Summary
Low G+C Gram-positive bacteria typically contain multiple LacI/GalR regulator family members which often have highly similar amino-terminal DNA binding domains suggesting significant overlap in target DNA sequences. The LacI/GalR family regulator catabolite control protein A (CcpA) is a global regulator of the Group A Streptococcus (GAS) transcriptome and contributes to GAS virulence in diverse infection sites. Herein, we studied the role of the maltose repressor (MalR), another LacI/GalR family member, in GAS global gene expression and virulence. MalR inactivation reduced GAS colonization of the mouse orophyarnx but did not detrimentally affect invasive infection. The MalR transcriptome was limited to only 25 genes, and a highly conserved MalR DNA-binding sequence was identified. Variation of the MalR binding sequence significantly reduced MalR binding in vitro. In contrast, CcpA bound to the same DNA sequences as MalR but tolerated variation in the promoter sequences with minimal change in binding affinity. Inactivation of pulA, a MalR regulated gene which encodes a cell-surface carbohydrate binding protein, significantly reduced GAS human epithelial cell adhesion and mouse oropharyngeal colonization but did not affect GAS invasive disease. These data delineate a molecular mechanism by which hierarchical regulation of carbon source utilization influences bacterial pathogenesis in a site-specific fashion.
Keywords: Streptococcus, maltodextrin, pharyngitis, adhesion
Introduction
Recently there has been a significant increase in understanding the underappreciated but important relationship between basic metabolic processes and virulence in pathogenic bacteria (Alteri et al., 2009, Majerczyk et al., 2010, Eisenreich et al., 2010, Marrero et al., 2010). A good example of the close ties between microbial metabolism and virulence is the LacI/GalR family of transcriptional regulators of which more than 1000 characterized and hypothetical members are currently known (Swint-Kruse & Matthews, 2009, Weickert & Adhya, 1992). LacI/GalR family regulators control a broad range of bacterial metabolic processes ranging from selective carbon source utilization to nucleotide synthesis to amino acid catabolism (Nguyen & Saier, 1995, Swint-Kruse & Matthews, 2009, Weickert & Adhya, 1992). Moreover, inactivation of members of the LacI/GalR family influences the virulence of several important human bacterial pathogens (Iyer & Camilli, 2007, Eswarappa et al., 2009, Li et al., 2010).
For low G+C Gram-positive pathogens, the best studied LacI/GalR family member is catabolite control protein A (CcpA) (Shelburne et al., 2010, Seidl et al., 2009, Kinkel & McIver, 2008, Varga et al., 2008, Abranches et al., 2008). CcpA is the master regulator of selective carbon source utilization for low G+C Gram-positive bacteria as well as a critical regulator of virulence factor genes in a diverse array of human Gram-positive pathogens (Shelburne et al., 2008a, Seidl et al., 2008, Varga et al., 2008, Abranches et al., 2008, Singh et al., 2008, Sonenshein, 2007, Kietzman & Caparon, 2010). All major low G+C Gram-positive organisms contain more than one LacI/GalR family member, which are characterized by an amino-terminal helix-turn-helix DNA binding domain, the DNA binding “Hinge-Helix”, and a carboxy-terminus domain that interacts with a variety of modulating agents (Weickert & Adhya, 1992, Swint-Kruse & Matthews, 2009). The relatively high degrees of homology in the DNA binding domains and the multiple LacI/GalR family members typically present in the same bacteria suggest that the distinct LacI/GalR regulators may target the same DNA sequences (Francke et al., 2008, Shelburne et al., 2008b). However, the molecular mechanisms underlying same-site DNA-targeting by distinct LacI/GalR regulators and if such targeting affects bacterial pathogenesis remain unknown.
Among pathogenic bacteria, the study of LacI/GalR family members has been relatively prominent in Group A Streptococcus (GAS), which causes infections in humans ranging from uncomplicated pharyngeal or skin infections to life-threatening bacteremia, pneumonia, and necrotizing fasciitis (Musser & Shelburne, 2009, Shelburne et al., 2008b, Kinkel & McIver, 2008, Almengor et al., 2007). The two LacI/GalR family members that have been studied in GAS are CcpA and the maltose repressor MalR (Shelburne et al., 2010, Shelburne et al., 2007b, Kietzman & Caparon, 2010, Almengor et al., 2007). CcpA influences the transcript level of several hundred GAS genes and affects GAS virulence at multiple different infection sites (Shelburne et al., 2010, Shelburne et al., 2008a, Kietzman & Caparon, 2010, Kinkel & McIver, 2008). The study of MalR has been much more limited, focusing mainly on the effects of MalR on the catabolism of α-1,4-linked glucose polymers known as maltodextrins (Shelburne et al., 2008b, Shelburne et al., 2007b).
Amino acid residues critical for CcpA binding to target DNA sequences have been elucidated via crystallographic analysis in Bacillus species (Schumacher et al., 2011, Schumacher et al., 2007, Schumacher et al., 2004). The amino-terminal DNA binding domain of GAS CcpA is highly homologous to CcpA from Bacillus species (86% identical, 97% similar) indicating that GAS CcpA likely functions in a similar fashion to Bacillus CcpA (Fig. S1). Similar to GAS CcpA, GAS MalR also contains several of the same amino acid residues previously shown to be critical for DNA binding in Bacillus CcpA (Fig. S1) suggesting that GAS CcpA and MalR may employ similar mechanisms to recognize cognate DNA sequences. Herein we report on studies designed to test the hypothesis that MalR has a significantly narrower effect on GAS virulence and a more restricted transcriptome compared to CcpA despite the similarities of the DNA binding domains of the two proteins. Our results provide new insights into the hierarchical control of carbon source utilization in pathogenic bacteria and further expand our understanding of the links between bacterial metabolic processes and virulence.
Results
Creation of MalR inactivated strains from a pharyngitis parental GAS strain
Previous work on MalR in GAS has used the parental invasive serotype M1 isolate MGAS5005 which contains an inactive control of virulence sensor kinase (CovS) (Shelburne et al., 2008b, Shelburne et al., 2007b, Sumby et al., 2006). It has recently been recognized that GAS strains isolated from pharyngitis tend to have a functional CovS protein, perhaps because an intact CovS imparts an increased ability to adhere to and colonize pharyngeal epithelium (Sumby et al., 2006, Hollands et al., 2010). Thus, to better understand the role of MalR in GAS pharyngeal disease, we replaced the malR gene with a spectinomycin resistance cassette in the pharyngitis serotype M1 isolate MGAS2221 (which contains an active CovS protein) to create strain 2221 ΔmalR-1 (see Table 1 for strain details). Similar to our previous work in strain MGAS5005, we were unable to genetically complement strain 2221 ΔmalR-1 in trans (Shelburne et al., 2007b). In an effort to ensure that the observed effects of MalR inactivation were not due to the introduction of spurious mutations, we repeated the entire mutant strain creation process to generate strain 2221 ΔmalR-2. The growth curves of the parental and MalR-inactivated strains during growth in a nutrient-rich medium were indistinguishable (Fig. S2).
Table 1.
Strains and plasmids.
| Strain or plasmid | Genotype | Reference |
|---|---|---|
| Strains | ||
| MGAS2221 | Pharyngeal isolate, serotype M1, wild-type CovS | (Sumby et al., 2006) |
| 2221 ΔmalR-1 | MGAS2221 ΔmalR::aad9, Spc+ | This study |
| 2221 ΔmalR-2 | MGAS2221 ΔmalR::aad9, Spc+ | This study |
| MGAS2221 | Pharyngeal isolate, serotype M1 | This study |
| MGAS2221(pSB100) | MGAS2221 gfp, Cm+ | This study |
| 2221 ΔpulA-1 | MGAS2221ΔpulA::aad9, Spc+ | This study |
| 2221 ΔpulA-2(pSB100) | MGAS2221ΔpulA::aad9 gfp, Spc+, Cm+ | This study |
| 2221 ΔpulA-2 | MGAS2221ΔpulA::aad9, Spc+ | This study |
| 2221 ΔpulA-2(pSB100) | MGAS2221ΔpulA::aad9 gfp, Spc+, Cm+ | This study |
| 2221 ΔcovS::7bp | MGAS2221 with 7 bp insertion in covS | (Sumby et al., 2006) |
| MGAS294 | Invasive isolate, serotype M1, V286F in CovS | (Sumby et al., 2006) |
| MGAS2217 | Invasive isolate, serotype M1, 1 bp insert in covS | (Sumby et al., 2006) |
| MGAS5005 | Invasive isolate, serotype M1, 1 bp deletion in covS | (Sumby et al., 2005) |
| MGAS5322 | Pharyngeal isolate, serotype M1, wild-type CovS | (Sumby et al., 2006) |
| MGAS5392 | Pharyngeal isolated, serotype M1, wild-type CovS | (Sumby et al., 2006) |
| MGAS6184 | Invasive isolate, serotype M1, A397V in CovS | (Sumby et al., 2006) |
| MGAS9506 | Pharyngeal isolate, serotype M1, wild-type CovS | (Sumby et al., 2006) |
| Plasmids | ||
| pSL60-1 | Vector containing aad9 gene, Spc+ | (Lukomski et al., 2000) |
| pSB100 | Gfp expressing plasmid, Cm+ | This study |
MalR-inactivation results in reduced colonization of the mouse oropharynx and decreased persistence in human saliva but does not affect invasive GAS disease
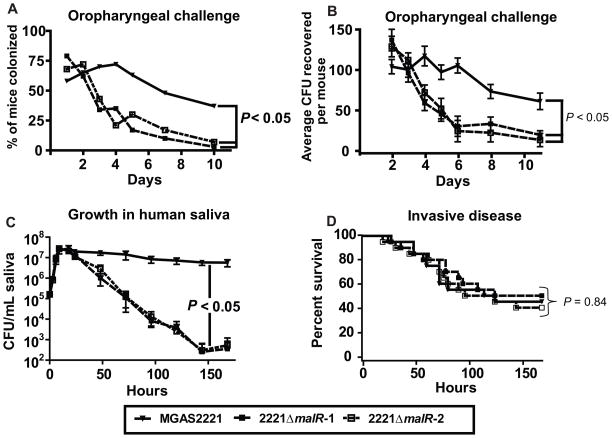
To examine the role of MalR in GAS host-pathogen interaction in the oropharynx, we compared the ability of strain MGAS2221 and its MalR-inactivated derivatives to colonize the mouse oropharynx. Following intranasal inoculation, the wild-type strain MGAS2221 was recovered from the oropharynx of a significantly higher proportion of mice over the time course of the study compared to either strains 2221 ΔmalR-1 or 2221 ΔmalR-2 (P < 0.05 for difference between strain MGAS2221 and either strain 2221 ΔmalR-1 or 2221 ΔmalR-2, Fig. 1A). Similarly, significantly more colony forming units (CFUs) were recovered over the course of the study from the mice infected with strain MGAS2221 compared to mice infected with either of the MalR-inactivated strains (P < 0.05 for difference between strain MGAS2221 and either strain 2221 ΔmalR-1 or 2221 ΔmalR-2, Fig. 1B).
Fig. 1.
MalR affects GAS pathogenesis in a site-specific fashion. (A, B) Indicated GAS strains were inoculated into the nares of 35 CD-1 outbred mice per strain. (A) Percent of mice colonized in the oropharynx with GAS by day. (B) Average number of GAS CFUs isolated from the oropharynx of mice by day with data graphed being mean ± standard error the mean. (C) Indicated GAS strains were grown in human saliva as described (Shelburne et al., 2005a). Strains were grown in duplicate on two separate occasions with data graphed being mean ± standard deviation. For (A–C) P values were derived from a repeated measures analysis followed by Bonferroni’s correction for multiple comparisons. (D) Indicated GAS strains were inoculated intraperitoneally into 20 CD-1 outbred mice per strain. Data graphed are Kaplan-Meier survival analysis with P value derived from a log-rank test.
Next, we tested the ability of the wild-type and MalR inactivated strains to grow in human saliva, an ex vivo model of human oropharyngeal conditions (Shelburne et al., 2005a). Strain MGAS2221 and its MalR-inactivated derivative strains proliferated in human saliva to a similar degree (Fig. 1C). However, the MalR-inactivated mutant strains were unable to persist at high levels compared to wild-type strain MGAS2221 (P < 0.05 for difference between strain MGAS2221 and either strain 2221 ΔmalR-1 or 2221 ΔmalR-2, Fig. 1C). To determine if MalR affects the virulence of strain MGAS2221 at multiple infection sites, we next challenged mice intraperitoneally with the wild-type and MalR-inactivated strains. There was no statistically significant difference in survival times for mice challenged with the three strains (P = 0.84 for difference among the three strains, Fig. 1D). Taken together, these data indicate that MalR contributes to GAS pathogenesis in a site-specific fashion.
The MalR transcriptome includes genes putatively involved in polysaccharide catabolism and genes of unknown function
The regulon of MalR or a related orthologue is unknown. Therefore, to increase understanding of mechanisms by which MalR influences GAS host-pathogen interaction, we determined the MalR transcriptome using a custom-made Affymetrix GeneChip® that contains 100% of the open reading frames of strain MGAS2221. Given that the two MalR inactivated mutant strains had identical phenotypes under multiple in vitro and in vivo conditions (Fig. 1 and Fig. S2), we used strain 2221 ΔmalR-1 as the sole MalR inactivated strain for the remainder of the studies. Strains MGAS2221 and 2221 ΔmalR-1 were grown in triplicate to mid-exponential phase (see Fig. S2A for RNA isolation point). Out of the 1,851 GAS genes present on the array, there were only 25 genes whose transcript levels were significantly different between the two strains, with 21 of the genes having higher transcript levels in the MalR-inactivated strain (Table 2). The majority of genes affected by MalR-inactivation encode proteins known to be or putatively involved in polysaccharide transport and metabolism, whereas most of the remaining genes encode hypothetical or phage proteins (Table 2). We conclude that the MalR transcriptome is relatively limited in GAS with most MalR-influenced genes being involved in carbon source acquisition and catabolism.
Table 2.
Microarray analysis of gene transcript level in strains MGAS2221 vs. 2221 ΔmalR-1.
| ORF in strain MGAS5005 | Putative function of encoded protein (name) | Fold changea | Putative MalR binding siteb |
|---|---|---|---|
| spy0015 | hypothetical protein | 4.2 | No |
| spy0150 | 3-keto-L-gluconate phosphotransferase | 0.42 | No |
| spy0177 | bioY protein | 0.46 | No |
| spy0428 | daunorubicin resistance ATP-binding protein | 0.16 | Yes |
| spy0429 | daunorubicin resistance transmembrane protein | 0.19 | Yes |
| spy0430 | ABC transporter permease protein | 0.13 | Yes |
| spy1021 | phage protein | 0.46 | No |
| spy1029 | phage protein | 0.42 | No |
| spy1049 | phage protein | 0.43 | No |
| spy1055 | maltodextrin phosphorylase (MalP) | 0.15 | Yes |
| spy1056 | 4-alpha-glucanotransferase (MalQ) | 0.09 | Yes |
| spy1058 | maltodextrin binding protein (MalE) | 0.40 | Yes |
| spy1059 | maltodextrin permease protein (MalF) | 0.38 | Yes |
| spy1060 | maltodextrin permease protein (MalG) | 0.39 | Yes |
| spy1456 | phage protein | 0.32 | No |
| spy1574 | universal stress response protein | 5.1 | No |
| spy1575 | MFS family transporter (NorA) | 7.1 | No |
| spy1632 | 6-phospho-beta-galactosidase (LacG) | 4.7 | No |
| spy1662 | putative transport protein | 0.34 | No |
| spy1678 | Thioredoxin | 0.48 | No |
| spy1679-80 | pulullanase (PulA) | 0.10 | Yes |
| spy1681 | 1,6-alpha-glucosidase | 0.07 | Yes |
| spy1691 | exonuclease family protein | 0.43 | Yes |
| spy1692-3 | maltose phosphotransferase (MalT) | 0.17 | Yes |
| spy1829 | phage protein | 0.20 | No |
Ratio indicates gene level in strain MGAS2221 relative to strain 2221 ΔmalR-1
Indicates whether a putative MalR binding site (NNGCAAYCGNTTGCYR) is present in the promoter region of the indicated gene
Identification of a putative MalR consensus DNA binding sequence
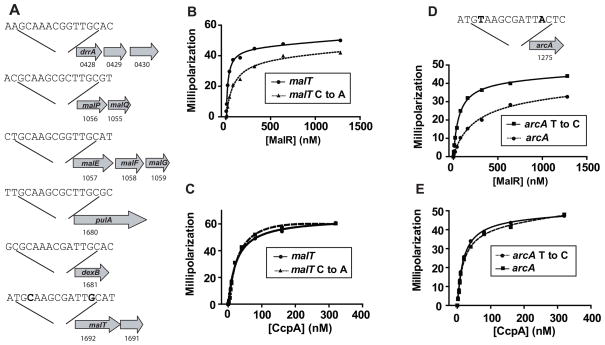
In low G+C Gram-positive organisms, MalR is thought to function mainly as a repressor protein (Shelburne et al., 2007b, Andersson & Radstrom, 2002, Nieto et al., 2001). Consistent with this idea, the microarray data revealed six operons for which the transcript levels of all genes in the operon were significantly higher in the MalR-inactivated strain indicating direct or indirect MalR repression of these genes (spy0428-30, spy1057-8, spy1060-2, spy1679-80, spy1681, and spy1691-3). The putative promoter regions of each of the six operons contained the sequence N1N2G3C4A5A6R7C8G9N10T11T12G13C14Y15R16 (where N stands for any base, R for A or G, and Y for C or T) suggesting a conserved MalR binding site (Fig. 2A). A search of multiple GAS genomes did not identify any additional locations with the aforementioned sequence. Bioinformatic analysis found that all GAS genomes published to date contain the identical putative MalR binding sequences in the six identified operons (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Moreover, the putative GAS MalR consensus DNA binding sequence is in good agreement with the MalR DNA binding sequence previously identified for two Streptococcus pneumoniae maltose utilization operons (Nieto et al., 2001).
Fig. 2.
Identification of MalR consensus binding sequence and functional MalR binding studies. (A) Schematic of putative MalR binding sites in the promoter regions of the indicated operons that were negatively influenced by MalR in the transcriptome analysis. Numbers below genes refer to designation in the MGAS5005 genome (Sumby et al., 2005). (B, C) Recombinant GAS MalR (B) or CcpA (C) was titrated into a binding buffer containing 1 nM fluorescein-labeled malT promoter DNA (circle) or 1 nM fluorescein labeled malT promoter DNA with the 4th/13th C/G changed to A/T (called malT C to A, triangle, dashed line). Note that the changed nucleotides are bold in the malT portion of (A). Millipolarization units are plotted against the protein concentration (nM). (D, E) Recombinant GAS MalR (D) or CcpA (E) was titrated into a binding buffer containing 1 nM fluorescein-labeled arcA promoter DNA (circle, dashed line) or 1 nM fluorescein labeled arcA promoter DNA with the 4th/13th T/A changed to C/G (called arcA T to C, triangle, solid line). The changed nucleotides are bold in the arcA promoter region schematic shown above the binding data. For (B–E), each experiment was done on three occasions with representative data from one experiment shown. Lines indicate fit of binding data derived from non-linear regression.
Identification of nucleotide residues critical to MalR DNA binding
Our proposed consensus MalR binding sequence is pseudopalindromic in nature similar to the CcpA consensus binding sequence from Bacillus species W1T2G3N4N5A6R7C8G9N10W11W12W13C14A15W16 (W = A or T) (Weickert & Chambliss, 1990, Miwa et al., 2000). However, at the 4th/13th positions of the DNA binding sequence, each of the six promoters from the MalR-influenced operons (Fig. 2A) contain a C/G whereas the CcpA consensus sequence tolerates significant heterogeneity in base composition at this site (N/W). We have previously demonstrated that recombinant MalR binds to DNA containing the putative consensus sequence from the malT promoter (Shelburne et al., 2008b). To test the hypothesis that the C/G at the 4th/13th base locations are critical for MalR DNA binding, we compared recombinant MalR binding to the malT site and to an altered malT site in which the 4th/13th base pairs had been changed from C/G to A/T. Consistent with our hypothesis, altering the 4th/13th base pairs decreased the DNA binding affinity of recombinant MalR (KD of 17.5 nM for wild-type malT sequence vs. 74.2 nM for altered malT sequence, Fig. 2B). In contrast, changing the malT sequence at the 4th/13th base positions did not markedly alter the DNA-binding affinity of recombinant CcpA (KD of 23.9 nM for wild-type MalT sequence and 29.8 nM for the altered MalT sequence) consistent with the flexible nature of CcpA for base pair composition at the 4th/13th position (Fig. 2C).
To determine whether the lack of a C/G at 4th/13th position impedes MalR-mediated gene regulation, we next examined the promoter of a gene influenced by CcpA but not MalR. CcpA, but not MalR, is known to affect expression of the arginine deiminase operon through the binding sequence A1T2G3T4A5A6G7C8G9A10T11T12A13C14T15C16 upstream of the arcA gene (Shelburne et al., 2010). The arcA CcpA binding site differs from a MalR consensus site in the presence of a T/A rather than C/G at the 4th/13th position. Therefore, we tested the hypothesis that changing the 4th/13th base positions of the arcA DNA binding site would increase the binding affinity of recombinant MalR. As predicted, substituting C/G for T/A at the 4th/13th base positions increased MalR affinity for the arcA site (KD decreased from 260 nM to 61 nM, Fig. 2D). However, no change in CcpA DNA binding affinity was observed between the wild-type and altered arcA binding sequences (Fig. 2E). Taken together, these data indicate that MalR tolerates less variability compared with CcpA at the 4th/13th position of the DNA binding sequence, which likely contributes to the comparatively narrow MalR transcriptome.
pulA transcript levels are elevated in human saliva and in humans with pharyngitis
Having established a site-specific contribution of MalR to GAS pathogenesis and having determined the MalR transcriptome, we next sought to better understand the mechanism by which specific MalR-regulated genes influences GAS host-pathogen interaction. The MalR regulon contains two putative cell-surface proteins, MalE, which encodes a maltodextrin-binding lipoprotein (Shelburne et al., 2007a, Shelburne et al., 2006), and PulA, which encodes a cell-wall anchored carbohydrate binding and degrading enzyme (van Bueren et al., 2007, Hytonen et al., 2006, Hytonen et al., 2003). Inasmuch as we had previously investigated the role of MalE in GAS virulence (Shelburne et al., 2008b, Shelburne et al., 2006) and that the role of PulA has yet to be determined, we next tested the hypothesis that PulA contributes to GAS pathogenesis.
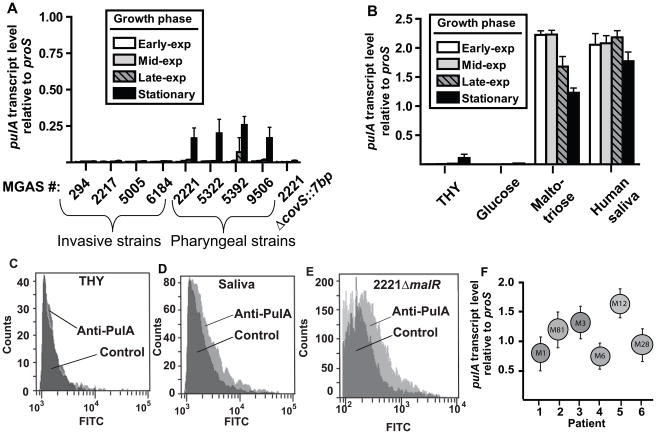
To begin to investigate the contribution of PulA to host-pathogen interaction we determined pulA transcript level in serotype M1 strains of defined clinical sources (Table 1). During cultivation in THY broth, pulA transcript was essentially undetectable except in pharyngitis strains during the stationary phase of growth (Fig. 3A). Consistent with pulA being positively influenced by CovS, at all growth phases pulA transcript levels were very low in the CovS-inactivated derivative of strain MGAS2221 (strain 2221 ΔcovS::7bp, Fig. 3A). When the pharyngitis strain MGAS2221 was grown in human saliva or a maltotriose-medium (i.e. a chemically defined medium in which maltotriose is the sole carbon source), pulA transcript level was significantly elevated compared to growth in THY broth or a glucose-medium (Fig. 3B). As expected, the increased pulA transcript level observed during growth in human saliva compared to growth in THY broth was translated into increased PulA cell-surface expression as determined by flow cytometry (Fig. 3C, Fig. 3D). Moreover, PulA cell-surface expression was easily detectable during growth in THY broth in the MalR-inactivated strain consistent with the idea that MalR represses PulA expression under nutrient-rich conditions (Fig. 3E). Finally, pulA transcript was readily detected in RNA extracted from oropharyngeal swabs collected from patients with confirmed GAS pharyngitis, suggesting in vivo derepression of pulA expression (Fig. 3F). These data indicate that the nutrient-limited conditions encountered in the human oropharynx result in increased PulA expression compared to a nutrient-rich environment such as a standard laboratory medium.
Fig. 3.
Analysis of pulA transcript levels in laboratory conditions and in vivo. (A) pulA transcript level in serotype M1 GAS strains of defined patient and CovR/S phenotype (Table 1). (B) pulA transcript level in strain MGAS2221 in indicated growth medium. For (A) and (B) white bars indicate early exponential growth, grey bars mid-exponential growth, striped bars late exponential growth, and black bars stationary phase. For (A) and (B) data graphed are mean ± standard deviation of 8 data points. (C–E) Flow cytometric analysis of PulA cell-surface expression. Light grey represents anti-PulA antibody whereas dark grey indicates a control antibody. (C) Strain MGAS2221 grown in THY. (D) Strain MGAS2221 grown in human saliva. (E) Strain 2221 malR-1 grown in THY. (F) pulA transcript levels for 6 patients with GAS pharyngitis were determined by TaqMan real-time PCR. The M serotype of the infecting GAS strain is shown in the circle. Error bars represent standard deviation with each experiment done in quadruplicate on two separate occasions.
Inability to detect a role for PulA in GAS α-glucan catabolism
PulA contains an amino-terminal domain previously shown to be involved in carbohydrate binding (van Bueren et al., 2007) and a carboxy-terminal domain putatively involved in carbohydrate degradation. Thus, one possible role for PulA is to allow GAS to use carbohydrates, specifically α-glucans such as glycogen, pullulan, or maltodextrins (i.e. maltose, maltotriose, etc.), as an energy source. We used non-polar insertional mutagenesis to independently create two PulA-inactivated strains from the parental strain MGAS2221 (growth curves for the PulA-inactivated strains in nutrient rich medium are shown in Fig. S2). We found no growth of strain MGAS2221 or its PulA(−) derivative strains in a chemically defined medium in which glycogen or pullulan were the sole carbon source (Fig. S2 and data not shown). Similarly, we observed no significant difference in growth between MGAS2221 and the PulA-inactivated strains when maltose, maltotriose, or maltotetraose were the sole carbon source (Fig. S2 and data not shown). Thus, under the conditions studied we could not discern a role for PulA in GAS α-glucan catabolism.
PulA contributes to GAS epithelial cell adhesion following growth in human saliva
Some microbial carbohydrate binding proteins facilitate adherence to eukaryotic cells by binding to cell-surface carbohydrates (Bouckaert et al., 2005, Uchiyama et al., 2009). Therefore, we next sought to test the hypothesis that PulA participates in GAS epithelial cell adherence (Hytonen et al., 2003, Hytonen et al., 2006). We speculated that low PulA expression on the GAS cell surface during growth in THY broth may have resulted in the modest role observed for PulA in previous eukaryotic cell adherence assays (Hytonen et al., 2006). Indeed, there was no significant difference in the adherence of strain MGAS2221 and its two isogenic ΔpulA derivatives to D562 pharyngeal epithelial cells when GAS was grown under standard laboratory conditions (i.e. in THY, Fig. 4A–C). However, when grown ex vivo in human saliva, adherence of strain MGAS2221 to D562 cells was significantly increased compared to strains 2221 ΔpulA-1 and 2221 ΔpulA-2 as measured by standard light microscopy (Fig. 4A, 4D–E). Similarly, after being grown in human saliva, strain MGAS2221 adhered significantly better than the ΔpulA strains to normal human tracheobronchial epithelial (NHTBE) cells (Fig. 4F–J). The increased adherence of GAS to D562 or NHTBE cells following growth in saliva did not result from the presence of saliva, as washing GAS grown in THY or a maltodextrin-medium (done to increase pulA expression) with human saliva decreased subsequent adherence compared to GAS not washed with saliva (data not shown). To determine whether our method of preparing or counting cells via light microscopy might have accounted for the observed differences, we next used fluorescence microscopy to study the adherence of the three strains transformed with a plasmid expressing green fluorescent protein (Gfp). Similar to our findings with light microscopy, strain MGAS2221(pSB100) grown in human saliva had increased adherence to D562 and NHTBE cells compared to strains 2221 ΔpulA-1(pSB100) or 2221 ΔpulA-2(pSB100) (Fig. 4K–P). There was no significant difference in the adherence of the three strains cultivated in THY to either D562 or NHTBE cells (data not shown).
Fig. 4.
PulA contributes to GAS eukaryotic cell adherence. (A) Adherence of wild-type (MGAS2221 – white bars) and ΔpulA derivative strains (2221 ΔpulA-1, grey bars; 2221 ΔpulA-2, striped bars) to D562 pharyngeal epithelial cells following cultivation in THY or human saliva as indicated. Representative pictures of adherence of strains 2221 (B) and 2221 ΔpulA-1 (C) to D562 cells following cultivation in THY. Representative pictures of adherence of strains 2221 (D) and 2221 ΔpulA-1 (E) to D562 cells following cultivation in human saliva. (F) Adherence of wild-type (MGAS2221 – white bars) and ΔpulA derivative strains (2221 ΔpulA-1, grey bars; 2221 ΔpulA-2, striped bars) to normal human tracheobronchial epithelial (NHTBE) cells following cultivation in THY or human saliva as indicated. Representative pictures of adherence of strains 2221 (G) and 2221 ΔpulA-1 (H) to NHTBE cells following cultivation in THY. Representative pictures of adherence of strains 2221 (I) and 2221 ΔpulA-2 (J) to NHTBE cells following cultivation in human saliva. For (A) and (F) data graphed are mean numbers of adherent bacteria with error bars indicating standard error of the mean with each experiment done in triplicate on four separate occasions. (K–P) Representative pictures of GAS adherence using strains expressing Gfp and fluorescence microscopy. (K–M) Adherence of strain MGAS2221(pSB100) to NHTBE cells following cultivation in human saliva. (K) Fluorescence alone; (L) Differential phase microscopy; (M) Merge of (K) and (L). (N–P) Adherence of strain 2221 ΔpulA-1(pSB100) to NHTBE cells following cultivation in human saliva. (N) Fluorescence alone; (O) Differential phase microscopy; (P) Merge of (N) and (O).
α-glucans and antibody to the PulA N-terminal α-glucan binding domain interfere with GAS eukaryotic cell adherence
To further investigate the mechanism by which PulA mediates GAS eukaryotic cell adhesion, we overexpressed and purified the PulA N-terminal carbohydrate binding domain designated as SpyDx in accordance with a previous publication (Fig. 5A) (van Bueren et al., 2007). We confirmed the functional activity of SpyDx by demonstrating its ability to bind the α-glucans glycogen and maltotetraoase whereas no specific binding was observed for glucose (Fig. 5B–D). Following growth in human saliva, the addition of purified SpyDx significantly inhibited the ability of strain MGAS2221 to bind D562 eukaryotic cells (Fig. 5F) whereas the presence of recombinant HPr (Fig. 5E), a GAS cytosolic protein (i.e. a negative control), had no significant effect. Similarly, polyclonal rabbit antibody raised against SpyDx significantly reduced GAS eukaryotic cell adhesion (Fig. 5F) whereas the presence of anti-HPr polyclonal antibody did not significantly affect GAS adherence (Fig. 5F). Finally, the presence of the α-glucans glycogen and maltotetraose significantly reduced GAS eukaryotic cell adherence (Fig. 5G) whereas glucose did not. These data strongly suggest that specific α-glucan binding by the N-terminal portion of PulA contributes to GAS eukaryotic cell adherence following growth in human saliva.
Fig. 5.
SpyDx contributes to GAS epithelial cell adhesion through binding of α-glucans. (A) The N-terminus, carbohydrate binding portion of PulA (SpyDx) was overexpressed and purified to apparent homogeneity (shown is purified SpyDx run on 4–15% gradient SDS-PAGE). (B–D) Isothermal titration calorimetry data of recombinant SpyDx for (B) glycogen, (C) maltotetraose, and (D) glucose. Note binding shape curve for (B) and (C) indicates heat-release upon binding whereas (D) shows no evidence of specific binding. (E) Recombinant HPr was purified to apparent homogeneity and analyzed as in (A). (F, G) Binding of strain MGAS2221 to D562 human epithelial cells following growth in human saliva. Indicated recombinant proteins (F), antibodies (F), and carbohydrates (G) were added as described in Material and Methods. For (F and G) data graphed are mean numbers of adherent bacteria with error bars indicating standard error of the mean with each experiment done in triplicate on four separate occasions.
PulA affects GAS oropharyngeal colonization but not invasive GAS disease
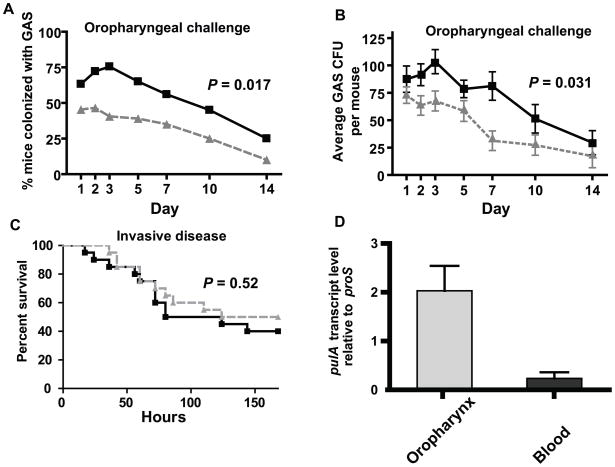
Given that PulA contributes to the epithelial cell adherence of strain MGAS2221, we next determined whether genetic inactivation of PulA affected the ability of strain MGAS2221 to colonize the mouse oropharynx. Following nasopharyngeal inoculation, strain MGAS2221 was recovered from more mice and at a higher density compared to strain 2221 ΔpulA at each time point tested (Fig. 6A, 6B). Similar to what we observed for MalR, however, PulA inactivation did not significantly affect the virulence of strain MGAS221 following intraperitoneal inoculation (Fig. 6C). Consistent with these findings, in strain MGAS2221 the pulA transcript level was significantly higher among GAS recovered from the mouse oropharynx compared to GAS isolated from the bloodstream of mice infected intraperitoneally (Fig. 6D). These data indicate that the contribution of PulA to GAS virulence and the expression of pulA occurs in a niche-specific fashion during GAS infection.
Fig. 6.
PulA contributes to GAS oropharyngeal colonization but not invasive disease. (A, B) Indicated GAS strains were inoculated into the nares of 35 CD-1 outbred mice per strain. (A) Percent of mice colonized in the oropharynx with GAS by day. (B) Average number of GAS CFUs isolated from the oropharynx of mice by day with data graphed being mean ± standard error the mean. For (A, B) P values were derived from a repeated measures analysis. (C) Indicated GAS strains were inoculated intraperitoneally into 20 CD-1 outbred mice per strain. Data graphed are Kaplan-Meier analysis with P value derived from log-rank test. (D) GAS RNA was isolated from mouse oropharynx on day 2 following intranasal inoculation and from mouse blood on day 2 following intraperitoneal inoculation. Data graphed are mean transcript level ± standard deviation for four animals analyzed in duplicate.
Discussion
LacI/GalR-type transcriptional regulators have long served as prototypes for studying basic physiologic processes in prokaryotes (Jacob & Monod, 1961). Recently, however, this large family of regulatory proteins has also gathered significant attention for their contribution to the virulence of numerous bacterial pathogens (Iyer et al., 2005, Li et al., 2010, Chapuy-Regaud et al., 2003, Shelburne et al., 2008a, Allen et al., 2000). In Gram-positive organisms, CcpA has been the best studied LacI/GalR member from a virulence standpoint, but Gram-positive pathogens generally contain multiple LacI/GalR family regulators whose pathogenic role has been only minimally investigated (Shelburne et al., 2008b). Herein we show that the LacI/GalR family member MalR plays a role in GAS virulence that is site-specific thereby extending knowledge of role of LacI/GalR family members in bacterial pathogenesis.
One of our key discoveries was that the GAS MalR transcriptome was quite limited, consisting of only 25 genes. This stands in marked contrast to the hundreds of genes influenced by CcpA-inactivation in GAS and related organisms despite the fact that the CcpA and MalR DNA binding motifs are quite similar (Fig. S1) (Shelburne et al., 2010, Shelburne et al., 2008a, Seidl et al., 2009, Kinkel & McIver, 2008, Abranches et al., 2008). The relatively few genes influenced by MalR appears to be explained by the highly conserved nature of the MalR consensus binding site (Fig. 2). For GAS, such binding sites only occur in the promoter regions of a select number of operons, most of which of involved in maltodextrin transport (Fig. 2). In contrast, we found that GAS CcpA has the flexibility to bind with high affinity to a less stringent consensus sequence providing a potential functional basis for the observation of the large CcpA transcriptome. Our data elucidate a mechanism for hierarchical control of carbon source utilization in which the global regulator CcpA can bind to a diverse array of promoters. In contrast, other LacI/GalR family members, such as MalR or the sucrose repressor ScrR, may target the same DNA sequences as CcpA but are capable of only binding to promoters that contain highly homologous sequences. Such a system would limit off-target binding of the numerous LacI/GalR family members while still permitting CcpA to be a master regulator.
Another key discovery of the work presented herein was the finding that the MalR-regulated carbohydrate-binding protein PulA contributes to GAS epithelial cell adhesion and to GAS oropharyngeal colonization but not invasive GAS disease. The cell-surface location of PulA means that it may contribute to GAS host-pathogen interaction (Reid et al., 2002), and it has been suggested that PulA could be part of a streptococcal vaccine strategy (Santi et al., 2008). A previous genome-wide approach to identify new GAS vaccine candidates did not study PulA because it was not found on the GAS cell-surface following growth in standard laboratory medium (Rodriguez-Ortega et al., 2006). We found that PulA cell-surface expression is minimal in a standard laboratory medium but increases markedly in distinct laboratory conditions and during oropharyngeal but not bacteremic infection (Figs. 3 and 6). The in vivo expression of PulA has been previously demonstrated by the finding that GAS infected human patients have anti-PulA antibodies (Reid et al., 2001, Fritzer et al., 2010). However, active immunization with recombinant PulA did not protect mice against intraperitoneal challenge with an invasive serotype M1 strain (Reid et al., 2002) or following intravenous challenge with GAS strains of three distinct M protein serotypes (Fritzer et al., 2010). Our data showing that PulA is expressed at low levels in mouse blood and that PulA inactivation did not affect GAS disease following intraperitoneal challenge provide potential explanations for the previous immunization studies. Moreover, our finding that PulA contributes to GAS epithelial cell adhesion only following growth in a medium that induces PulA expression may provide a rationale for why PulA-inactivation only slightly affected GAS adherence in a previous investigation (Hytonen et al., 2006). Our data suggest that the pro-adherence effect of PulA is mediated via its amino-terminus carbohydrate binding domain. The specific carbohydrate epitopes on human epithelial cells targeted by amino-terminus of PulA are not currently known but probably are part of cell-surface glycoproteins.
Analysis of the multi-domain structure of PulA suggests that its carboxy-terminus is involved in α-glucan degradation and catabolism. However, under the conditions studied GAS did not proliferate in a defined medium in which α-glucans were the sole carbon source. A previous study in group B Streptococcus (GBS) found that GBS was able to grow in a medium in which pullulan was the sole carbon source and that inactivation of pulA abrogated such growth (Santi et al., 2008). However, in that study, the observed growth phenotype consisted of only a two-fold increase in colony forming unit (CFU) density, as opposed to the 50–100 fold increase typically seen in streptococcal growth studies (Santi et al., 2008). Thus whether PulA-mediated carbon source catabolism is physiologically relevant in streptococci remains unclear. The carboxy-terminus of PulA is highly conserved among various GAS strains (BLAST analysis of publicly available GAS PulA sequences), however, indicating selective pressure to maintain the putative α-glucan degrading domain of PulA which suggests a currently unknown role for this portion of the protein.
In summary, we have discovered that the LacI/GalR regulatory protein MalR contributes to GAS virulence in a site-specific fashion and that at least part of this contribution is likely to be mediated by the carbohydrate binding activity of PulA. These findings shed new light into the molecular mechanisms of selective carbon source utilization in pathogenic bacteria and how such processes influence bacterial pathogenesis.
Experimental procedures
Bacterial strains and culture media
Bacterial strains and plasmids used in this study are listed in Table 1. The genome of the pharyngeal isolate, serotype M1 strain MGAS2221 has been sequenced (Sumby et al., 2006). The additional serotype M1 strains and CovS-inactivated derivative of strain MGAS2221 (strain 2221 ΔcovS::7bp) studied herein have been previously described (Sumby et al., 2006). Isogenic MalR- and PulA-inactivated strains were created by non-polar insertional mutagenesis from strain MGAS2221 using the PCR splicing overlap extension method described by Kuwayama et al. with the spectinomycin resistance cassette being amplified from plasmid pSL60 (Kuwayama et al., 2002, Lukomski et al., 2000). Primers used to create the isogenic mutant strains are listed in Table S1.
Growth of GAS strains in various media
GAS was grown on Trypticase soy agar containing 5% sheep blood (BSA; Becton Dickinson) or in Todd-Hewitt broth containing 0.2% (wt/vol) yeast extract (THY; Difco). Spectinomycin (Sigma) and chloramphenicol (Sigma) was added when appropriate to THY agar or broth at a concentration of 150 μg/mL and 4 μg/mL, respectively. Ultra-pure carbohydrates (Sigma) were added at a concentration of 0.5% (wt/vol) to a carbohydrate-free preparation of a commercially available chemically-defined medium (CDM; JR Biosciences) (Vise et al., 2003). For the purposes of this manuscript, we have used the terms glucose-medium, maltotriose-medium, pullulan-medium etc., to refer to the carbohydrate-free CDM supplemented with the specified carbohydrate. We have previously demonstrated that GAS cannot grow in the CDM without the addition of exogenous carbohydrates (Shelburne et al., 2007a). All comparative growth experiments in THY or CDM were done as previously described in triplicate on three separate occasions for a total of nine replicates (Shelburne et al., 2006). GAS was grown in human saliva as previously described (Shelburne et al., 2005a). Healthy volunteers who gave their informed consent prior to participation in the study donated saliva according to a protocol for human subjects approved by the Baylor College of Medicine Institutional Review Board.
Overexpression and purification of GAS proteins
GAS MalR, CcpA, and HPr were overexpressed and purified via C-terminus His-tags as described (Shelburne et al., 2008b, Shelburne et al., 2008a, Shelburne et al., 2010). HPr phosphorylated at Ser46 (HPr-Ser46-P) was created as described using recombinant GAS HPr kinase/phosphorylase (Shelburne et al., 2010). To work with recombinant SpyDx protein that lacked a tag, we cloned the N-terminal portion of the pulA gene from MGAS2221 into plasmid pTXB1 (New England BioLabs) which resulted in a fusion protein with an intein tag and a chitin binding domain. Recombinant SpyDx was obtained following the manufacturer’s instructions with release of the intein tag using DTT (Fig. 5A).
Fluorescence polarization
The DNA binding activities of MalR and CcpA were measured by fluorescence polarization on a Beacon 2000 fluorescence polarization instrument (Pavera, Madison, WI) as described (Shelburne et al., 2008b). For CcpA, 50 μM phosphorylated HPr (HPr-Ser46-P), which is key to high affinity DNA binding, was included in the binding buffer (Shelburne et al., 2008b). Equilibrium binding constants were determined in triplicate as described with all experiments carried out at 25 °C (Newberry and Brennan, 2004).
Mouse challenge studies
All mouse experiments were performed according to protocols approved by the Methodist Hospital Research Institute Institutional Animal Care and Use Committee and were conducted with adult (18- to 20-g) female outbred CD-1 Swiss mice (Harlan Sprague-Dawley Inc.). For the oropharyngeal challenge studies, both nares of each mouse were inoculated with 50 μL of the GAS suspension (total inoculation 100 μl, 1 × 107 CFU) with 35 mice in each group (Shelburne et al., 2006). The throat of each mouse was swabbed before inoculation and then at specific time periods thereafter, spread onto BSA, incubated overnight, and tested for GAS as described (Shelburne et al., 2006). For the invasive challenge studies, 1 × 107 CFUs of each GAS strain were inoculated intraperitoneally and mice were monitored for near mortality (Shelburne et al., 2008a).
RNA isolation and transcript level analysis
For transcript level analysis under diverse growth conditions, various GAS strains were grown as indicated. RNA was isolated and purified using an RNeasy kit (Qiagen) (Shelburne et al., 2005b). To determine pulA transcript levels in vivo, GAS RNA was isolated from throat swabs obtained from six patients with GAS pharyngitis and analyzed as previously described (Virtaneva et al., 2003). The throat swabs were obtained from patients who gave their informed consent prior to participation in the study according to a protocol for human subjects approved by the Baylor College of Medicine Institutional Review Board. Similarly, GAS RNA was isolated from the mouse oropharynx using swabs obtained 48 hrs after challenge. GAS RNA was isolated from mouse blood 48 hrs after inoculation using the QIAamp RNA Blood MiniKit (Qiagen). The quality and the concentration of RNA were assessed with an Agilent 2100 Bioanalyzer and by analysis of the A260/A280 ratio. cDNA was reverse transcribed from RNA using Superscript III (Invitrogen) following the manufacturer’s instructions. TaqMan quantitative real-time PCR (QRT-PCR) was performed with an ABI Thermocycler 7700 (Applied Biosystems) using the ΔCT method of analysis with the proS gene used as the internal control (Chaussee et al., 2001). Primers and probes used are listed in Table S1. Unless indicated, transcript level analyses were performed in duplicate biologic replicates on two separate occasions and analyzed in duplicate (8 total data points).
Expression microarray analysis
A custom-made Affymetrix GeneChip® that contains probes for 100% of the genes encoded by strain MGAS2221 was used for expression microarray (transcriptome) studies using three biologic replicates of indicated strains grown to mid-exponential growth phase (Fig. S2A). (44). To compare gene transcript levels between the wild-type and mutant strains, a two-sample t-test (unequal variance) was applied followed by a false discovery rate correction (Q < 0.05) to account for multiple testing using Partek Genomics Suite version 6.4. Transcript levels were considered significantly different when the corrected P value was < 0.05 and the mean difference was at least 2-fold.
Creation of anti-SpyDx and anti-HPr antibodies
Purified recombinant SpyDx and HPr were provided to Covance Laboratories for generation of polyclonal antibody using immunization in rabbits (Reid et al., 2002). Purified antibody was obtained from rabbit hyperimmune serum using purified protein (either SpyDx or HPr) as an immunosorbent (Reid et al., 2002).
Assessment of PulA on cell-surface
Surface localization of PulA was assessed with an LSR-II flow cytometer (BD Biosciences) using affinity-purified SpyDx-specific polyclonal antibody (Covance Laboratories) as described (Shelburne et al., 2006). Antibody against HPr, a GAS cytosolic protein, was used as a control for nonspecific antibody binding.
Eukaryotic cell adherence assays
The ability of GAS to adhere to D562 pharyngeal cells was determined by growing various GAS strains to mid-logarithmic phase in either THY or saliva. GAS cells were pelleted, washed in PBS, resuspended in antibiotic free minimal medium and added to the D562 cells at 1:100 multiplicity of infection (MOI). After 2 hrs, non-adherent bacteria were removed via triplicate washing with PBS, and the cells were fixed by adding 4% formaldehyde (VWR) for 1 hr. Formaldehyde was removed, a Gram-stain was performed using standard protocol (Becton-Dickinson), and GAS cells were counted in five high power fields (1000×) by two independent observers blinded as to the strains being analyzed. The same protocol was employed to analyze GAS adhesion to normal human tracheobronchial epithelial cells (NHBTE, Lonza). A similar protocol was used to compare adherence of strains MGAS2221(pSB100), 2221 ΔpulA-1(pSB100), and 2221 ΔpulA-2(pSB100) expressing Gfp via epiflourescence except that the Gram-stain step was omitted. The effect of purified proteins on GAS adherence was determined as above with the additional step of adding 20 μg of the indicated recombinant protein to the D562 cells for 15 mins prior to adding GAS (Jensch et al., 2010). Similarly, when indicated polyclonal antibody from rabbits immunized with purified, recombinant GAS proteins was added to GAS cell suspensions at a final concentration of 10 μg/mL for 15 mins prior to adding GAS to the D562 cells. The effect of various carbohydrates on GAS adhesion was performed by pre-incubating GAS in the indicated carbohydrate (25 mM for maltotetraose or glucose and 5 mg/mL for glycogen) as described (Ryan et al., 2001). All adherence studies were done in triplicate on four separate occasions with microscopy done on a Zeiss AxiostarPlus microscope.
Isothermal titration calorimetry
Analysis of SpyDx binding to various carbohydrates purchased from Sigma was performed as described (Shelburne et al., 2007a) using a VP-ITC (GE Healthcare) in 20 mM Tris (pH 8.0) and 0.5 M NaCl at 25 °C. For glycogen, 2 mM SpyDx was titrated into type IV glycogen (bovine liver, 0.84 mg/ml) in the same buffer as SpyDx (van Bueren et al., 2007). For maltotetraose and glucose, 13.5 mM of the indicated carbohydrate dissolved in the same buffer was titrated into 400 μM SpyDx (van Bueren et al., 2007). Each experiment was performed on at least three occasions with representative data being presented in Fig. 5.
Statistical analyses
Growth, RNA transcript levels, and eukaryotic cell adhesion assays were compared using Student’s two-sided t-test. For the murine mucosal challenge model, a repeated measures analysis was used to test for differences in the percent of animals colonized and in the number of CFU recovered per animal. Statistical significance was assigned a two-sided P value of 0.05 using Bonferroni’s adjustment for multiple comparisons when appropriate. Statistical calculations were performed using GraphPad version 5.01.
Supplementary Material
Acknowledgments
This work was supported by American Heart Association grant 09GRNT2280109 (S.A.S), National Institute Allergy and Infectious Diseases K08 Career Development Award AI-064564 (S.A.S.) and funds from the Welch Foundation (G-0040 to R.G.B.). . The authors thank S.B. Beres for the use of plasmid pSB100 and Paul Sumby for use of strain 2221 ΔcovS::7bp.
References
- Abranches J, Nascimento MM, Zeng L, Browngardt CM, Wen ZT, Rivera MF, Burne RA. CcpA regulates central metabolism and virulence gene expression in Streptococcus mutans. J Bacteriol. 2008;190:2340–2349. doi: 10.1128/JB.01237-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JH, Utley M, van Den Bosch H, Nuijten P, Witvliet M, McCormick BA, Krogfelt KA, Licht TR, Brown D, Mauel M, Leatham MP, Laux DC, Cohen PS. A functional cra gene is required for Salmonella enterica serovar typhimurium virulence in BALB/c mice. Infect Immun. 2000;68:3772–3775. doi: 10.1128/iai.68.6.3772-3775.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almengor AC, Kinkel TL, Day SJ, McIver KS. The catabolite control protein CcpA binds to Pmga and influences expression of the virulence regulator Mga in the group A Streptococcus. J Bacteriol. 2007;189:8405–8416. doi: 10.1128/JB.01038-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alteri CJ, Smith SN, Mobley HL. Fitness of Escherichia coli during urinary tract infection requires gluconeogenesis and the TCA cycle. PLoS Pathog. 2009;5:e1000448. doi: 10.1371/journal.ppat.1000448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson U, Radstrom P. Physiological function of the maltose operon regulator, MalR, in Lactococcus lactis. BMC Microbiol. 2002;2:28. doi: 10.1186/1471-2180-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouckaert J, Berglund J, Schembri M, De Genst E, Cools L, Wuhrer M, Hung CS, Pinkner J, Slattegard R, Zavialov A, Choudhury D, Langermann S, Hultgren SJ, Wyns L, Klemm P, Oscarson S, Knight SD, De Greve H. Receptor binding studies disclose a novel class of high-affinity inhibitors of the Escherichia coli FimH adhesin. Mol Microbiol. 2005;55:441–455. doi: 10.1111/j.1365-2958.2004.04415.x. [DOI] [PubMed] [Google Scholar]
- Chapuy-Regaud S, Ogunniyi AD, Diallo N, Huet Y, Desnottes JF, Paton JC, Escaich S, Trombe MC. RegR, a global LacI/GalR family regulator, modulates virulence and competence in Streptococcus pneumoniae. Infect Immun. 2003;71:2615–2625. doi: 10.1128/IAI.71.5.2615-2625.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaussee MS, Watson RO, Smoot JC, Musser JM. Identification of Rgg-regulated exoproteins of Streptococcus pyogenes. Infect Immun. 2001;69:822–831. doi: 10.1128/IAI.69.2.822-831.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenreich W, Dandekar T, Heesemann J, Goebel W. Carbon metabolism of intracellular bacterial pathogens and possible links to virulence. Nat Rev Microbiol. 2010;8:401–412. doi: 10.1038/nrmicro2351. [DOI] [PubMed] [Google Scholar]
- Eswarappa SM, Karnam G, Nagarajan AG, Chakraborty S, Chakravortty D. lac repressor is an antivirulence factor of Salmonella enterica: its role in the evolution of virulence in Salmonella. PLoS ONE. 2009;4:e5789. doi: 10.1371/journal.pone.0005789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francke C, Kerkhoven R, Wels M, Siezen RJ. A generic approach to identify transcription factor-specific operator motifs; inferences for LacI-family mediated regulation in Lactobacillus plantarum WCFS1. BMC Genomics. 2008;9:145. doi: 10.1186/1471-2164-9-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzer A, Senn BM, Minh DB, Hanner M, Gelbmann D, Noiges B, Henics T, Schulze K, Guzman CA, Goodacre J, von Gabain A, Nagy E, Meinke AL. Novel conserved group A streptococcal proteins identified by the antigenome technology as vaccine candidates for a non-M protein-based vaccine. Infect Immun. 2010;78:4051–4067. doi: 10.1128/IAI.00295-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollands A, Pence MA, Timmer AM, Osvath SR, Turnbull L, Whitchurch CB, Walker MJ, Nizet V. Genetic switch to hypervirulence reduces colonization phenotypes of the globally disseminated group A Streptococcus M1T1 clone. J Infect Dis. 2010;202:11–19. doi: 10.1086/653124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hytonen J, Haataja S, Finne J. Streptococcus pyogenes glycoprotein-binding strepadhesin activity is mediated by a surface-associated carbohydrate-degrading enzyme, pullulanase. Infect Immun. 2003;71:784–793. doi: 10.1128/IAI.71.2.784-793.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hytonen J, Haataja S, Finne J. Use of flow cytometry for the adhesion analysis of Streptococcus pyogenes mutant strains to epithelial cells: investigation of the possible role of surface pullulanase and cysteine protease, and the transcriptional regulator Rgg. BMC Microbiol. 2006;6:18. doi: 10.1186/1471-2180-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer R, Baliga NS, Camilli A. Catabolite control protein A (CcpA) contributes to virulence and regulation of sugar metabolism in Streptococcus pneumoniae. J Bacteriol. 2005;187:8340–8349. doi: 10.1128/JB.187.24.8340-8349.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer R, Camilli A. Sucrose metabolism contributes to in vivo fitness of Streptococcus pneumoniae. Mol Microbiol. 2007;66:1–13. doi: 10.1111/j.1365-2958.2007.05878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob F, Monod J. Genetic regulatory mechanisms in the synthesis of proteins. J Mol Biol. 1961;3:318–356. doi: 10.1016/s0022-2836(61)80072-7. [DOI] [PubMed] [Google Scholar]
- Jensch I, Gamez G, Rothe M, Ebert S, Fulde M, Somplatzki D, Bergmann S, Petruschka L, Rohde M, Nau R, Hammerschmidt S. PavB is a surface-exposed adhesin of Streptococcus pneumoniae contributing to nasopharyngeal colonization and airways infections. Mol Microbiol. 2010;77:22–43. doi: 10.1111/j.1365-2958.2010.07189.x. [DOI] [PubMed] [Google Scholar]
- Kietzman CC, Caparon MG. CcpA and LacD.1 affect temporal regulation of Streptococcus pyogenes virulence genes. Infect Immun. 2010;78:241–252. doi: 10.1128/IAI.00746-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinkel TL, McIver KS. CcpA-mediated repression of streptolysin S expression and virulence in the group A Streptococcus. Infect Immun. 2008;76:3451–3463. doi: 10.1128/IAI.00343-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwayama H, Obara S, Morio T, Katoh M, Urushihara H, Tanaka Y. PCR-mediated generation of a gene disruption construct without the use of DNA ligase and plasmid vectors. Nucleic Acids Res. 2002;30:E2. doi: 10.1093/nar/30.2.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Sun F, Cho H, Yelavarthi V, Sohn C, He C, Schneewind O, Bae T. CcpA mediates proline auxotrophy and is required for Staphylococcus aureus pathogenesis. J Bacteriol. 2010;192:3883–3892. doi: 10.1128/JB.00237-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukomski S, Hoe NP, Abdi I, Rurangirwa J, Kordari P, Liu M, Dou SJ, Adams GG, Musser JM. Nonpolar inactivation of the hypervariable streptococcal inhibitor of complement gene (sic) in serotype M1 Streptococcus pyogenes significantly decreases mouse mucosal colonization. Infect Immun. 2000;68:535–542. doi: 10.1128/iai.68.2.535-542.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majerczyk CD, Dunman PM, Luong TT, Lee CY, Sadykov MR, Somerville GA, Bodi K, Sonenshein AL. Direct targets of CodY in Staphylococcus aureus. J Bacteriol. 2010;192:2861–2877. doi: 10.1128/JB.00220-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrero J, Rhee KY, Schnappinger D, Pethe K, Ehrt S. Gluconeogenic carbon flow of tricarboxylic acid cycle intermediates is critical for Mycobacterium tuberculosis to establish and maintain infection. Proc Natl Acad Sci U S A. 2010;107:9819–9824. doi: 10.1073/pnas.1000715107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa Y, Nakata A, Ogiwara A, Yamamoto M, Fujita Y. Evaluation and characterization of catabolite-responsive elements (cre) of Bacillus subtilis. Nucleic Acids Res. 2000;28:1206–1210. doi: 10.1093/nar/28.5.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musser JM, Shelburne SA., 3rd A decade of molecular pathogenomic analysis of group A Streptococcus. J Clin Invest. 2009;119:2455–2463. doi: 10.1172/JCI38095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen CC, Saier MH., Jr Phylogenetic, structural and functional analyses of the LacI-GalR family of bacterial transcription factors. FEBS Lett. 1995;377:98–102. doi: 10.1016/0014-5793(95)01344-x. [DOI] [PubMed] [Google Scholar]
- Nieto C, Puyet A, Espinosa M. MalR-mediated regulation of the Streptococcus pneumoniae malMP operon at promoter PM. Influence of a proximal divergent promoter region and competition between MalR and RNA polymerase proteins. J Biol Chem. 2001;276:14946–14954. doi: 10.1074/jbc.M010911200. [DOI] [PubMed] [Google Scholar]
- Reid SD, Green NM, Buss JK, Lei B, Musser JM. Multilocus analysis of extracellular putative virulence proteins made by group A Streptococcus: population genetics, human serologic response, and gene transcription. Proc Natl Acad Sci U S A. 2001;98:7552–7557. doi: 10.1073/pnas.121188598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid SD, Green NM, Sylva GL, Voyich JM, Stenseth ET, DeLeo FR, Palzkill T, Low DE, Hill HR, Musser JM. Postgenomic analysis of four novel antigens of group A Streptococcus: growth phase-dependent gene transcription and human serologic response. J Bacteriol. 2002;184:6316–6324. doi: 10.1128/JB.184.22.6316-6324.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Ortega MJ, Norais N, Bensi G, Liberatori S, Capo S, Mora M, Scarselli M, Doro F, Ferrari G, Garaguso I, Maggi T, Neumann A, Covre A, Telford JL, Grandi G. Characterization and identification of vaccine candidate proteins through analysis of the group A Streptococcus surface proteome. Nat Biotechnol. 2006;24:191–197. doi: 10.1038/nbt1179. [DOI] [PubMed] [Google Scholar]
- Ryan PA, Pancholi V, Fischetti VA. Group A streptococci bind to mucin and human pharyngeal cells through sialic acid-containing receptors. Infect Immun. 2001;69:7402–7412. doi: 10.1128/IAI.69.12.7402-7412.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santi I, Pezzicoli A, Bosello M, Berti F, Mariani M, Telford JL, Grandi G, Soriani M. Functional characterization of a newly identified group B Streptococcus pullulanase eliciting antibodies able to prevent alpha-glucans degradation. PLoS ONE. 2008;3:e3787. doi: 10.1371/journal.pone.0003787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher MA, Allen GS, Diel M, Seidel G, Hillen W, Brennan RG. Structural basis for allosteric control of the transcription regulator CcpA by the phosphoprotein HPr-Ser46-P. Cell. 2004;118:731–741. doi: 10.1016/j.cell.2004.08.027. [DOI] [PubMed] [Google Scholar]
- Schumacher MA, Seidel G, Hillen W, Brennan RG. Structural mechanism for the fine-tuning of CcpA function by the small molecule effectors glucose 6-phosphate and fructose 1,6-bisphosphate. J Mol Biol. 2007;368:1042–1050. doi: 10.1016/j.jmb.2007.02.054. [DOI] [PubMed] [Google Scholar]
- Schumacher MA, Sprehe M, Bartholomae M, Hillen W, Brennan RG. Structures of carbon catabolite protein A-(HPr-Ser46-P) bound to diverse catabolite response element sites reveal the basis for high-affinity binding to degenerate DNA operators. Nucleic Acids Res. 2011;39:2931–2942. doi: 10.1093/nar/gkq1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidl K, Bischoff M, Berger-Bachi B. CcpA mediates the catabolite repression of tst in Staphylococcus aureus. Infect Immun. 2008;76:5093–5099. doi: 10.1128/IAI.00724-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidl K, Muller S, Francois P, Kriebitzsch C, Schrenzel J, Engelmann S, Bischoff M, Berger-Bachi B. Effect of a glucose impulse on the CcpA regulon in Staphylococcus aureus. BMC Microbiol. 2009;9:95. doi: 10.1186/1471-2180-9-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelburne SA, 3rd, Fang H, Okorafor N, Sumby P, Sitkiewicz I, Keith D, Patel P, Austin C, Graviss EA, Musser JM, Chow DC. MalE of group A Streptococcus participates in the rapid transport of maltotriose and longer maltodextrins. J Bacteriol. 2007a;189:2610–2617. doi: 10.1128/JB.01539-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelburne SA, 3rd, Granville C, Tokuyama M, Sitkiewicz I, Patel P, Musser JM. Growth characteristics of and virulence factor production by group A Streptococcus during cultivation in human saliva. Infect Immun. 2005a;73:4723–4731. doi: 10.1128/IAI.73.8.4723-4731.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelburne SA, 3rd, Keith D, Horstmann N, Sumby P, Davenport MT, Graviss EA, Brennan RG, Musser JM. A direct link between carbohydrate utilization and virulence in the major human pathogen group A Streptococcus. Proc Natl Acad Sci USA. 2008a;105:1698–1703. doi: 10.1073/pnas.0711767105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelburne SA, 3rd, Keith DB, Davenport MT, Horstmann N, Brennan RG, Musser JM. Molecular characterization of group A Streptococcus maltodextrin catabolism and its role in pharyngitis. Mol Microbiol. 2008b;69:436–452. doi: 10.1111/j.1365-2958.2008.06290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelburne SA, 3rd, Okorafor N, Sitkiewicz I, Sumby P, Keith D, Patel P, Austin C, Graviss EA, Musser JM. Regulation of polysaccharide utilization contributes to the persistence of group A Streptococcus in the oropharynx. Infect Immun. 2007b;75:2981–2990. doi: 10.1128/IAI.00081-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelburne SA, 3rd, Sumby P, Sitkiewicz I, Granville C, DeLeo FR, Musser JM. Central role of a bacterial two-component gene regulatory system of previously unknown function in pathogen persistence in human saliva. Proc Natl Acad Sci USA. 2005b;102:16037–16042. doi: 10.1073/pnas.0505839102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelburne SA, 3rd, Sumby P, Sitkiewicz I, Okorafor N, Granville C, Patel P, Voyich J, Hull R, DeLeo FR, Musser JM. Maltodextrin utilization plays a key role in the ability of group A Streptococcus to colonize the oropharynx. Infect Immun. 2006;74:4605–4614. doi: 10.1128/IAI.00477-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelburne SA, Olsen RJ, Suber B, Sahasrabhojane P, Sumby P, Brennan RG, Musser JM. A combination of independent transcriptional regulators shapes bacterial virulence gene expression during infection. PLoS Pathog. 2010;6:e1000817. doi: 10.1371/journal.ppat.1000817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh KD, Schmalisch MH, Stulke J, Gorke B. Carbon catabolite repression in Bacillus subtilis: quantitative analysis of repression exerted by different carbon sources. J Bacteriol. 2008;190:7275–7284. doi: 10.1128/JB.00848-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenshein AL. Control of key metabolic intersections in Bacillus subtilis. Nat Rev Microbiol. 2007;5:917–927. doi: 10.1038/nrmicro1772. [DOI] [PubMed] [Google Scholar]
- Sumby P, Porcella SF, Madrigal AG, Barbian KD, Virtaneva K, Ricklefs SM, Sturdevant DE, Graham MR, Vuopio-Varkila J, Hoe NP, Musser JM. Evolutionary origin and emergence of a highly successful clone of serotype M1 group A Streptococcus involved multiple horizontal gene transfer events. J Infect Dis. 2005;192:771–782. doi: 10.1086/432514. [DOI] [PubMed] [Google Scholar]
- Sumby P, Whitney AR, Graviss EA, DeLeo FR, Musser JM. Genome-wide analysis of group A streptococci reveals a mutation that modulates global phenotype and disease specificity. PLoS Pathog. 2006;2:e5. doi: 10.1371/journal.ppat.0020005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swint-Kruse L, Matthews KS. Allostery in the LacI/GalR family: variations on a theme. Curr Opin Microbiol. 2009;12:129–137. doi: 10.1016/j.mib.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama S, Carlin AF, Khosravi A, Weiman S, Banerjee A, Quach D, Hightower G, Mitchell TJ, Doran KS, Nizet V. The surface-anchored NanA protein promotes pneumococcal brain endothelial cell invasion. J Exp Med. 2009;206:1845–1852. doi: 10.1084/jem.20090386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bueren AL, Higgins M, Wang D, Burke RD, Boraston AB. Identification and structural basis of binding to host lung glycogen by streptococcal virulence factors. Nat Struct Mol Biol. 2007;14:76–84. doi: 10.1038/nsmb1187. [DOI] [PubMed] [Google Scholar]
- Varga JJ, Therit B, Melville SB. Type IV pili and the CcpA protein are needed for maximal biofilm formation by the gram-positive anaerobic pathogen Clostridium perfringens. Infect Immun. 2008;76:4944–4951. doi: 10.1128/IAI.00692-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virtaneva K, Graham MR, Porcella SF, Hoe NP, Su H, Graviss EA, Gardner TJ, Allison JE, Lemon WJ, Bailey JR, Parnell MJ, Musser JM. Group A Streptococcus gene expression in humans and cynomolgus macaques with acute pharyngitis. Infect Immun. 2003;71:2199–2207. doi: 10.1128/IAI.71.4.2199-2207.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vise PD, Kodali K, Hoe N, Paszczynski A, Musser JM, Daughdrill GW. Stable isotope labeling of a group A Streptococcus virulence factor using a chemically defined growth medium. Protein Expr Purif. 2003;32:232–238. doi: 10.1016/S1046-5928(03)00235-3. [DOI] [PubMed] [Google Scholar]
- Weickert MJ, Adhya S. A family of bacterial regulators homologous to Gal and Lac repressors. J Biol Chem. 1992;267:15869–15874. [PubMed] [Google Scholar]
- Weickert MJ, Chambliss GH. Site-directed mutagenesis of a catabolite repression operator sequence in Bacillus subtilis. Proc Natl Acad Sci USA. 1990;87:6238–6242. doi: 10.1073/pnas.87.16.6238. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.