Abstract
Objective
To assess the accuracy of transvaginal sonographic cervical length (CL) in predicting spontaneous preterm birth in women with twin pregnancies.
Study design
Systematic review and metaanalysis of predictive test accuracy.
Results
Twenty-one studies (16 in asymptomatic women and 5 in symptomatic women) with a total of 3523 women met the inclusion criteria. Among asymptomatic women, a CL ≤20 mm 20-24 weeks’ gestation was the most accurate in predicting preterm birth <32 and <34 weeks’ gestation (pooled sensitivities, specificities, and positive and negative likelihood ratios of 39% and 29%, 96% and 97%, 10.1 and 9.0, and 0.64 and 0.74, respectively). A CL ≤25 mm 20-24 weeks’ gestation had a pooled positive likelihood ratio of 9.6 to predict preterm birth <28 weeks’ gestation. The predictive accuracy of CL for preterm birth was low in symptomatic women.
Conclusion
Transvaginal sonographic CL 20-24 weeks’ gestation is a good predictor of spontaneous preterm birth in asymptomatic women with twin pregnancies.
Keywords: Cervical length, preterm birth, prediction, metaanalysis, systematic review
INTRODUCTION
Despite advancing knowledge of the risk factors and mechanisms associated with preterm labor and delivery, the preterm birth rate has risen 36% in the United States during the last quarter century (from 9.4% in 1981 to 12.8% in 2006).1 This increase has been explained in part by a rise in the number of indicated preterm births in singleton gestations and preterm delivery of multiple pregnancies that occurred as a result of assisted reproductive technologies.2 Furthermore, the twin birth rate has risen 70% from 1980 (18.9 per 1000 live births) to 2006 (32.1 per 1000 live births).1
In the United States, the rates of preterm birth <37 and <32 weeks of gestation for twin pregnancies (60.4% and 12.1%, respectively) were 5.4 and 7.6 times the rates for singleton pregnancies (11.1% and 1.6%, respectively).1 Overall, twin pregnancies comprise 15% of all preterm births1 accounting for a disproportionate share of preterm births. Therefore, there is an urgent need to develop cost-effective tests for the prediction of preterm birth in twin pregnancies. The ability to identify women at high risk for spontaneous preterm birth could allow for patients to undergo targeted interventions such as transfer to a tertiary care center, antenatal corticosteroid administration and tocolysis, which might improve perinatal outcomes among twins. Previous reviews have suggested that transvaginal sonographic assessment of cervical length (CL) is an effective tool for predicting preterm birth, particularly in asymptomatic women or those at a higher risk of spontaneous preterm birth.3-5 However, these reviews largely evaluated the use of CL in singleton pregnancies. In addition, published studies on predictive accuracy of CL for preterm birth in twin pregnancies report conflicting conclusions on the value of this test.
The objective of this study was to assess the value of transvaginal sonographic CL for the prediction of spontaneous preterm birth in women with twin pregnancies through the use of formal methods for systematic reviews and metaanalytic techniques.
MATERIALS AND METHODS
This systematic review was conducted following a prospectively prepared protocol and reported using recently recommended guidelines for systematic reviews of diagnostic test accuracy.6
Literature search
Electronic searches, without language restrictions, were performed in the MEDLINE (January 1966-November 2009), EMBASE (January 1980- November 2009), CINAHL (January 1982- November 2009), LILACS (January 1982-November 2009), and Medion (January 1974-November 2009) databases to identify potentially eligible studies. We applied the following algorithm both in Medical Subject Headings and in free-text words in MEDLINE: {cervical length OR [(transvaginal OR vaginal OR cervix OR cervical) AND (ultrasound OR ultrasonography OR ultrasonographic OR sonography OR sonographic)]} AND (preterm OR premature). This search strategy was also used for the other databases, adjusted according to specific requirements for the particular electronic database. Proceedings of the Society for Maternal-Fetal Medicine and international meetings on preterm birth and twin or multiple pregnancy, reference lists of identified studies, textbooks, and previously published systematic reviews were also searched. In addition, we contacted experts in the field to obtain unpublished studies. All searches were conducted independently by two of the authors (A.C-A and R.R) and results were merged.
Inclusion criteria
Studies were included if they met the following minimal criteria: (1) a cohort or cross-sectional study that evaluated the accuracy of transvaginal sonographic CL measurement to predict spontaneous preterm birth in asymptomatic or symptomatic pregnant women with twin pregnancies; (2) the outcome measures included any category of spontaneous preterm birth <37 weeks of gestation; (3) the studies provided the necessary information to generate 2 × 2 tables; and (4) the women had no therapeutic intervention resulting from the test result. When a study based its results on mixed (singleton and twin) pregnancies, unless data for twins were extractable separately, it was not considered for inclusion in the review. In cases of duplicate publication we selected the most recent and complete versions and supplemented if additional information appeared in the other publications
Studies were excluded from the systematic review if they: (1) were case-control studies because these tend to overestimate the predictive or diagnostic accuracy of a test;7 (2) did not provide data on predictive estimates and sufficient information to calculate them could not be retrieved; or (3) they included women with cervical cerclage, previous cervical surgery, or premature rupture of membranes.
Studies deemed suitable were retrieved and reviewed independently by two authors (A.C-A and R.R) to determine inclusion. Disagreements were resolved by consensus.
Quality assessment
Methodological quality of included studies was assessed independently by two reviewers (A.C-A and R.R) using 4 of the 14 items of the Quality Assessment of Diagnostic Accuracy Studies(QUADAS) tool.8 The remaining 10 QUADAS items were not used because they were not relevant to our review. Each item was scored as “yes”, “no”, or “unclear.”
The items of the QUADAS tool evaluated and their interpretation were as follows:
Representative spectrum of patients. This item was scored “yes” when pregnant women with twin pregnancies were consecutively selected in a prospective way. Convenience sampling, such as arbitrary recruitment or nonconsecutive recruitment, was scored as “no.”
Description of the test: This item was scored as “yes” if the study described sufficient details of the technique used for measuring CL such as plane in which images were obtained, anatomic references for the determination of CL, and number of measurements. If this information was not reported, then this item was scored as “no.”
Blinding of index test result: This item was scored “yes” if the study clearly stated that clinicians treating the patient did not have knowledge of the CL results. If this did not appear to be the case, this item was scored as “no.”
Reporting of study withdrawals. If there were withdrawals from the study, this item was scored as ”yes” if withdrawals were explained or if a flow diagram of study participants was reported. If it appeared that some of the participants did not complete the study and these patients were not accounted for, then this item was scored as “no.”
If there was insufficient information available to make a judgment of these items, then they were scored as “unclear.” Summary score estimating the overall quality of an article was not calculated because the interpretation of such summary scores is problematic and potentially misleading.9
Discrepancies in quality assessment were resolved by discussion.
Data extraction
Data extraction was performed independently by 2 investigators (A.C-A and R.R) and recorded on a standardized form. There was no blinding of authorship. Information was extracted on study characteristics, study quality, and participant characteristics. With regard to transvaginal ultrasonography, we extracted data on the technique used for measuring CL, gestational age(s) at testing, and cutoff values used. Gestational age at testing was divided into 3 groups: <20, 20-24, and >24 weeks of gestation. For studies in which the reported gestational age at testing encompassed ≥2 of the groups, we classified them according to their mean gestational age at testing. For each study, for all reported cutoff values for CL, and for all categories of spontaneous preterm birth, we then extracted the number of true-positive, false-positive, true-negative, and false-negative results. When predictive accuracy data were not available, we recalculated them from the reported results.
Studies reporting on spontaneous preterm birth <35 weeks of gestation were considered with those reporting spontaneous preterm birth <34 weeks of gestation, as both these gestational ages have similar neonatal outcomes. In the same way, studies reporting spontaneous preterm birth <36 weeks of gestation were considered with those reporting spontaneous preterm birth <37 weeks of gestation. We extracted data separately for asymptomatic women and for women with threatened preterm labor.
Disagreements in data extraction were resolved by discussion among authors.
Statistical analysis
For both asymptomatic and symptomatic women, we synthesized data for spontaneous preterm birth at <34 and <37 weeks of gestation. In addition, for asymptomatic women we synthesized data for spontaneous preterm birth at <28 and <32 weeks of gestation.
Data extracted from each study were arranged in 2 × 2 contingency tables. When these tables contained cells for which the value was 0, we added 0.5 to those cells to allow for the calculation of variances.10 Sensitivity and specificity were calculated for each study and for all reported cutoff values. For asymptomatic women, we plotted sensitivities and specificities in receiver operating characteristic (ROC) plots according to the timing of transvaginal ultrasonography (20-24, and >24 weeks of gestation) and definition of spontaneous preterm birth as outcome measure (<28, <32, <34, and <37 weeks of gestation). We then constructed summary ROC curves for each outcome using a bivariate random-effects approach11 and calculated area under the summary ROC curves with their corresponding 95% confidence intervals (CIs).12 This measure allows for comparison of the predictive accuracy of the test for different outcomes and cutoff values (two-sided P < .05 was considered to be statistically significant).
Metaanalyses were performed using subgroups of studies with a similar gestational age at testing, cutoff values, and outcome measures to minimize clinical heterogeneity. Pooled estimates of sensitivity and specificity with 95% CIs were calculated using bivariate, random-effects meta-regression model.11 Thereafter, we derived likelihood ratios with 95% CIs from the pooled sensitivities and specificities for each outcome reported.13 Likelihood ratios indicate by how much a given test result raises or lowers the probability of having the disease and thus allow interpretation of the results for use in clinical practice.14 Likelihood ratios for a positive test result >10 and likelihood ratios for a negative test result <0.1 have been noted as providing convincing predictive evidence. Moderate prediction can be achieved with likelihood ratio values of 5-10 and 0.1-0.2, whereas those <5 and > 0.2 would provide only minimal prediction.14 Likelihood ratios are more clinically meaningful than sensitivities or specificities because they are less likely to change with the prevalence of the disorder, they can be calculated for several levels of the test, and they can also be used in conjunction with pretest probability of disease to estimate the post-test probability of disease for individual patients.
Likelihood ratios generated from metaanalyses were used to determine post-test probabilities of spontaneous preterm birth <28, <32, <34, and <37 weeks of gestation for positive and negative CL results as follows:14
Posttest probability of preterm birth = likelihood ratio × pretest probability/[1-pretest probability × (1-likelihood ratio)]
Estimates of pretest probabilities of spontaneous preterm birth <28, <32, <34, and <37 weeks of gestation were obtained from the global prevalence of these outcomes among included studies.
Heterogeneity of the results among studies was investigated through visual examination of forest plots of sensitivities and specificities, and ROC plots. In addition, heterogeneity was assessed by means of the quantity I2, which describes the percentage of total variation across studies that is due to heterogeneity rather than chance.15 Statistical heterogeneity was defined as an I2 statistic value of ≥50%.15 We explored potential sources of heterogeneity by performing meta-regression analysis of subgroups as defined a priori:16 study setting (those conducted in North America vs Europe), sample size (<100 versus ≥100 in studies of asymptomatic women and <50 versus ≥50 in studies of symptomatic women), and study’s year of publication (<2000 vs ≥ 2000). In addition, we examined the impact of study quality on estimation of predictive accuracy according to individual quality items and also according to an overall quality level incorporating these items (those that met all 4 methodological criteria vs <4).
We assessed publication and related biases visually by examining the symmetry of funnel plots and statistically by using the Egger’s regression test.17 P<.1 indicated significant asymmetry.
The bivariate models were fitted using the NLMIXED procedure (SAS 9.1 for Windows [SAS Institute, Inc., Cary, NC]). The summary ROC curves were constructed using Review Manager 5.0.21 (The Nordic Cochrane Centre, Copenhagen, Denmark). The remaining analyses were performed using SPSS version 15.0 (SPSS Inc, Chicago, IL).
RESULTS
The searches produced 1027 citations of which 314 were considered relevant (Figure 1). In all, 293 studies were excluded, the main reasons being the inclusion of only singleton pregnancies (37%), not a test accuracy study (27%), and the lack of original data (24%). A total of 21 studies, including 3523 women with twin pregnancies,18-38 met the inclusion criteria of which 15 (3001 women) provided data for metaanalyses. Sixteen studies (3213 women) provided data on asymptomatic women18-33 and 5 studies (310 women) on women with symptoms of preterm labor.34-38 One of the included articles30 was an extended analysis of an initial study39 from which we extracted data for metaanalyses of CL ≤25 and ≤35 mm at 20-24 weeks of gestation to predict preterm birth <28 weeks of gestation. Six studies19-21,28,32,37 could not be included in the metaanalyses because the CL cutoff values used,19-21,32 and outcome measures evaluated28,37 did not match those of other studies that provided data for metaanalyses. Predictive accuracy of CL for spontaneous preterm birth reported in studies not included in the metaanalyses is provided in the supplementary Appendix (available online at www.ajog.org).
Figure 1.
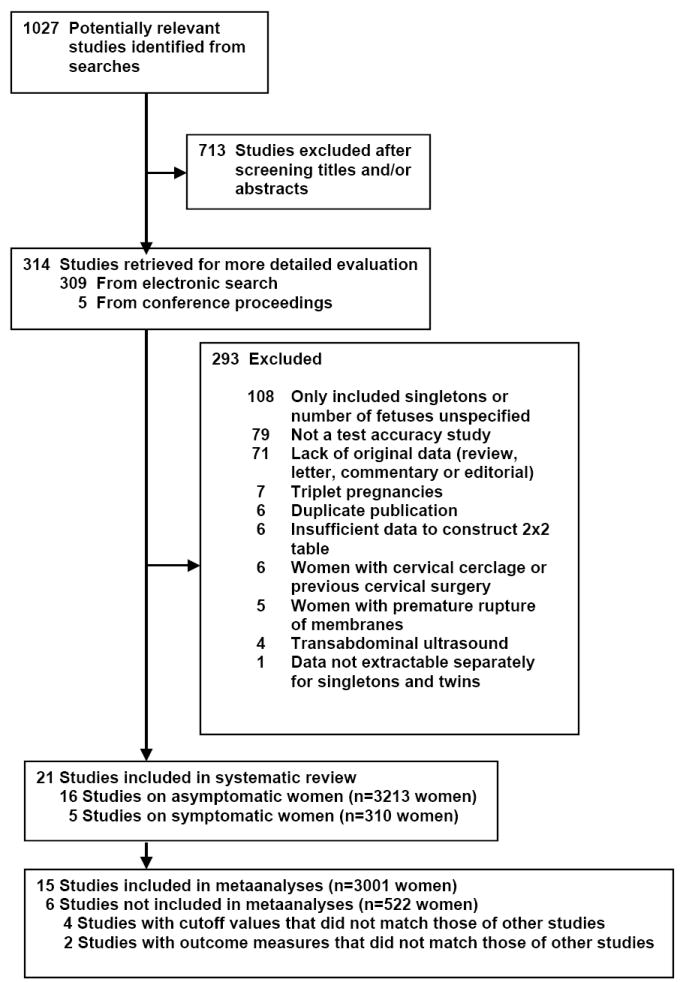
Study selection process
The main characteristics of studies included in the review are shown in Table 1. Nine studies (43%) were performed in Europe, 8 (38%) in the United States, 2 in Israel, and 1 each in Egypt and the French West Indies. The sample size ranged from 1828 to 113530 in asymptomatic women (median, 139) and from 2634,36 to 10535 in symptomatic women (median, 66). The range for outcome definitions was preterm birth at <28 to <37 weeks of gestation. Three studies provided data on preterm birth <28 weeks, 7 on preterm birth <32 weeks, 16 on preterm birth <34 or <35 weeks, and 8 on preterm birth <36 or <37 weeks. Five studies reported results using a cervical length cutoff value of 20 mm, 13 using 25 mm, 9 using 30 mm, and 8 using 35 mm. Among asymptomatic women, 3 studies reported data on CL at <20 weeks, 12 on CL at 20-24 weeks, and 8 on CL at >24 weeks.
TABLE 1.
Characteristics of studies included in the systematic review
| Study, year, | Country | No of women | Inclusion criteria | Exclusion criteria | Gestational age at testing (weeks) | Cervical length cut-off (mm) | Outcome |
|---|---|---|---|---|---|---|---|
| Asymptomatic women | |||||||
| Goldenberg,18 1996 | United States | 147 | Twins | Cervical cerclage,placenta previa, major fetal anomaly | 24, 28 | 25 | Preterm birth <32, <35 and <37 weeks |
| Imseis,19 1997 | United States | 85 | Twins | Cervical cerclage | 24-26 | 35 | Preterm birth <34 weeks |
| Wennerholm,20 1997 | Sweden | 101 | Twins | Not reported | 24-34 | 33 | Preterm birth <35 and <37 weeks |
| Grisaru-Granovsky,21 1998 | Canada | 38 | Twins, triplets, quadruplets | Not reported | 18-29 (mean, 25) | 30 | Preterm birth <34 weeks |
| Yang,22 2000 | United States | 65 | Twins | Cervical cerclage, placenta previa or bleeding | 18-26 (91% at <24 weeks) | 25, 30, 35 | Preterm birth <32 and <35 weeks |
| Guzman,23 2000 | United States | 131 | Twins | Cervical cerclage | 15-20, 21-24, 25-28 | 20 | Preterm birth <28,<30, <32, and <34 weeks |
| Soriano,24 2002 | Israel | 44 | Twins | Not reported | 18-24 (mean, 22.7) | 35 | Preterm birth <34 weeks |
| Vayssiere,25 2002 | France | 251 | Twins | Cervical cerclage, placenta previa, major fetal anomaly, twin-twin transfusion syndrome, premature rupture of membranes | 21-23, 26-28 | 25, 30 | Preterm birth <32 and <35 weeks |
| Gibson,26 2004 | United Kingdom | 91 | Twins | fetal anomaly, twin-twin transfusion syndrome | 18,24,28, and 32 | 25, 22 | Preterm birth <35 weeks |
| Sperling,27 2005 | Denmark and Sweden | 383 | Twins | Cervical cerclage, prior conization | 23 | 20, 25, 30, 35 | Preterm birth <28,<32, <33, <34 and <35 weeks |
| Fait,28 2005 | Israel | 18 | Triplets reduced to twins | Cervical cerclage | 15.9 ± 0.3 | 35 | Preterm birth <33 weeks |
| Arabin,29 2006 | The Netherlands | 153 | Twins | Not reported | 20-25 | 25, 30 | Preterm birth <36 weeks |
| To,30 2006 | United Kingdom | 1135 | Twins | Cervical cerclage, major fetal abnormalities, premature rupture of membranes, monochorionic twins with severe twin-twin transfusion syndrome | 22-24 | 5-55a | Preterm birth <30, <32, and <34 weeks |
| Klein,31 2008 | Austria | 223 | Twins | Not reported | 20-25 | 25, 30, 35 | Preterm birth <34 weeks |
| Aboulghar,32 2009 | Egypt | 193 | Twin ICSI pregnancies | Cervical cerclage | Mean, 20 | 38 | Preterm birth <34 weeks |
| Fox,33 2009 | United States | 155 | Twins | Monoamniotic twins, fetal aneuploidy, major fetal abnormalities | 22-24, 25-32 | 20, 25, 35 | Preterm birth <28,<32, <34,and <37 weeks |
| Women with symptoms of preterm labor | |||||||
| Crane,34 1997 | Canada | 26 | Twins, regular uterine contractions with cervical changes (dilatation, effacement, or change in consistency) | Active vaginal bleeding, placenta previa, premature rupture of membranes, cervical cerclage, stillbirth, cervical dilatation >3 cm | 23-33 | 25, 30 | Preterm birth <34 and <37 weeks |
| Persutte,35 2000 | United States | 105 | Twins, spontaneous onset of labor and vaginal delivery | Not reported | 20-32 | 25 | Preterm birth <37 weeks |
| Vendittelli,36 2001 | French West Indies | 26 | Twins, uterine contractions on external tocodynamometry at least one every 10 min and/or with changes in manual cervical effacement or dilatation | Cervical dilatation >3 cm, premature rupture of membranes, cervical cerclage, active vaginal bleeding, placenta previa, stillbirth, fetal malformation, | 18-36 | 25 | Preterm birth <37 weeks |
| Fuchs,37 2004 | Germany, United Kingdom | 87 | Twins, painful and regular uterine contractions | Cervical dilatation >3 cm, cervical cerclage, premature rupture of membranes | 24-36 | 25 | Delivery within 7 days of testing |
| Gonzalez,38 2004 | France | 66 | Twins, regular uterine contractions with cervical changes (effacement of at least 50% or dilatation of at least one finger) | Cervical cerclage, maternal or fetal disorder contraindicating continuation of pregnancy | <34 | 20, 30 | Preterm birth <34 and <37 weeks |
For the review, we chose <20, <25, <30, and <35 mm as cutoff values
Methodological quality of studies included in the systematic review is summarized in Figure 2. Six studies (29%), 5 among asymptomatic and 1 among symptomatic women, met all 4 criteria; and 9 studies (43%), 7 among asymptomatic and 2 among symptomatic women, met 3 criteria (Figure 2). The most common shortcoming was failure to blind investigators to CL results. In 7 (33%) studies, results of CL were made available to the physicians involved in the clinical treatment of the patients, whereas in 8 (38%) studies it was unclear whether CL results were provided to caregivers.
Figure 2.
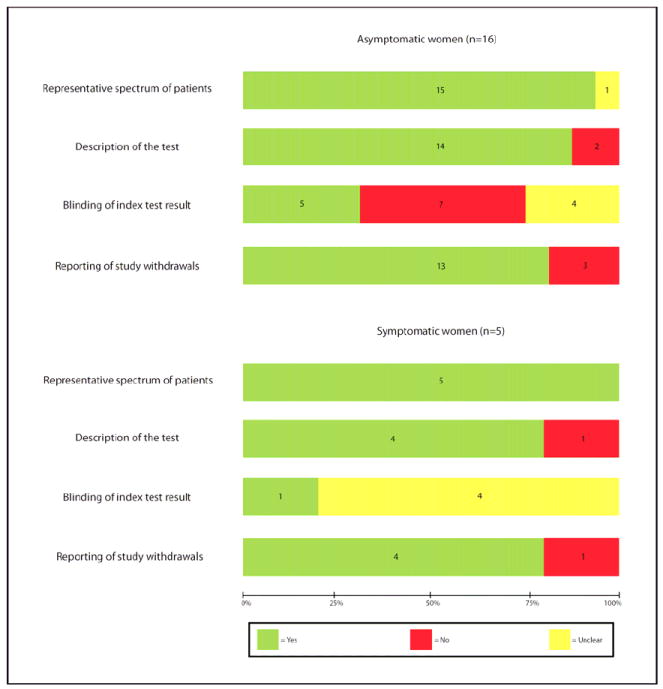
Methodological quality of studies included in the systematic review. Data presented as percentages across all included studies. Figures in the stacks represent number of studies.
Figure 3 shows the summary ROC curves of CL for the prediction of spontaneous preterm birth in asymptomatic women tested at 20-24 weeks of gestation. The greatest area under the summary ROC curve was for a CL ≤25 mm to predict preterm birth <28 weeks (0.86) (Figure 3a), followed by CL ≤20 and ≤25 mm to predict preterm birth <32 weeks (both 0.80) (Figure 3b), and cervical length ≤20 and ≤35 mm to predict preterm birth <34 weeks (both 0.77) (Figure 3c). The remaining areas under the summary ROC curves ranged between 0.64 and 0.76 (Figure 3). Among asymptomatic women tested > 24 weeks of gestation, the areas under the summary ROC curves for a cervical length ≤25 mm to predict preterm birth <32, 34, and 37 weeks of gestation were 0.77, 0.62 and 0.60, respectively (figures not shown).
Figure 3.
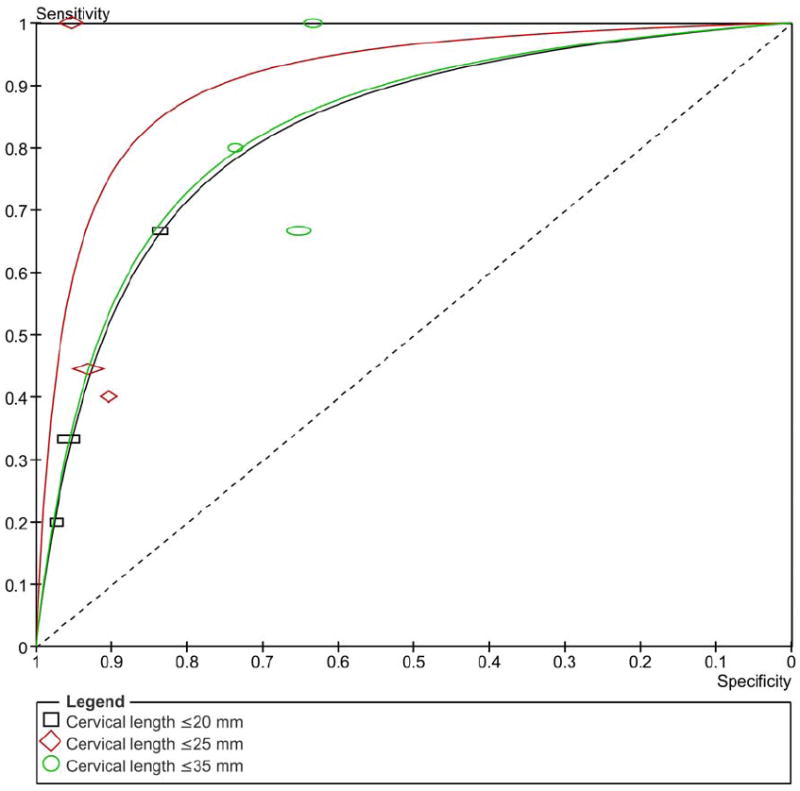
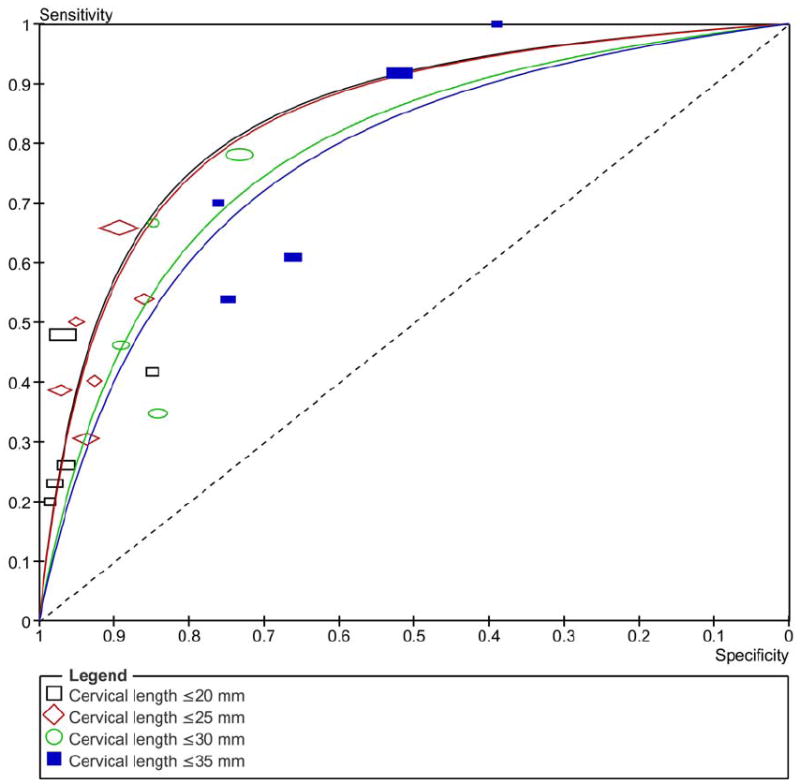
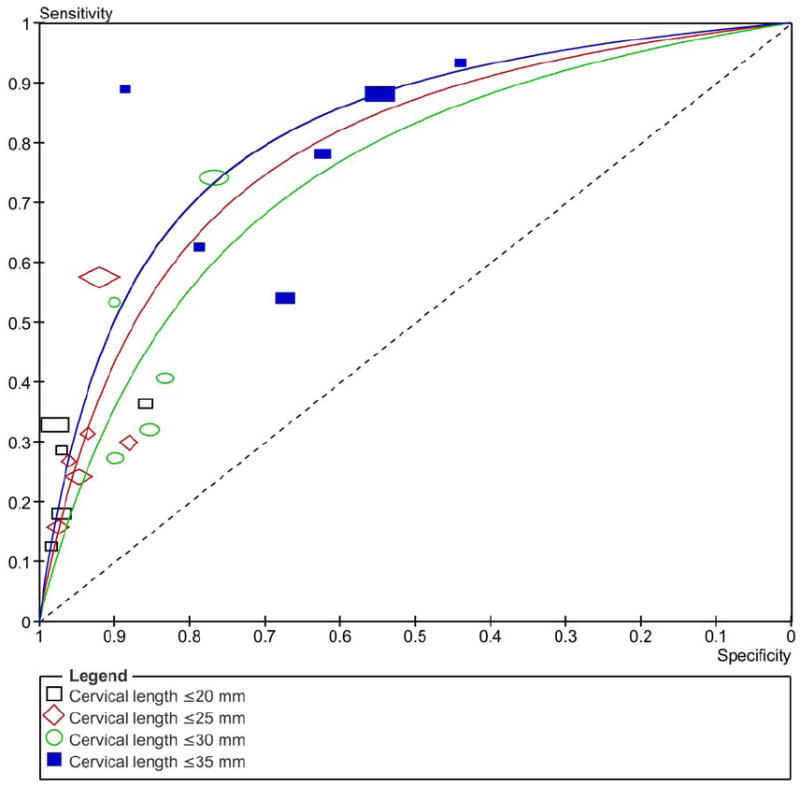
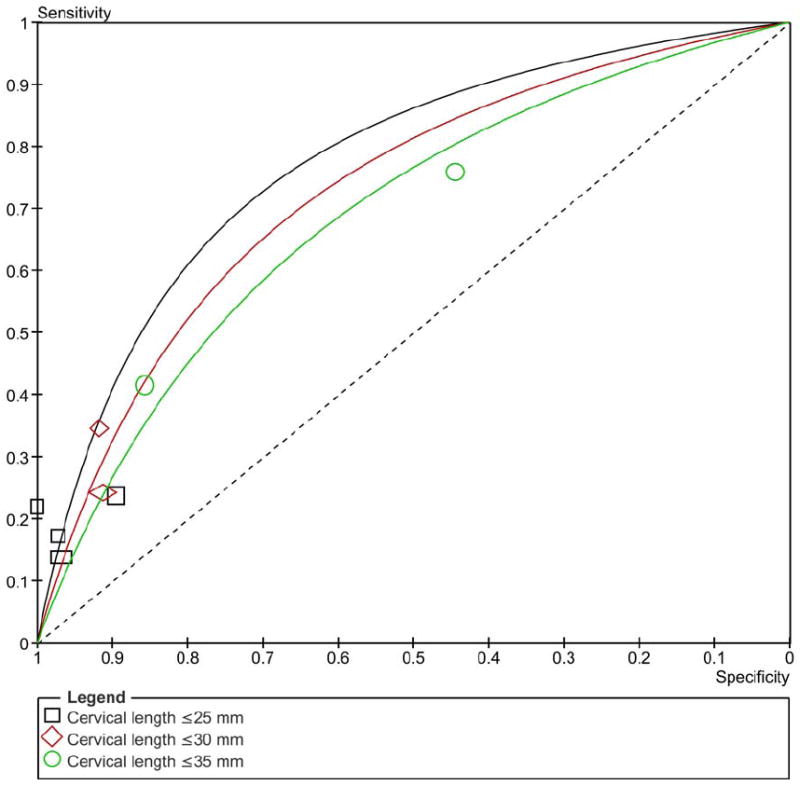
Summary receiver operating characteristic (ROC) curves of cervical length tested at 20-24 weeks of gestation in asymptomatic women to predict spontaneous preterm birth: A) <28; B) <32; C) <34; D) <37 weeks of gestation. The area of each circle, rectangle and diamond is proportional to study’s sample size.
Pooled estimates of accuracy of CL for the prediction of spontaneous preterm birth in women with twin pregnancies are presented in Table 2. Among asymptomatic women, pooled sensitivities and specificities ranged from 21% to 82% and from 58% to 97%, respectively. Among women with symptoms of preterm labor, pooled sensitivities and specificities varied from 49% to 79% and from 32% to 74%, respectively. Preterm birth <32 and <34 weeks of gestation in asymptomatic women were best predicted at 20-24 weeks of gestation by a CL ≤20 mm (pooled positive likelihood ratios of 10.1 and 9.0; pooled negative likelihood ratios of 0.64 and 0.74). Similar pooled estimates of accuracy were obtained for CL ≤25 mm to predict preterm birth <28 weeks of gestation (positive and negative likelihood ratios of 9.6 and 0.40, respectively). CL was less accurate to predict preterm birth <37 weeks of gestation (positive and negative likelihood ratios between 1.5 and 4.4, and between 0.71 and 0.83, respectively). A CL ≤25 mm at > 24 weeks of gestation had pooled positive and negative likelihood ratios of 1.8-2.7 and 0.47-0.75, respectively, for predicting preterm birth from <32 to <37 weeks of gestation. Among women with threatened preterm labor, the measurement of CL had a minimal predictive accuracy for preterm birth <34 and <37 weeks of gestation (pooled positive and negative likelihood ratios between 1.2 and 1.9, and between 0.65 and 0.69, respectively). It was not possible to perform metaanalysis of studies reporting data on cervical length at <20 weeks of gestation because of the differences in cutoff values and outcome measures used in the 3 studies23,26,28 (217 women) that met the minimal inclusion criteria.
TABLE 2.
Pooled estimates for cervical length in predicting spontaneous preterm birth in women with twin pregnancies
| Outcome | Cervical length cut-off (mm) | No of studies | Sample size | Sensitivity (%) (95% CI) | Specificity (%) (95% CI) | Positive Likelihood ratio (95% CI) | Negative Likelihood ratio (95% CI) |
|---|---|---|---|---|---|---|---|
| Asymptomatic women | |||||||
| Testing at 20-24 weeks’ gestation | |||||||
| Preterm birth <28 weeks | 20 | 3 23,27,33 | 591 | 35 (14-62) | 93 (91-95) | 5.2 (2.6-10.6) | 0.69 (0.49-1.01) |
| 25 | 327,33,39 | 637 | 64 (41-83) | 93 (91-95) | 9.6 (5.8-14.8) | 0.40 (0.23-0.68) | |
| 35 | 327,33,39 | 637 | 82 (60-95) | 66 (62-69) | 2.4 (1.9-3.0) | 0.28 (0.11-0.67) | |
| Preterm birth <32 weeks | 20 | 523,25,27,30,33 | 1955 | 39 (31-48) | 96 (95-97) | 10.1 (7.4-13.9) | 0.64 (0.55-0.73) |
| 25 | 618,22,25,27,30,33 | 2036 | 54 (45-62) | 91 (90-92) | 6.0 (4.8-7.4) | 0.51 (0.43-0.61) | |
| 30 | 422,25,27,30 | 1812 | 65 (56-74) | 78 (76-80) | 3.0 (2.5-3.5) | 0.45 (0.35-0.57) | |
| 35 | 522,25,27,30,33 | 1889 | 81 (73-87) | 58 (56-61) | 1.9 (1.7-2.2) | 0.33 (0.23-0.48) | |
| Preterm birth <34 weeks | 20 | 523,26,27,30,33 | 1760 | 29 (23-35) | 97 (96-98) | 9.0 (6.1-12.7) | 0.74 (0.68-0.80) |
| 25 | 618,22,27,30,31,33 | 1987 | 40 (38-46) | 93 (92-94) | 5.8 (4.5-7.2) | 0.64 (0.58-0.71) | |
| 30 | 522,25,27,30,31 | 2014 | 56 (50-62) | 81 (79-83) | 3.0 (2.6-3.4) | 0.55 (0.48-0.63) | |
| 35 | 622,24,27,30,31,33 | 1884 | 79 (74-84) | 60 (57-62) | 2.0 (1.8-2.2) | 0.35 (0.27-0.44) | |
| Preterm birth <37 weeks | 25 | 418,22,29,33 | 434 | 21 (15-27) | 95 (92-98) | 4.4 (2.4-8.2) | 0.83 (0.75-0.92) |
| 30 | 222,29 | 218 | 29 (18-43) | 91 (86-95) | 3.4 (1.6-6.7) | 0.78 (0.65-0.92) | |
| 35 | 222,33 | 134 | 56 (43-68) | 63 (50-74) | 1.5 (1.0-2.2) | 0.71 (0.51-0.98) | |
| Testing after 24 weeks’ gestation | |||||||
| Preterm birth <32 weeks | 25 | 318,25,33 | 511 | 65 (45-81) | 76 (72-79) | 2.7 (2.0-3.6) | 0.47 (0.29-0.76) |
| Preterm birth <34 weeks | 25 | 418,25,26,33 | 594 | 44 (34-53) | 81 (78-85) | 2.3 (1.8-3.1) | 0.70 (0.59-0.83) |
| Preterm birth <37 weeks | 25 | 218,33 | 276 | 43 (35-51) | 77 (68-84) | 1.8 (1.3-2.6) | 0.75 (0.63-0.89) |
| Women with symptoms of preterm labor | |||||||
| Preterm birth <34 weeks | 30 | 234,38 | 92 | 79 (62-91) | 32 (20-45) | 1.2 (0.9-1.7) | 0.67 (0.31-1.44) |
| Preterm birth <37 weeks | 25 | 234,35 | 131 | 49 (38-60) | 74 (59-87) | 1.9 (1.1-3.3) | 0.69 (0.52-0.90) |
| 30 | 334,36,38 | 118 | 76 (65-84) | 37 (19-58) | 1.2 (0.9-1.7) | 0.65 (0.29-1.31) | |
Study # 39 is an initial report of study # 30
Table 3 summarizes pooled estimates of pretest and post-test probabilities of having spontaneous preterm birth in women with twin pregnancies after measurement of CL. In asymptomatic women, a CL ≤20 mm at 20-24 weeks of gestation increased the pretest probability of preterm birth <32 and <34 weeks of gestation from 6.8-42.4%, and from 15.3-61.9%, respectively, whereas a CL >20 mm decreased the risk to 4.5% and 11.8%, respectively. A cervical length ≤25 mm at 20-24 weeks of gestation increased the pretest probability of preterm birth <28 and <37 weeks of gestation from 3.5%-25.8%, and from 41.2%-75.5%, respectively, whereas a CL >25 mm decreased the risk to 1.4% and 36.8%, respectively. In both asymptomatic women tested > 24 weeks of gestation and women with symptoms of threatened labor, the likelihood ratios only produced minimal changes in the pretest probabilities of preterm birth.
TABLE 3.
Pooled estimates of pretest probabilities, likelihood ratios, and post-test probabilities for cervical length in the prediction of spontaneous preterm birth in women with twin pregnancies
| Outcome | Cervical length cut-off (mm) | Pretest probability (%) | Likelihood ratios | Post-test probabilities (%) | ||
|---|---|---|---|---|---|---|
| Positive test result | Negative test result | Positive test result | Negative test result | |||
| Asymptomatic women (testing at 20-24 weeks’ gestation) | ||||||
| Preterm birth <28 weeks | 25 | 3.5 | 9.6 | 0.40 | 25.8 | 1.4 |
| Preterm birth <32 weeks | 20 | 6.8 | 10.1 | 0.64 | 42.4 | 4.5 |
| Preterm birth <34 weeks | 20 | 15.3 | 9.0 | 0.74 | 61.9 | 11.8 |
| Preterm birth <37 weeks | 25 | 41.2 | 4.4 | 0.83 | 75.5 | 36.8 |
| Asymptomatic women (testing after 24 weeks’ gestation) | ||||||
| Preterm birth <32 weeks | 25 | 6.1 | 2.7 | 0.47 | 14.9 | 3.0 |
| Preterm birth <34 weeks | 25 | 18.2 | 2.3 | 0.70 | 33.9 | 13.5 |
| Preterm birth <37 weeks | 25 | 53.6 | 1.8 | 0.75 | 67.5 | 46.4 |
| Women with symptoms of threatened labor | ||||||
| Preterm birth <34 weeks | 30 | 35.9 | 1.2 | 0.67 | 40.2 | 27.3 |
| Preterm birth <37 weeks | 25 | 67.2 | 1.9 | 0.69 | 79.6 | 58.6 |
| 30 | 67.2 | 1.2 | 0.65 | 71.1 | 57.1 | |
There was graphical and statistical heterogeneity of predictive performance among studies as confirmed by I2 values > 50% in some of the meta-analyses performed. An explanation for heterogeneity was not provided by the study setting, sample size, and study’s year of publication. Most women included in the current metaanalyses were enrolled in a single study.30 Sensitivity analyses, in which we removed this study, revealed similar pooled accuracy results (data not shown). Individual quality items did not have effect on predictive ability of CL at 20-24 weeks of gestation. In addition, pooled predictive accuracy estimates obtained from studies that met <4 methodological criteria did not differ significantly from those obtained from studies that met all 4 criteria. All funnel plots showed no asymmetry, either visually or in terms of statistical significance (P>.10 for all, by Egger test), indicating that publication and related biases were not present.
COMMENT
This systematic review and metaanalysis gives the strongest evidence to date that transvaginal sonographic measurement of CL at 20-24 weeks of gestation is a good predictor of spontaneous preterm birth in asymptomatic women with twin pregnancies. A CL ≤20 mm predicts spontaneous preterm birth at <32 and <34 weeks of gestation, whereas a CL ≤25 mm predicts preterm birth at <28 weeks of gestation. A “normal” CL, however, was less accurate in predicting the absence of preterm birth because the likelihood ratios for negative test results generated only minimal changes in the pretest probabilities of preterm birth. In addition, transvaginal sonographic CL has limited accuracy in predicting spontaneous preterm birth in women with twin pregnancies and threatened preterm labor, and in asymptomatic women in which the test is performed >24 weeks of gestation. There were limited data on the predictive accuracy of cervical length performed <20 weeks of gestation.
The strength of our review is based upon its compliance with stringent criteria for performing a rigorous systematic review of predictive test accuracy. These included, among others, the use of a prospective protocol designed to address a research question; the extensive search of relevant studies without language restrictions; the use of well developed methods for quality assessment and techniques recently recommended for metaanalysis of diagnostic and predictive tests, and the investigation for possible sources of heterogeneity. However, some potential limitations of our study must also be considered. First, like any systematic review, it is limited by the quality of included studies. The main area where quality was poor was in the area of blinding of the results of the sonographic CL measurement. There is evidence to suggest that this bias can lead to a significant overestimation of predictive accuracy.7,40,41 Nevertheless, both subgroup and meta-regression analyses did not reveal the blinding of test results to significantly affect predictive performance. Second, there was significant heterogeneity among individual studies in some of the metaanalyses performed. Homogeneity is one of the desired prerequisites for metaanalysis, but it is not an absolute requirement. We explored the sources of heterogeneity as thoroughly as possible but we were unable to explain it. In the presence of unexplained heterogeneity, the use of a random-effects meta-regression model, which we did, provides the most useful estimate for informing practice. Third, the statistical power of some of our metaanalyses was limited by the small number of studies within each subgroup and the relatively small sample size of some included studies.
Only 1 previous systematic review, which included 14 studies involving 1593 women, has evaluated the accuracy of transvaginal sonographic CL in predicting spontaneous preterm birth in twin pregnancies.4 In 2003, Honest et al4 reported pooled positive and negative likelihood ratios between 1.5 and 5.0 and between 0.56 and 1.17, respectively, to predict spontaneous preterm birth from <32 to <37 weeks of gestation in asymptomatic women by using cutoff values for CL ranging between 20-35 mm at 20-24 weeks of gestation. Pooled positive and negative likelihood ratios for predicting preterm birth at <34 weeks of gestation in asymptomatic women using cutoff values for CL ranging between 25-35 mm at >24 weeks of gestation varied between 1.8 and 2.1 and between 0.29 and 0.83, respectively. This review, however, included 2 studies with duplicate data and one study in which a cervical cerclage was placed in women with a CL <30 mm at <27 weeks of gestation.
The results of our systematic review suggest that transvaginal sonographic measurement of CL is a better predictor of spontaneous preterm birth in twin pregnancies than in singleton pregnancies. In fact, the metaanalysis by Honest et al4 reported that among asymptomatic women with singleton pregnancies, a CL ≤25 mm at 20-24 weeks of gestation had pooled positive and negative likelihood ratios of 4.2 and 0.40, and 4.4 and 0.67, respectively, to predict preterm birth <32 and <34 weeks of gestation, respectively. At <20 weeks of gestation, a CL ≤25 mm had pooled positive and negative likelihood ratios of 4.1 and 0.75, and 6.3 and 0.79, respectively, to predict preterm birth <32 and <34 weeks of gestation, respectively, whereas at >24 weeks of gestation the pooled positive and negative likelihood ratios for predicting preterm birth at <34 weeks of gestation were 4.1 and 0.62, respectively. In contrast, the present study showed that among asymptomatic women with twin pregnancies, a CL ≤20 mm at 20-24 weeks of gestation had pooled positive and negative likelihood ratios of 10.1 and 0.64, and 9.0 and 0.64, respectively, to predict preterm birth at <32 and <34 weeks of gestation, respectively.
Although our metaanalyses have demonstrated that transvaginal sonographic measurement of CL is predictive of preterm birth in asymptomatic women with twin pregnancies, it is unclear if antenatal management of these pregnancies based on the results of this test can prevent preterm birth. In 2006, Gordon et al42 conducted a study in which 125 women with twin pregnancies were randomly assigned to undergo a transvaginal sonographic CL measurement and a cervical digital exam every 4 weeks starting at 16-20 weeks until 28 weeks gestation (n=63) or to a digital cervical exam without cervical assessment by ultrasound at the same intervals (n=62). Women who underwent transvaginal sonographic cervical examinations were treated with a predetermined algorithm for the use of cerclage and bedrest. Treatment decisions of women allocated to the control group were not based upon a predetermined algorithm. There was no significant difference between groups in mean length of gestation (35.7±2.2 weeks in the study group versus 35.5±3.1 weeks in the control group, P=0.60) but life table analysis revealed that preterm birth <35 weeks of gestation in the transvaginal sonographic CL group was significantly reduced (P=0.02). In addition, it should be emphasized that as of late 2009, there is no therapy that effectively prevents preterm birth in twin pregnancies. This includes the use of bed rest,43 oral betamimetics,44 cervical cerclage,45 and progesterone.46
Further well-designed randomized controlled trials are required to evaluate the effectiveness of antenatal management of women with twin pregnancies based upon transvaginal sonographic CL results for the prevention of preterm birth. In addition, these studies should include a clear protocol for the management of women based on the results of the test.
Supplementary Material
Acknowledgments
This research was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services. We are very grateful to Dr Percy Pacora for his assistance in obtaining the articles. We would like to thank Dr Nathan Fox for assistance in providing unpublished data from his study and for clarification of other queries.
References
- 1.Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Kirmeyer S, Mathews TJ. Births: final data for 2006. Natl Vital Stat Rep. 2009;57:1–102. [PubMed] [Google Scholar]
- 2.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leitich H, Brunbauer M, Kaider A, Egarter C, Husslein P. Cervical length and dilatation of the internal cervical os detected by vaginal ultrasonography as markers for preterm delivery: A systematic review. Am J Obstet Gynecol. 1999;181:1465–72. doi: 10.1016/s0002-9378(99)70407-2. [DOI] [PubMed] [Google Scholar]
- 4.Honest H, Bachmann LM, Coomarasamy A, Gupta JK, Kleijnen J, Khan KS. Accuracy of cervical transvaginal sonography in predicting preterm birth: a systematic review. Ultrasound Obstet Gynecol. 2003;22:305–22. doi: 10.1002/uog.202. [DOI] [PubMed] [Google Scholar]
- 5.Crane JM, Hutchens D. Transvaginal sonographic measurement of cervical length to predict preterm birth in asymptomatic women at increased risk: a systematic review. Ultrasound Obstet Gynecol. 2008;31:579–87. doi: 10.1002/uog.5323. [DOI] [PubMed] [Google Scholar]
- 6.Leeflang MM, Deeks JJ, Gatsonis C, Bossuyt PM. Cochrane Diagnostic Test Accuracy Working Group. Systematic reviews of diagnostic test accuracy. Ann Intern Med. 2008;149:889–97. doi: 10.7326/0003-4819-149-12-200812160-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lijmer JG, Mol BW, Heisterkamp S, Bonsel GJ, Prins MH, van der Meulen JHP, et al. Empirical evidence of design-related bias in studies of diagnostic tests. JAMA. 1999;282:1061–6. doi: 10.1001/jama.282.11.1061. [DOI] [PubMed] [Google Scholar]
- 8.Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol. 2003;3:25. doi: 10.1186/1471-2288-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whiting P, Harbord R, Kleijnen J. No role for quality scores in systematic reviews of diagnostic accuracy studies. BMC Med Res Methodol. 2005;5:19. doi: 10.1186/1471-2288-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sankey S, Weisfiels L, Fine M, Kapoor W. An assessment of the use of the continuity correction for sparse data in meta-analysis. Commun Stat Simulation Computation. 1996;25:1031–56. [Google Scholar]
- 11.Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol. 2005;58:982–90. doi: 10.1016/j.jclinepi.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 12.Walter SD. Properties of the summary receiver operating characteristic (SROC) curve for diagnostic test data. Stat Med. 2002;21:1237–56. doi: 10.1002/sim.1099. [DOI] [PubMed] [Google Scholar]
- 13.Zwinderman AH, Bossuyt PM. We should not pool diagnostic likelihood ratios in systematic reviews. Stat Med. 2008;27:687–97. doi: 10.1002/sim.2992. [DOI] [PubMed] [Google Scholar]
- 14.Jaeschke R, Guyatt GH, Sackett DL. Users’ guides to the medical literature. III. How to use an article about a diagnostic test. B. What are the results and will they help me in caring for my patients? The Evidence-Based Medicine Working Group. JAMA. 1994;271:703–7. doi: 10.1001/jama.271.9.703. [DOI] [PubMed] [Google Scholar]
- 15.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lijmer JG, Bossuyt PM, Heisterkamp SH. Exploring sources of heterogeneity in systematic reviews of diagnostic tests. Stat Med. 2002;21:1525–37. doi: 10.1002/sim.1185. [DOI] [PubMed] [Google Scholar]
- 17.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analyses detected by a simple graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldenberg RL, Iams JD, Miodovnik M, Van Dorsten JP, Thurnau G, Bottoms S, Mercer BM, Meis PJ, Moawad AH, Das A, Caritis SN, McNellis D. The preterm prediction study: risk factors in twin gestations. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am J Obstet Gynecol. 1996;175:1047–53. doi: 10.1016/s0002-9378(96)80051-2. [DOI] [PubMed] [Google Scholar]
- 19.Imseis HM, Albert TA, Iams JD. Identifying twin gestations at low risk for preterm birth with a transvaginal sonographic cervical measurement at 24 to 26 weeks’ gestation. Am J Obstet Gynecol. 1997;177:1149–55. doi: 10.1016/s0002-9378(97)70032-2. [DOI] [PubMed] [Google Scholar]
- 20.Wennerholm UB, Holm B, Mattsby-Baltzer I, Nielsen T, Platz-Christensen J, Sundell G, Hosseini N, Hagberg H. Fetal fibronectin, endotoxin, bacterial vaginosis and cervical length as predictors of preterm birth and neonatal morbidity in twin pregnancies. Br J Obstet Gynaecol. 1997;104:1398–404. doi: 10.1111/j.1471-0528.1997.tb11010.x. [DOI] [PubMed] [Google Scholar]
- 21.Grisaru-Granovsky S, Farine D, Barrett J, Van Eyk N, Ryan G, Seaward PGR, Windrim R. Is a single ultrasound measurement of cervical length a predictor of the risk of preterm delivery in multifetal pregnancy? Am J Obstet Gynecol. 1998;178(1S):191S. [Google Scholar]
- 22.Yang JH, Kuhlman K, Daly S, Berghella V. Prediction of preterm birth by second trimester cervical sonography in twin pregnancies. Ultrasound Obstet Gynecol. 2000;15:288–91. doi: 10.1046/j.1469-0705.2000.00087.x. [DOI] [PubMed] [Google Scholar]
- 23.Guzman ER, Walters C, O’reilly-Green C, Kinzler WL, Waldron R, Nigam J, Vintzileos AM. Use of cervical ultrasonography in prediction of spontaneous preterm birth in twin gestations. Am J Obstet Gynecol. 2000;183:1103–7. doi: 10.1067/mob.2000.108896. [DOI] [PubMed] [Google Scholar]
- 24.Soriano D, Weisz B, Seidman DS, Chetrit A, Schiff E, Lipitz S, Achiron R. The role of sonographic assessment of cervical length in the prediction of preterm birth in primigravidae with twin gestation conceived after infertility treatment. Acta Obstet Gynecol Scand. 2002;81:39–43. doi: 10.1046/j.0001-6349.2001.00466.x. [DOI] [PubMed] [Google Scholar]
- 25.Vayssière C, Favre R, Audibert F, Chauvet MP, Gaucherand P, Tardif D, Grangé G, Novoa A, Descamps P, Perdu M, Andrini E, Janse-Marec J, Maillard F, Nisand I. Cervical length and funneling at 22 and 27 weeks to predict spontaneous birth before 32 weeks in twin pregnancies: a French prospective multicenter study. Am J Obstet Gynecol. 2002;187:1596–604. doi: 10.1067/mob.2002.127380. [DOI] [PubMed] [Google Scholar]
- 26.Gibson JL, Macara LM, Owen P, Young D, Macauley J, Mackenzie F. Prediction of preterm delivery in twin pregnancy: a prospective, observational study of cervical length and fetal fibronectin testing. Ultrasound Obstet Gynecol. 2004;23:561–6. doi: 10.1002/uog.1048. [DOI] [PubMed] [Google Scholar]
- 27.Sperling L, Kiil C, Larsen LU, Qvist I, Bach D, Wojdemann K, Bladh A, Nikkila A, Jørgensen C, Skajaa K, Bang J, Tabor A. How to identify twins at low risk of spontaneous preterm delivery. Ultrasound Obstet Gynecol. 2005;26:138–44. doi: 10.1002/uog.1938. [DOI] [PubMed] [Google Scholar]
- 28.Fait G, Har-Toov J, Gull I, Lessing JB, Jaffa A, Wolman I. Cervical length, multifetal pregnancy reduction, and prediction of preterm birth. J Clin Ultrasound. 2005;33:329–32. doi: 10.1002/jcu.20159. [DOI] [PubMed] [Google Scholar]
- 29.Arabin B, Roos C, Kollen B, van Eyck J. Comparison of transvaginal sonography in recumbent and standing maternal positions to predict spontaneous preterm birth in singleton and twin pregnancies. Ultrasound Obstet Gynecol. 2006;27:377–86. doi: 10.1002/uog.2694. [DOI] [PubMed] [Google Scholar]
- 30.To MS, Fonseca EB, Molina FS, Cacho AM, Nicolaides KH. Maternal characteristics and cervical length in the prediction of spontaneous early preterm delivery in twins. Am J Obstet Gynecol. 2006;194:1360–5. doi: 10.1016/j.ajog.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 31.Klein K, Gregor H, Hirtenlehner-Ferber K, Stammler-Safar M, Witt A, Hanslik A, Husslein P, Krampl E. Prediction of spontaneous preterm delivery in twin pregnancies by cervical length at mid-gestation. Twin Res Hum Genet. 2008;11:552–7. doi: 10.1375/twin.11.5.552. [DOI] [PubMed] [Google Scholar]
- 32.Aboulghar MM, Aboulghar MA, Mourad L, Serour GI, Mansour RT. Ultrasound cervical measurement and prediction of spontaneous preterm birth in ICSI pregnancies: a prospective controlled study. Reprod Biomed Online. 2009;18:296–300. doi: 10.1016/s1472-6483(10)60269-6. [DOI] [PubMed] [Google Scholar]
- 33.Fox NS, Saltzman DH, Klauser CK, Peress D, Gutierrez CV, Rebarber A. Prediction of spontaneous preterm birth in asymptomatic twin pregnancies with the use of combined fetal fibronectin and cervical length. Am J Obstet Gynecol. 2009;201:313.e1–5. doi: 10.1016/j.ajog.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 34.Crane JM, Van den Hof M, Armson BA, Liston R. Transvaginal ultrasound in the prediction of preterm delivery: singleton and twin gestations. Obstet Gynecol. 1997;90:357–63. doi: 10.1016/s0029-7844(97)00277-9. [DOI] [PubMed] [Google Scholar]
- 35.Persutte WH, Chyu J, Cioffi-Ragan D, Hobbins JC. Cervical length in twins. Am J Obstet Gynecol. 2000;182:S118. [Google Scholar]
- 36.Vendittelli F, Mamelle N, Munoz F, Janky E. Transvaginal ultrasonography of the uterine cervix in hospitalized women with preterm labor. Int J Gynaecol Obstet. 2001;72:117–25. doi: 10.1016/s0020-7292(00)00313-1. [DOI] [PubMed] [Google Scholar]
- 37.Fuchs I, Tsoi E, Henrich W, Dudenhausen JW, Nicolaides KH. Sonographic measurement of cervical length in twin pregnancies in threatened preterm labor. Ultrasound Obstet Gynecol. 2004;23:42–5. doi: 10.1002/uog.951. [DOI] [PubMed] [Google Scholar]
- 38.Gonzalez N, Bige V, Kandoussi S, Graesslin O, Quereux C, Gabriel R. Sonographic measurement of cervical length in twin pregnancies with preterm labor: comparison with singleton pregnancies [Article in French] Gynecol Obstet Fertil. 2004;32:122–7. doi: 10.1016/j.gyobfe.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 39.Souka AP, Heath V, Flint S, Sevastopoulou I, Nicolaides KH. Cervical length at 23 weeks in twins in predicting spontaneous preterm delivery. Obstet Gynecol. 1999;94:450–4. doi: 10.1016/s0029-7844(99)00277-x. [DOI] [PubMed] [Google Scholar]
- 40.Whiting P, Rutjes AW, Reitsma JB, Glas AS, Bossuyt PM, Kleijnen J. Sources of variation and bias in studies of diagnostic accuracy: a systematic review. Ann Intern Med. 2004;140:189–202. doi: 10.7326/0003-4819-140-3-200402030-00010. [DOI] [PubMed] [Google Scholar]
- 41.Rutjes AW, Reitsma JB, Di Nisio M, Smidt N, van Rijn JC, Bossuyt PM. Evidence of bias and variation in diagnostic accuracy studies. CMAJ. 2006;174:469–76. doi: 10.1503/cmaj.050090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gordon M, Robbins A, McKenna D, Howard B, Barth W. Cervical length assessment as a resource to identify twins at risk for preterm delivery (Clarity study) Am J Obstet Gynecol. 2006;195:S55. [Google Scholar]
- 43.Crowther CA. Hospitalisation and bed rest for multiple pregnancy. Cochrane Database Syst Rev. 2001;1 doi: 10.1002/14651858.CD000110. CD000110. [DOI] [PubMed] [Google Scholar]
- 44.Yamasmit W, Chaithongwongwatthana S, Tolosa JE, Limpongsanurak S, Pereira L, Lumbiganon P. Prophylactic oral betamimetics for reducing preterm birth in women with a twin pregnancy. Cochrane Database Syst Rev. 2005;3 doi: 10.1002/14651858.CD004733.pub2. CD004733. [DOI] [PubMed] [Google Scholar]
- 45.Jorgensen AL, Alfirevic Z, Tudur Smith C, Williamson PR. cerclage IPD Meta-analysis Group. Cervical stitch (cerclage) for preventing pregnancy loss: individual patient data meta-analysis. BJOG. 2007;114:1460–76. doi: 10.1111/j.1471-0528.2007.01515.x. [DOI] [PubMed] [Google Scholar]
- 46.Norman JE, Mackenzie F, Owen P, Mactier H, Hanretty K, Cooper S, Calder A, Mires G, Danielian P, Sturgiss S, MacLennan G, Tydeman G, Thornton S, Martin B, Thornton JG, Neilson JP, Norrie J. Progesterone for the prevention of preterm birth in twin pregnancy (STOPPIT): a randomised, double-blind, placebo-controlled study and meta-analysis. Lancet. 2009;373:2034–40. doi: 10.1016/S0140-6736(09)60947-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


