black (M4750, Cabot Corp., Billerica, MA). Selected samples were washed with toluene to check for the effects of adsorbed organic material (Supporting Information).
Larval Food Preparation
Standard Drosophila food was prepared as described in the Supporting Information. Nanomaterials were placed in 200 μL of ethanol or tetrahydrofuran (THF) at doses of 0, 5, and 50 mg/mL and dispersed by bath sonication for 2–4 h. These 200 μL suspensions were stirred into ~10 mL of liquid Drosophila larval food, which had been converted from the gel to sol phase in an 80 °C hot water bath followed by overnight cooling covered by a cheesecloth to create nanomaterial-containing gels with doses of 0, 100, and 1000 μg/g. Six replicate samples were prepared at each dose, and Drosophila eggs were added. This exposure method (gel-imbedding) avoids the conventional requirement to create stable nanomaterial suspensions in liquids using surfactant stabilization, functionalization, or, for C60, long-time stirring.
Nanomaterial-free solvents of the same volume were used to prepare negative control samples. Ethanol was used as the standard solvent, except where THF is noted below.
Characterization
The nanomaterial-containing larval foods were examined by optical microscopy for uniformity and to inspect for visible aggregates. The C60-containing food was sectioned at a thickness of 80 nm on a Reichert ultramicrotome with a diamond knife, placed on copper grids, stained with uranyl acetate and lead, and viewed on a Phillips 420 transmission electron microscope (TEM) at 120 kV. Morphologies of all carbon nanomaterials and Drosophila were characterized on a LEO 1530 field-emission scanning electron microscope (FE-SEM).
Drosophila Strain
The OregonR strain of Drosophila melanogaster was used for all experiments. Flies were cultured on standard cornmeal, dextrose food for one generation at constant density prior to exposure of eggs or adults.
Toxicity Assays
Populations of adult flies were housed in grape-plate chambers with yeast paste to collect fresh eggs. Individual eggs were gently picked from the surface of the grape plate into each replicate vial with nanomaterial-containing food. A minimum of 4 replicate vials of 50 eggs was used for each material type and concentration. For the adult fly experiments, replicate samples of 10 males were dropped directly into 40 μL of dry nanomaterial loaded as a powder bed into the bottom of a glass vial sealed with parafilm with no access to food or water. The corresponding mass doses, which depend on material-specific bulk powder density, were 3.3 mg (CB), 1.0 mg (SWNT), 28 mg (C60), 2.4 mg (MWNT arrays), and 3.1 mg (MWNT spherical aggregates). The number of living flies was scored for mortality at multiple time points over about 2 days.
Locomotor Function Assay
Adult Drosophila were exposed to the same set of dry nanopowders in test tubes sealed with parafilm. The tubes were placed in a test tube rotator rack at 25 °C with 12:12 light/dark cycles to ensure complete exposure to the flies. A rack containing all test tubes was placed against a white background and tapped against the lab bench causing all flies to drop to the bottom of the tube. Digital videos were acquired and the times recorded for the first fly and the first five flies to reach a line marked on each tube. The time taken for the first fly to cross the line was highly correlated with the time taken by the first five flies to cross the line (r2 > 0.8), so only the first fly data are shown.
Results and Discussion
Dietary Exposure of Nanomaterials to Drosophila Larvae
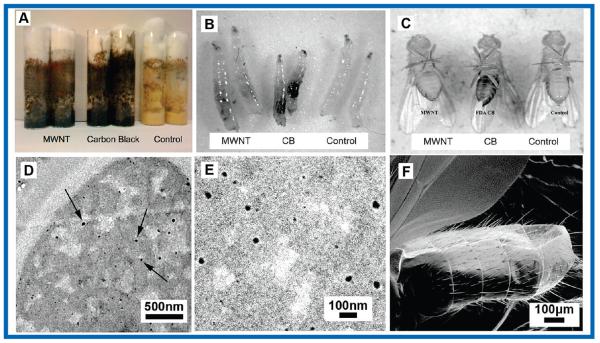
Carbon nanomaterials were suspended in standard Drosophila food at different concentrations, and the food was seeded with 50 eggs of Drosophila melanogaster. After egg hatching (24 h), the larvae were observed to crawl through the food and ingest the suspended nanomaterials, whose small aggregate size and uniform dispersion were engineered to make it difficult or impossible for the larvae to sense and avoid the synthetic materials. The feeding phase of the Drosophila larval period is ~4 days, in which they range in size from ~1 to 4 mm with mouth openings from ~50 to ~200 μm. During this time, larvae consume nutrients in preparation for pupation. Optical and electron micrographs indicate uniform dispersion of nanomaterials in the food matrix at aggregate sizes below 1 μm and the postexposure localization in the lumen of the larval gut (Figure 1). Nanomaterials were also observed as dark concentrations in tissues of adults that hatched from these larval-diet-exposed flies. Scanning electron microscopy confirmed that these darkened tissues were not the result of external particle/tube coating the adult cuticle. These results suggest that nanomaterials consumed by the larvae were assimilated into the tissues of the developing fly and are sequestered into the tissues (Figure 1, top right).
FIGURE 1.
Drosophila larval exposure assay for carbon nanoparticles. (A) Drosophila food with suspended carbon nanomaterials. (B) Evidence that carbon nanomaterials in the food are taken into the larval gut (black areas compared to control). (C) Adult Drosophila hatched from nanomaterial-containing food have sequestered particles in tissues associated with external bristles (darkened abdominal regions, compared to control, most evident in CB sample). (D, E) Thin-section TEM showing C60 particles dispersed in the Drosophila food at an aggregate size ~1000 times smaller than a larval mouth opening (~20 nm vs >50 μm) permitting nanomaterials to readily enter the larval gut. (F) SEM showing absence of CB particles on the exterior of adult flies hatched from CB-containing food, indicating that the dark patches in C represent sequestration in internal tissue.
Larval Toxicity Assay
From each vial containing 50 eggs, the number of flies emerging was counted twice a day as a measure of nanomaterial effects on egg-to-adult viability and development time. In no case did the carbon nanomaterials alter total survivorship (Figure 2A). Males and females emerged at statistically indistinguishable rates (all terms in an ANOVA for particle mass concentration, sex and interactions were not significant; e.g., P > 0.2). The suspension of C60 in different solvents prior to dispersal in the food matrix had no detectable effect on toxicity in this egg to adult assay (Figure 2B; P > 0.2). Development time was delayed slightly at high nanomaterial doses, but the effect was not significant even if all data for different materials (CB, SWNTs, MWNTs) were pooled and the control, 100 μg/g and 1000 μg/g doses were pooled (data not shown). It is significant that the nanomaterials were not toxic at 1000 μg/g in food, which is a much higher environmental concentration than would be expected in most scenarios associated with the release of nanoparticles by consumers or manufacturers.
FIGURE 2.
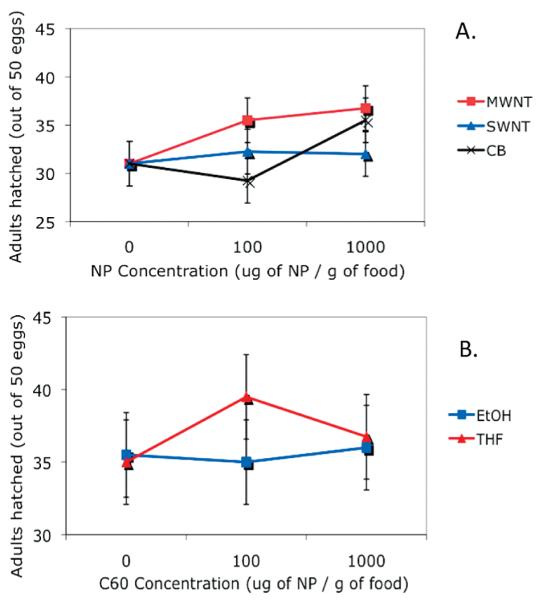
Drosophila larvae exposed to carbon nanoparticles (NP) in food have little or no toxic effects. (A) No significant effect of material type or concentration on egg-to-adult viability for Drosophila raised in food containing 0, 100, or 1000 ug-nanomaterial/g-food. Means and SE from least-squares ANOVA model are shown (n = 4 vials of 50 eggs per condition; all terms of ANOVA have P > 0.2) (B) No effect of C60 concentration or solvent type (ethanol or tetrahydrofuran) used to disperse C60 on egg-to-adult survival. Means and SE from least-squares ANOVA model are shown (n = 4 vials of 50 eggs per condition; all terms of ANOVA have P > 0.3).
There are few studies to which the present results can be directly compared. The study of Leeuw et al. (1) showed no loss of viability or adult weight upon exposure of Drosophila larvae to 9 ppm SWNTs dispersed in food paste - consistent with our results which extend to 1000 ppm. Recent studies using other organisms include Velzeboer et al. (15), who report low toxicities of various nanomaterials including C60 and SWNT at concentrations up to 100 μg/L in aquatic systems, and Blickley and McClellan-Green (16), who report low toxicity of fullerene to embryo, larvae and adult Fundulus heteroclitus. Johansen et al. (17) report modest but persistent effects of C60 on rapidly growing soil bacteria, while Brunet et al. (18) report bacterial toxicity of some fullerene formulations - THF/nC60, PVP/C60 but not aqueous C60 or fullerol. The recent comparative study by Kang et al. (19) report toxicity to gram-negative bacteria for the carbon nanomaterial family in rank order SWNT > C60 > MWNT. Petersen et al. (20) studied uptake of 14C labeled SWNTs and MWNTs by aquatic worms and found that the nanomaterials entered the organisms but were purged within a few days and did not persist in tissues. Petersen et al. (21) further report bioaccumulation and limited depuration of MWNTs after uptake by Daphnia magna.
Low toxicity may reflect a low bioavailability of nanomaterials to internal tissue and organs (1). We did not attempt to quantify uptake, but the optical microscopy showed that at least some of our test nanomaterials do become sequestered in adult Drosophila tissue after larval exposure (Figure 1), which indicates transport across the gut lining. It is notable that nanomaterials (carbon black) appear localized in tissues where sensory bristles are dense, and are not evenly distributed across adult tissues. These flies were tested for differences in fecundity after emerging from the food by allowing them to lay eggs for replicate broods of 24 h. No significant effect of nanomaterial exposure was detected (data not shown).
Adult Fly Exposure to Nanomaterials
The different nanomaterials types varied considerably in their effect on adult behavior and survivorship following physical exposure to powder beds in the dry state. CB, toluene washed CB, and SWNTs spontaneously adhered to external surfaces of the flies on contact and engulfed the flies in a fine particle coating (Figure 3), killing them within several hours. Other materials (C60, MWNT arrays and toluene washed SWNTs), did not adhere to the outer surface of the flies as extensively, and could be removed by the flies through natural grooming behaviors.
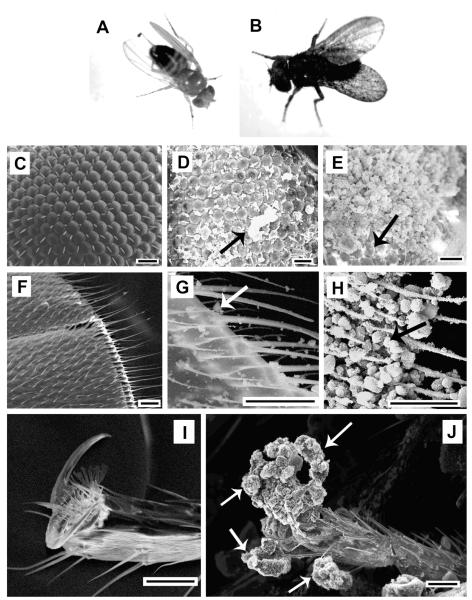
FIGURE 3.
Representative morphologies observed in adult Drosophila exposed to carbon nanomaterials as dry powders. Top row: whole flies. (A) unexposed; (B) after SWNT exposure with whole body blackened by adherent nanoparticles (particles not visible at this scale). Second row; Drosophila compound eye: (C) unexposed; (D) after SWNT exposure; (E) after CB exposure. SWNTs form discontinuous adhesive clusters (see arrow, D), while carbon black causes near complete eye coverage with only a small patch of uncovered eye cells remaining visible (see arrow, E). Third row; Drosophila wing: (F) unexposed; (G) after crushed C60 exposure; (H) after carbon black exposure. Bottom row; Drosophila leg and foot. (I) unexposed; (J) after MWNT spherical aggregates exposure. MWNTs decorate the Drosophila pincer structure used for gripping (see arrows). All scale bars 20 μm. Note that the nanomaterial structures here are primary aggregates of varying size and structure as discussed later in detail. Example nanoparticle aggregates are pointed out by arrows in D, G, H, and J. The arrow in E shows uncovered eye cells.
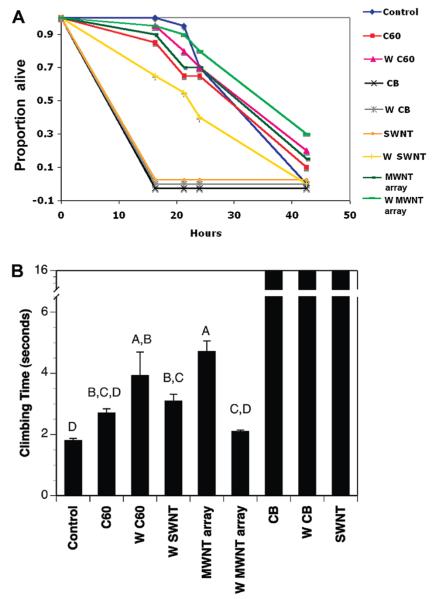
The strongly adhering nanomaterials (CB, toluene washed CB and SWNT) significantly reduced survivorship (Figure 4A) relative to unexposed controls. Survivorship for the less-adhering nanomaterials (C60, MWNT arrays, toluene washed SWNTs), was statistically indistinguishable from unexposed control flies (for six treatments excluding CB, W CB and SWNT, χ2 = 7.3, df = 5, P > 0.19). This experiment was repeated in two other assays using slightly different scoring times, with qualitatively similar results. The estimate of time to complete mortality for CB and SWNT (Figure 4A) is conservative, as all such flies were dead within ~6 h in repeat experiments where mortality was scored more frequently (not shown).
FIGURE 4.
(A) Differential toxicity of nanomaterials to adult Drosophila. Abbreviations as in Figure 2. “W” denotes toluene-washed samples. For CB and SWNTs, values are staggered around zero on the Y-axis to show different NPs. Toluene washing of the single-wall nanotube sample (W SWNT vs SWNT) significantly increases survivorship (log rank and Wilcoxon χ2 = 18.7, df = 1, P < 0.0001). (B) Locomotor effects of nanomaterial exposure on adult Drosophila. The time required for a fly to climb to 6 cm in a glass test tube after nanomaterial exposure is shown. Values are means from four recordings in each of two replicate sets of flies; error bars are standard errors. The three materials on the right side of each graph with high values exhibited whole-body coating completely preventing climbing (16 s shown but no climbing occurred). The six other materials showed significant variation in their effects on climbing (due to non-normality of the data, a nonparametric Wilcoxon/Kruskal–Wallis test was used: χ2 = 31.72, df = 5, P < 0.0001 for the six NPs that slowed climbing). Bars with the same letter on the plot are not significantly different, while bars with different letters are significant, based on a Tukey-Kramer HSD test correcting for multiple comparisons.
Effects of Nanomaterial Exposure on Fly Locomotor Performance
The ability of adult Drosophila to climb test tube walls in the presence of nanomaterial powders was recorded using digital videography. Figure 4B gives the results plotted as average time required for the first fly to reach a height of 6 cm after being knocked into the nanomaterial powder at the bottom of the test tube. In parallel with the adhesion and mortality effects (Figure 4A), CB and SWNTs immobilized the flies at the bottom of the tube completely preventing climbing, while the other nanomaterials that did not adhere to the flies allowed climbing but showed significant material-to-material differences on climbing rates (completion times). In general, control flies (no exposure) climbed the fastest, and exposure to any material slowed climbing time, with toluene washed C60 (W C60) and MWNT arrays having the greatest impact on climbing, and C60, toluene washed SWNT and MWNT (W SWNT; W MWNT) having less effect (Figure 4B). The duration of NP exposure altered the impact of the NPs on climbing speed, as did the use of glass vs plastic vials, or parafilm vs cotton plugs to seal the vials. However, the immobilization effect of CB and SWNT was highly repeatable. The details of these time- and chamber-effects on locomotor performance will be reported in a separate study.
The mechanism of the locomotor impairment warrants further experimentation but may be related to the fine-structure of the coated fly foot. Studies have indicated that secretions from the pads of the foot are important in adhering to smooth surfaces, and that the surface area of contact is related to the magnitude of the attractive force (22). If nanomaterials interfere with the fine scale pads on the fly foot or hinder the release of fluid, this could limit the adhesive force for climbing up the smooth wall of the glass tubes we used in our experiments.
Material-to-material differences are striking (Figure 4), with toxicity trends summarized as: CB ~ SWNTs ≫ C60 ~ MWNT-arrays. The effect of toluene washing was material dependent, having no effect for C60 but significantly reducing the toxicity of SWNTs and MWNT arrays. The intention behind toluene washing was to control for the effects of soluble organic matter, if any, present on these high-surface-area hydrophobic materials. We observed, however, that toluene washing collapsed the low-density aggregate structure of the SWNT sample by the action of surface tension during drying to result in a much denser material superstructure. In this form, the availability of tube bundles or small aggregates to detach from the bulk powder and adhere to external fly surfaces appears to be greatly reduced, which is the likely cause of increased survivorship.
Further, we noticed that the main toxicity trend (SWNTs ≫ C60 ~ MWNT-arrays) is consistent with the availability of fine (<20 μm) aggregates or free primary nanoparticles. The C60 powder consisted of very large (~ 20–50 μm) aggregates with smooth and faceted crystal faces (Supporting Info.), and was poorly adherent. In contrast, the CB samples (Figure S1B, Supporting Info.) consist of small aggregates (~2–10 μm) with porous, nanostructured surfaces due to irregular packing of the CB 20–50 nm primary nanoparticles. Some insect surfaces have natural micro/nanotextures (23) including the Drosophila wing and foot (Figure 3), and these are capable of interpenetration and interlacing interactions with the textured carbon black aggregates.
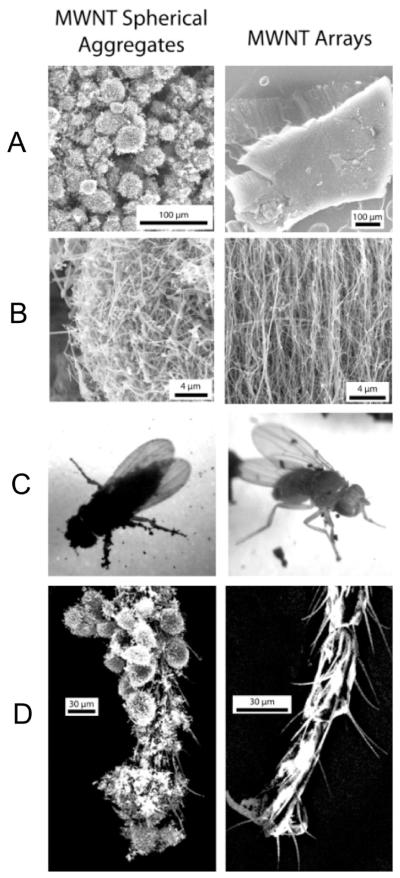
The effect of aggregate size is particularly apparent when comparing two different MWNT samples (Figure 5). Here adult Drosophila interact with: (i) massive vertically aligned MWNT arrays of typical array size >100 μm, and (ii) spherical disordered aggregates of nominal size 5–20 μm. The small spherical aggregates adhere much more extensively, cause whole-body coverage of the flies, and are as toxic as the most toxic materials in Figure 4 (CB, SWNTs). The initial low toxicity of MWNTs in Figure 4A was clearly due to their large-aggregate array structure (MWNT-arrays), and not to an intrinsic property of MWNTs relative to SWNTs or CB.
FIGURE 5.
Effect of aggregation state on adhesion and toxicity in adult Drosophila. Columns show two contrasting MWNT aggregation states: left, spherical aggregates of randomly entangled nanotubes; right, large vertically aligned arrays. The small random aggregates show strong adhesion and whole-body coverage (C, left) and richly decorate the fly leg and foot in a manner that completely alters the external morphology of the active gripping structures (D, left). The MWNT arrays show limited adhesion and coverage (C, right), no interference with gripping structures on the fly foot (D, right), and low toxicity (Figure 4A).
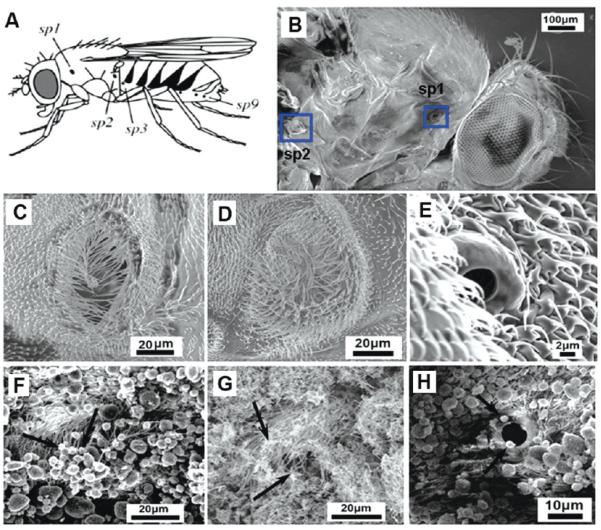
Such physical mechanisms of nanotoxicity have not been widely reported, one exception being the observation of airway blockage by aggregated nanotubes during intratracheal instillation in rats (24). Lehmann et al. (25, 26) report that Drosophila use spiracle opening to regulate respiratory gas flux in response to metabolic requirements; Figure 6 shows that nanomaterials can partially block spiracles (F,G vs unexposed controls in C-E), which may in turn hinder oxygen diffusion and impair metabolism.
FIGURE 6.
Possible mechanism of nanoparticle-induced mortality in adult Drosophila. (A) Location of spiracles in Drosophila: sp1, mesothoracic spiracle; sp2, metathoracic spiracle; sp3 to sp9, abdominal spiracles, image from Lehnmann (25). (B) SEM image shows mesothoracic and metathoracic spiracle of an adult Drosophila (blue square) Center row (C–E): SEM images of spiracles in unexposed Drosophila; sp1 (C), sp2 (D), both 20–50 μm, and an abdominal spiracle (E) at 5 μm. Bottom row (F–H): spiracles are covered/decorated with nanomaterials (see arrows) after dry exposure of adults to CB (F); MWNTs (G); CB (H).
Overall, aggregate size appears to be the most important variable determining adhesion and toxicity to adult Drosophila. This is not unexpected as the ratio of adhesion forces to gravitational and inertial detachment forces increases with decreasing particle size. The largest class of adhering aggregates is about 20 μm, which is on the order of the threshold between free-flowing (nonaggregating) powders that can be size classified by dry methods (~30 μm) and finer particles that undergo spontaneous aggregation. Particles of mean size 7–10 μm have been used in pesticide applications where adhesion to insects can cause active transport of the active ingredient to microhabitats that are hard to reach directly (27). Nanoroughness in the contact area is also known to influence adhesion (28), and our data (Figure 3) suggest intrinsically stronger adhesion of the nanorough carbon black (aggregates up to 20 μm adhere) compared to nanosmooth C60 (only finer aggregates adhere).
In the environment, contact with pure nanomaterial deposits is expected only in hot spots near manufacturing point sources or during intentional application of pest control agents (27) and, thus, the adult Drosophila results presented here represent a high-dose limiting case. We anticipate much lower doses in other environmental scenarios, where insects contact airborne nanoparticles or contact soils, sediments, and surfaces containing a nanoparticle fraction through prior deposition. In these scenarios, we expect nanoparticle/insect adhesion and transport similar to microbial transport by flies acting as disease vectors (29). Figure S2 (in the Supporting Information) shows a simple experiment in which flies exposed to MWNT spherical aggregates in one test tube move to an adjacent connected clean test tube and through grooming behaviors contaminate the second test tube with easily identifiable nanotube deposits. Clean unexposed flies were observed to be contaminated with nanoparticles through fly-to-fly contact and grooming behaviors. In the environment, such transport and redeposition may bring nanoparticles into contact with human or environmental receptors that would not otherwise be exposed. It is noteworthy that there is an overlapping length scale between the adherent nanoparticle aggregates observed in this study (typically 1–20 μm) and pollen (6–100 μm) or bacteria (~1 μm).
In summary, exposure to the major members of the carbon nanomaterial family (C60, carbon black, SWNTs, and MWNTs) elicit responses in Drosophila that depend sensitively on exposure route (larval ingestion, adult dry contact) and material aggregation state. Larval ingestion leads to systemic uptake and tissue sequestration but is without other significant consequences even at high doses (1 mg/g of food). In contrast, dry exposure of adults to primary particles or small aggregates (<20 μm) leads to whole-body coverage, loss of locomotor function, and mortality, while lower doses lead to active transport and fly-to-fly transmission.
Supplementary Material
Acknowledgments
Financial support was provided by the Superfund Research Program Grant P42 ES013660, a National Science Foundation NIRT Grant DMI-0506661, and the Brown University Office of Vice President for Research Seed Fund Program. The technical contributions of Anthony McCormick and Paula Weston at Brown are gratefully acknowledged. Although the research described in the Article was funded in part by the NIEHS, it does not necessarily reflect the views of the Institute, and no official endorsement should be inferred.
Footnotes
Supporting Information Available Additional details concerning the experimental procedures, nanomaterial aggregate structures, and fly borne nanoparticle transport. This material is available free of charge via the Internet at http://pubs.acs.org.
Literature Cited
- (1).Leeuw TK, Reith RM, Simonette RA, Harden ME, Cherukuri P, Tsyboulski DA, Beckingham KM, Weisman RB. Single-walled carbon nanotubes in the intact organism: Near-IR imaging and biocompatibility studies in Drosophila. Nano Lett. 2007;7(9):2650–2654. doi: 10.1021/nl0710452. [DOI] [PubMed] [Google Scholar]
- (2).Baun A, Hartmann NB, Grieger K, Kusk KO. Ecotoxicity of engineered nanoparticles to aquatic invertebrates: a brief review and recommendations for future toxicity testing. Ecotoxicology. 2008;17(5):387–395. doi: 10.1007/s10646-008-0208-y. [DOI] [PubMed] [Google Scholar]
- (3).Klaine SJ, Alvarez PJJ, Batley GE, Fernandes TF, Handy RD, Lyon DY, Mahendra S, McLaughlin MJ, Lead JR. Nanomaterials in the environment: Behavior, fate, bioavailability, and effects. Environ. Toxicol. Chem. 2008;27(9):1825–1851. doi: 10.1897/08-090.1. [DOI] [PubMed] [Google Scholar]
- (4).Metz KM, Mangham AN, Bierman MJ, Jin S, Hamers RJ, Pedersen JA. Engineered nanomaterial transformation under oxidative environmental conditions: development of an in vitro biomimetic assay. Environ. Sci. Technol. 2009;43(5):1598–1604. doi: 10.1021/es802217y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Diallo MS, Glinka CJ, Goddard WA, Johnson JH. Characterization of nanoparticles and colloids in aquatic systems 1. Small angle neutron scattering investigations of Suwannee River fulvic acid aggregates in aqueous solutions. J. Nanopart. Res. 2005;7(4-5):435–448. [Google Scholar]
- (6).Zhu SQ, Oberdorster E, Haasch ML. Toxicity of an engineered nanoparticle (fullerene, C-60) in two aquatic species, Daphnia and fathead minnow. Mar. Environ. Res. 2006;62:S5–S9. doi: 10.1016/j.marenvres.2006.04.059. [DOI] [PubMed] [Google Scholar]
- (7).King-Heiden TC, Wiecinski PN, Mangham AN, Metz KM, Nesbit D, Pedersen JA, Hamers RJ, Heideman W, Peterson RE. Quantum Dot Nanotoxicity Assessment Using the Zebrafish Embryo. Environ. Sci. Technol. 2009;43(5):1605–1611. doi: 10.1021/es801925c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Cummings A, Kavlock R. A systems biology approach to developmental toxicology. Reprod. Toxicol. 2005;19(3):281–290. doi: 10.1016/j.reprotox.2004.10.001. [DOI] [PubMed] [Google Scholar]
- (9).Peterson RT, Nass R, Boyd WA, Freedman JH, Dong K, Narahashi T. Use of non-mammalian alternative models for neurotoxicological study. Neurotoxicology. 2008;29(3):546–555. doi: 10.1016/j.neuro.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Cohen CA, Katfakis JA, Kurnick MD, Hockey KS, Rzigalinski BA. Cerium oxide nanoparticles reduce free Radical-mediated toxicity in drosophila melanogaster. Free Radic. Biol. Med. 2007;43:S68–S68. [Google Scholar]
- (11).Rosenthal SJ, Tomlinson A, Adkins EM, Schroeter S, Adams S, Swafford L, McBride J, Wang YQ, DeFelice LJ, Blakely RD. Targeting cell surface receptors with ligand-conjugated nanocrystals. J. Am. Chem. Soc. 2002;124(17):4586–4594. doi: 10.1021/ja003486s. [DOI] [PubMed] [Google Scholar]
- (12).Biju V, Muraleedharan D, Nakayama K, Shinohara Y, Itoh T, Baba Y, Ishikawa M. Quantum dot-insect neuropeptide conjugates for fluorescence imaging, transfection, and nucleus targeting of living cells. Langmuir. 2007;23(20):10254–10261. doi: 10.1021/la7012705. [DOI] [PubMed] [Google Scholar]
- (13).Hurt RH, Monthioux M, Kane A. Toxicology of carbon nanomaterials: Status, trends, and perspectives on the special issue. Carbon. 2006;44(6):1028–1033. [Google Scholar]
- (14).Helland A, Wick P, Koehler A, Schmid K, Som C. Reviewing the environmental and human health knowledge base of carbon nanotubes. Environ. Health Perspect. 2007;115(8):1125–1131. doi: 10.1289/ehp.9652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Velzeboer I, Hendriks AJ, Ragas AMJ, Van de Meent D. Aquatic ecotoxicity tests of some nanomaterials. Environ. Toxicol. Chem. 2008;27(9):1942–1947. doi: 10.1897/07-509.1. [DOI] [PubMed] [Google Scholar]
- (16).Blickley TM, McClellan-Green P. Toxicity of aqueous fullerene in adult and larval Fundulus heteroclitus. Environ. Toxicol. Chem. 2008;27(9):1964–1971. doi: 10.1897/07-632.1. [DOI] [PubMed] [Google Scholar]
- (17).Johansen A, Pedersen AL, Jensen KA, Karlson U, Hansen BM, Scott-Fordsmand JJ, Winding A. Effects of C-60 fullerene nanoparticles on soil bacteria and protozoans. Environ. Toxicol. Chem. 2008;27(9):1895–1903. doi: 10.1897/07-375.1. [DOI] [PubMed] [Google Scholar]
- (18).Brunet L, Lyon DY, Hotze EM, Alvarez PJJ, Wiesner MR. Comparative Photoactivity and Antibacterial Properties of C-60 Fullerenes and Titanium Dioxide Nanoparticles. Environ. Sci. Technol. 2009;43(12):4355–4360. doi: 10.1021/es803093t. [DOI] [PubMed] [Google Scholar]
- (19).Kang S, Mauter MS, Elimelech M. Microbial Cytotoxicity of Carbon-Based Nanomaterials: Implications for River Water and Wastewater Effluent. Environ. Sci. Technol. 2009;43(7):2648–2653. doi: 10.1021/es8031506. [DOI] [PubMed] [Google Scholar]
- (20).Petersen EJ, Huang QG, Weber WJ. Ecological uptake and depuration of carbon nanotubes by Lumbriculus variegatus. Environ. Health Perspect. 2008;116(4):496–500. doi: 10.1289/ehp.10883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Petersen EJ, Akkanen J, Kukkonen JVK, Weber WJ. Biological uptake and depuration of carbon nanotubes by Daphia magna. Environ. Sci. Technol. 2009;43(8):2969–2975. doi: 10.1021/es8029363. [DOI] [PubMed] [Google Scholar]
- (22).Langer MG, Ruppersberg JP, Gorb S. Adhesion forces measured at the level of a terminal plate of the fly’s seta. Proc. R. Soc. London, Ser. B. 2004;271(1554):2209–2215. doi: 10.1098/rspb.2004.2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Watson GS, Myhra S, Cribb BW, Watson JA. Putative functions and functional efficiency of ordered cuticular nanoarrays on insect wings. Biophys. J. 2008;94(8):3352–3360. doi: 10.1529/biophysj.107.109348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Warheit DB, Laurence BR, Reed KL, Roach DH, Reynolds GAM, Webb TR. Comparative pulmonary toxicity assessment of single-wall carbon nanotubes in rats. Toxicol. Sci. 2004;77(1):117–125. doi: 10.1093/toxsci/kfg228. [DOI] [PubMed] [Google Scholar]
- (25).Lehmann FA. Matching spiracle opening to metabolic need during flight in Drosophila. Science. 2001;294(5548):1926–1929. doi: 10.1126/science.1064821. [DOI] [PubMed] [Google Scholar]
- (26).Heymann N, Lehmann FO. The significance of spiracle conductance and spatial arrangement for flight muscle function and aerodynamic performance in flying Drosophila. J. Exp. Biol. 2006;209(9):1662–1677. doi: 10.1242/jeb.02203. [DOI] [PubMed] [Google Scholar]
- (27).Barton L, Armsworth C, Baxter I, Poppy G, Gaunt L, Nansen C. Adhesive powder uptake and transfer by Mediterranean fruit flies, Ceratitis capitata (Dipt., Tephritidae) J. Appl. Entomol. 2006;130(5):257–262. [Google Scholar]
- (28).Sonnenberg JP, Schmidt E. Numerical calculation of Londonvan der waals adhesion force distributions for different super-quadric shaped particles. Part. Part. Syst. Charact. 2005;22(1):45–51. [Google Scholar]
- (29).Graczyk TK, Cranfield MR, Fayer R, Bixler H. House flies (Musca domestica) as transport hosts of Cryptosporidium parvum. Am. J. Trop. Med. Hyg. 1999;61(3):500–504. doi: 10.4269/ajtmh.1999.61.500. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.