Abstract
Objective
Acrylamide, a probable human carcinogen, is formed during high-heat cooking of many common foods. The validity of food frequency questionnaire (FFQ) measures of acrylamide intake has not been established. We assessed the validity of acrylamide intake calculated from an FFQ using a biomarker of acrylamide exposure.
Methods
We calculated acrylamide intake from an FFQ in the Nurses' Health Study II. We measured hemoglobin adducts of acrylamide and its metabolite, glycidamide, in a random sample of 296 women. Correlation and regression analyses were used to assess the relationship between acrylamide intake and adducts.
Results
The correlation between acrylamide intake and the sum of acrylamide and glycidamide adducts was 0.31 (95% CI: 0.20 – 0.41), adjusted for laboratory batch, energy intake, and age. Further adjustment for BMI, alcohol intake, and correction for random within-person measurement error in adducts gave a correlation of 0.34 (CI: 0.23 – 0.45). The intraclass correlation coefficient for the sum of adducts was 0.77 in blood samples collected 1 to 3 years apart in a subset of 45 women. Intake of several foods significantly predicted adducts in multiple regression.
Conclusions
Acrylamide intake and hemoglobin adducts of acrylamide and glycidamide were moderately correlated. Within-person consistency in adducts was high over time.
Keywords: acrylamide, glycidamide, diet, hemoglobin adducts
Introduction
Acrylamide is classified as a probable human carcinogen. Before 2002, human acrylamide exposure was thought to be mainly from occupational and tobacco sources. (1,2) In 2002, acrylamide was found to be formed by high-temperature cooking of carbohydrate-containing foods, including potato chips, French fries, and cold breakfast cereal.(3) This finding caused substantial alarm, and led to studies to assess whether acrylamide intake through foods increases cancer risk in humans.
Eight epidemiological studies have since been published on dietary acrylamide intake and risk of various cancers.(4–11) Of these, only one has reported a significant increase in cancer risk among those consuming more acrylamide.(11)
Acrylamide intake in these reports was calculated using individuals’ responses to food frequency questionnaires (FFQs) and data on the acrylamide content of foods. The acrylamide content of foods varies widely depending on specific cooking methods and other parameters (for review, see Stadler, et al.(12)). For instance, acrylamide is formed in roasting or frying, but not in boiling. Parameters such as length of cooking, cooking temperature, and even the water content or age of ingredients also affect acrylamide formation. Because of this wide variability in acrylamide content of foods, it is not clear how well typical FFQs measure dietary acrylamide exposure. Several studies have examined the validity of FFQ acrylamide measurements with varying results,(13–15) making it difficult to know whether the null results observed for acrylamide intake and cancer risk were the result of misclassified exposure or a true lack of effect.
Therefore, we conducted a validation study of FFQ-measured acrylamide in the Nurses' Health Study 2 cohort. We chose to use hemoglobin (Hb) adducts of acrylamide and its primary metabolite, glycidamide, to validate FFQ-measured acrylamide intake. Both acrylamide and glycidamide form bonds with the N-terminal valine of globin chains in hemoglobin.(16) These hemoglobin adducts would be expected to provide a time-integrated measure of exposure because the half-life of red blood cells is approximately 120 days.(17) A major advantage of comparing FFQ acrylamide intake to Hb adducts is that measurement errors in the FFQ are likely to be independent of errors in adduct levels. However, the two measures are not directly comparable, as the FFQ measures dietary intake while adduct levels are also influenced by absorption and metabolism. Given this difference, the correlations between the FFQ and adduct measures can be seen as a lower bound of the true validity of the questionnaire assessment of acrylamide intake.
For the validation analysis, we constructed an acrylamide food composition database for the NHS II FFQ and calculated acrylamide intake for each woman. We compared these intakes with Hb adducts of acrylamide and glycidamide in a random sample of women in the Nurses’ Health Study II. We were also able to assess the consistency of Hb adducts over time in a subset of women. This allowed us to correct our validation analysis for random within-person error in the adducts and to assess the utility of Hb adducts as an exposure measure for future studies.
Materials and Methods
Study population
The Nurses’ Health Study II cohort was established in 1989 when 116,609 female nurses between 25 and 42 years old completed a mailed questionnaire. The women have since been followed every two years to update exposure and disease information. Every four years since 1991, participants have filled out a semi-quantitative food frequency questionnaire (FFQ) including over 130 food items. Participants are asked how frequently they have consumed each food item over the previous year and select from nine possible responses ranging from less than once per month to six or more times per day. We used the 1999 FFQ for this analysis because it was closest in time to collection of blood samples.
Between 1998 and 2000, 29611 cohort members provided a blood sample. A subgroup of 304 women provided two to three blood samples over a period of 1 to 3 years. Blood samples were shipped overnight to our laboratory, where they were separated into plasma, red blood cell, and white blood cell components. All samples have been stored in continuously monitored liquid nitrogen freezers since collection. We selected a random sample of 342 women who had provided blood samples and completed the 1999 questionnaire. We measured acrylamide and glycidamide adducts to hemoglobin using red blood cell samples from these women. The median time from blood draw until the completion of the 1999 FFQ was 8 months; the timing ranged from blood draw 34 months prior to the FFQ to blood draw 6 months after the FFQ. In a subgroup of 45 women, we measured two blood samples in order to assess the reproducibility of blood acrylamide over time. The median time between blood samples for these women was 23 months with a range of 10 to 32 months. The blood sample collected closest in time to the 1999 FFQ was used as the main validation sample for these women.
Creation of acrylamide database
When possible, we used acrylamide values from the FDA’s Exploratory Analysis of Acrylamide in Foods, which began in 2002 and continued as part of the FDA's Total Diet Study.(18,19) We assigned values to foods not included in the FDA analyses in one of 3 ways: (1) imputation from a similar food with an analyzed value, (2) assumption of a zero value, based on knowledge of the conditions needed to form acrylamide, or (3) laboratory analysis by the laboratory of Karl-Erik Hellenäs at the Swedish National Food Administration in Uppsala, Sweden.
The foods sent for laboratory analysis were chosen either because the category of food is known to contain acrylamide but exact values were not available (for example, specific brands of cold breakfast cereal; low-fat muffins; roasted pistachios), or because analyses to date had not established whether the food contained acrylamide (for example, dried fruits). Foods consumed in larger amounts in the Nurses’ Health Study and Health Professionals Follow-up Study were given priority for analysis. Foods analyzed included breakfast cereals (29 types), baked goods (10 types of pies, cakes, muffins), nuts (5 types), dried fruit (5 types), bread (4 types), grains (4 types), potato chips (2 types), chocolate-containing candy (mix of 4 types), power/energy bars, chocolate ice cream, microwaved potatoes, and microwaved and baked sweet potatoes.
Breakfast cereals were given particular emphasis because they are a major source of acrylamide in American diets(20) and because the acrylamide content of breakfast cereals varies widely depending on ingredients and processing methods. As part of the FFQ, nurses report the brand of breakfast cereal they eat most often, allowing us to tailor our intake calculations for each participant. Acrylamide content of breakfast cereals without analyzed values were imputed based on analyzed cereals with similar grain composition and processing method.
All foods sent for analysis were a combination of at least three different samples of the food, either from different brands or from different production dates of the same brand. Samples were processed at the Harvard School of Public Health and sent by express mail to the Swedish National Food Administration for analysis. Foods were analyzed for acrylamide content using the LC-MS-MS method described elsewhere.(21) The quantification limit (LOQ) was 1–5 µg/kg, depending on which type of food that was analyzed. The method has been evaluated in an international collaborative trial.(22) The relative repeatability (r) values were 4–9% for potato chip samples and 3–8% for bakery products. The corresponding reproducibility (R) values were 9–13% and 5–12%, respectively. Each food item was analyzed two or three times and the average acrylamide value was used.
Many FFQ items include multiple foods or multiple preparation methods for one food. For instance “bagels, English muffins, soft pretzels, and rolls” and “potatoes, baked, broiled, or mashed” are both single FFQ items which combine foods with different acrylamide contents. Other items, such as ice cream, cookies, or nuts, include a variety of food types or flavors which may vary in acrylamide content. For these types of items, we averaged values for all foods using weights based on either market share data or data on the consumption of different types of foods from pilot studies conducted every four years within the cohort. In all, 42 items from the 149-item FFQ contributed to acrylamide intake. The content of other food items was either zero or below the limit of quantitation of currently available analytic methods.
Measurement of blood acrylamide levels
We assessed acrylamide exposure by measuring hemoglobin adducts of acrylamide (AA-Hb) and glycidamide (GA-Hb), its primary metabolite, in red blood cells. These adducts reflect acrylamide exposure over the previous four months, the lifetime of red blood cells, and is a measure of the average internal dose.(17) Hemoglobin adducts were measured as described elsewhere.(23,24) In brief, the N-terminal valines of hemoglobin with acrylamide and glycidamide attached were cleaved from the protein chain using modified Edman reaction with pentafluorophenylisothioisocyanate, PFPITC as Edman reagent. The resulting Edman products (AA-Val-PFPTH, GA-Val-PFPTH) were extracted using liquid-liquid extraction and analyzed by HPLC/MS/MS. Calibrators, reagent blanks, and quality-control materials were processed the same way as the samples. Hemoglobin adduct concentrations were reported relative to the amount of hemoglobin used in the analysis. The detection limit for this method was 3 pmol/g Hb and 7 pmol/g Hb for AA-Hb and GA-Hb, respectively. The average inter-day variability of this method expressed as percent coefficient of variation was 13% for AA-Hb and 14% for GA-Hb determined with 3 blood pools (AA-Hb and GA-Hb concentrations in pmol/g Hb: Pool 1: 182 and 151, Pool 2: 119 and 101, Pool 3: 45.1 and 37.3) measured over 20 days.
Samples were processed in twelve batches. Samples for the women in the reproducibility subgroup were assayed in the same batch. The inter-batch coefficient of variation from masked replicate samples in each batch was 9.8% for acrylamide adducts and 10.9% for glycidamide adducts. A pilot study established that the collection methods used for blood samples in this cohort did not affect adduct levels.
Statistical analysis
Of the 342 women in the analysis, 7 were excluded due to incomplete responses to the 1999 FFQ which prevented acrylamide intake from being calculated. Because smokers are exposed to much higher levels of acrylamide through tobacco use than through the diet (median adduct levels were 97.3 for AA-Hb and 137.5 for GA-Hb among current smokers, compared to 43.9 and 49.4 among never and past smokers), we excluded 36 women who smoked at the time of the blood draw or 1999 questionnaire, and 3 other women whose smoking status at the time of blood draw was unclear. This left 296 women in the validation sample. Reproducibility data was available for 45 of these women.
Hemoglobin adducts from acrylamide (AA-Hb) and from glycidamide (GA-Hb) were measured. We also calculated the sum of these adducts as these were present in approximately the same amounts and we hypothesized that this would provide the best overall measure of dietary acrylamide exposure by accounting for differences among individuals in metabolism of acrylamide to glycidamide. All blood values were log-transformed to improve normality for correlation and regression analyses. Acrylamide intake was adjusted for energy intake using the residual method for most analyses.(25) We calculated partial Pearson correlation coefficients (with p-values) between the three Hb adduct measures and dietary acrylamide intake, adjusting for laboratory batch in all analyses, and adjusting for age and energy intake in some analyses. We also conducted correlation analyses stratified by time between blood draw and FFQ, by BMI category (≥26 vs. <26), and by alcohol intake (none vs. some). There were no material differences in observed correlations among these groups, so only overall results are shown.
For the reproducibility analysis, we used data from the 45 women who gave two blood samples to calculate the intraclass correlation coefficient for each of the Hb adduct measures. We used data from the reproducibility study to de-attenuate validation correlations with the calculated intake for within-person error in blood levels. Confidence intervals for these corrected correlations were calculated according to Rosner and Willett.(26)
We used the top 20 acrylamide-contributing foods in the cohort to perform stepwise regression to find the best food predictors of acrylamide and glycidamide adducts. A food entered the model if it was individually predictive of adduct level with p<0.15; foods stayed in the model if the p-value remained below 0.15 with the inclusion of other food variables. Age, laboratory batch, and energy intake were included in these models to reduce extraneous variation in adduct levels of acrylamide and glycidamide.
Results
Characteristics of the 296 women in the validation study subgroup are shown in Table 1 along with characteristics of the full cohort. Mean acrylamide intake was 20.1 mcg/day in the full cohort and 19.3 mcg/day in the validation group. Intake in relation to body weight was 0.3 mcg/kg body weight/day, somewhat lower than the estimated 0.4 mcg/kg/d for the overall U.S. population including men and children.(20) Women in the validation group were slightly older and more likely to be postmenopausal than women in the full cohort. The two groups were very similar in macro- and micro-nutrient intakes and servings per day of major food groups.
Table 1.
Characteristics of the validation study subgroup and the full NHS II Cohort (Mean ± SD)
| VALIDATION SUBGROUP |
FULL NHS II COHORT |
|
|---|---|---|
| n=296 | n=85,092 | |
| Acrylamide intake (mcg/d)* | 19.3 ± 7.9 | 20.1 ± 8.4 |
| AA intake by body weight (mcg/kg/d) | 0.27 ± 0.12 | 0.29 ± 0.14 |
| COVARIATES | ||
| Age (yrs) | 47 ± 4 | 44 ± 5 |
| BMI | 27 ± 6 | 26 ± 6 |
| % Past smokers | 29% | 26% |
| Parity | 1.8 ± 1.2 | 1.9 ± 1.2 |
| % Postmenopausal | 48% | 17% |
| Activity (MET-h/wk) | 16 ± 18 | 18 ± 23 |
| BLOOD LEVELS | ||
| Median AA-Hb (pmol/g) [range] | 43.9 [14 –148] | - |
| Median GA-Hb (pmol/g) [range] | 49.4 [23–157] | - |
| Median AA+GA Sum (pmol/g) [range] | 94.3 [46–274] | - |
| Median GA:AA Ratio [range] | 1.10 [0.6–2.6] | - |
Acrylamide intake adjusted for total energy intake.
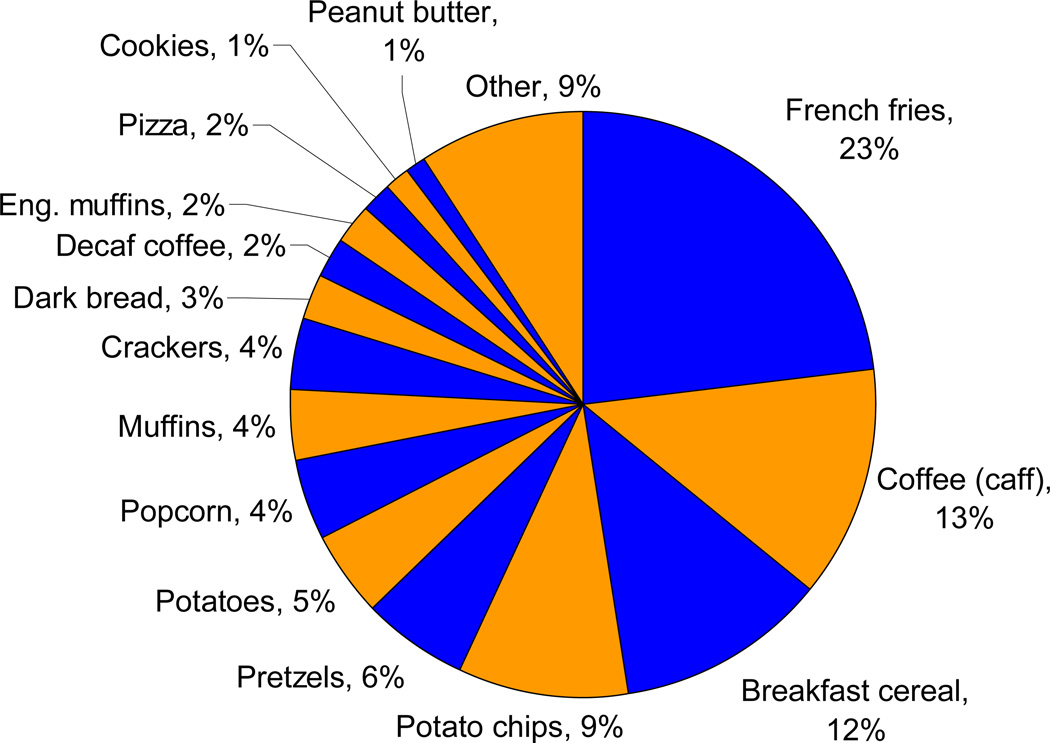
Figure 1 shows the contribution of various foods to acrylamide intake in this cohort. The major food sources of acrylamide were French fries (23%), caffeinated coffee (13%), cold breakfast cereal (12%), and potato chips (9%). These are similar to FDA estimates of food sources of acrylamide in the US.(20)
Figure 1.
Contribution of foods to acrylamide intake in the Nurses' Health Study 2 cohort (1999).
Table 2 shows characteristics of the validation group according to quartile of total acrylamide and glycidamide adducts (AA+GA-Hb). Acrylamide intake as measured by the FFQ increased across blood quartiles from 15.5 mcg/day [0.22 mcg/kg/day] in the lowest quartile to 21.9 mcg/day [0.31 mcg/kg/day] in the highest quartile. Servings per day of major acrylamide-contributing foods also increased across quartiles. Women in the highest versus lowest quartile ate more French fries, coffee, cold breakfast cereal, and potato chips. Macronutrient intake was similar across quartiles, but alcohol intake was lower in the highest quartile. Alcohol and BMI were both associated with adduct levels independent of acrylamide intake. Adjusting for laboratory batch, age, and calorie-adjusted acrylamide intake, BMI was positively correlated with AA-Hb (r=0.17, p=0.006) and negatively correlated with GA-Hb (r=−0.11, p=0.07). Alcohol intake was negatively correlated with AA-Hb (r=−0.18, p=0.002). Neither alcohol nor BMI were significantly correlated with the sum of AA+GA-Hb.
Table 2.
Characteristics of the study population by quartile of blood adducts levels
| QUARTILE OF AA-Hb+GA-Hb | ||||
|---|---|---|---|---|
| Q1 (low) | Q2 | Q3 | Q4 (high) | |
| n=77 | n=74 | n=74 | n=71 | |
| Acrylamide intake (mcg/d)* | 15.5 | 19.0 | 20.8 | 21.9 |
| AA intake by body weight (mcg/kg/d) | 0.22 | 0.26 | 0.29 | 0.31 |
| AA-Hb (pmol/g) | 31.6 | 40.6 | 49.6 | 71.0 |
| GA-Hb (pmol/g) | 36.3 | 46.3 | 54.6 | 71.6 |
| AA + GA Sum | 67.9 | 86.9 | 104.3 | 142.6 |
| GA:AA Ratio | 1.21 | 1.17 | 1.13 | 1.05 |
| COVARIATES | ||||
| Age (yrs) | 47 | 47 | 47 | 47 |
| BMI | 27 | 28 | 27 | 28 |
| % Past smokers | 26% | 20% | 33% | 35% |
| Parity | 1.8 | 1.9 | 1.7 | 1.7 |
| Activity (MET-h/wk) | 17 | 14 | 18 | 17 |
| NUTRIENT INTAKES (per day) * | ||||
| Calories (kcal) | 1789 | 1776 | 1786 | 1840 |
| Carbohydrates (g) | 239 | 239 | 237 | 238 |
| Protein (g) | 80 | 78 | 80 | 82 |
| Total fat (g) | 60 | 60 | 60 | 60 |
| Alcohol (g) | 4 | 4 | 5 | 2 |
| FOODS AND FOOD GROUPS (servings/day) | ||||
| French fries | 0.05 | 0.1 | 0.1 | 0.1 |
| Coffee | 1.0 | 1.3 | 1.5 | 1.3 |
| Cold breakfast cereal | 0.3 | 0.3 | 0.4 | 0.5 |
| Potato chips | 0.1 | 0.1 | 0.2 | 0.2 |
| Pretzels | 0.2 | 0.1 | 0.2 | 0.2 |
| Potatoes (boiled, baked) | 0.3 | 0.3 | 0.3 | 0.3 |
| Popcorn | 0.1 | 0.1 | 0.2 | 0.2 |
| Muffins | 0.1 | 0.1 | 0.1 | 0.1 |
| Crackers | 0.2 | 0.2 | 0.2 | 0.3 |
| Dark bread | 0.4 | 0.6 | 0.4 | 0.6 |
| English muffins/bagels | 0.3 | 0.3 | 0.3 | 0.3 |
| Pizza | 0.1 | 0.1 | 0.1 | 0.1 |
| Cookies | 0.4 | 0.4 | 0.4 | 0.3 |
| Peanut butter | 0.1 | 0.2 | 0.1 | 0.2 |
Except for data on mean age, all data shown are standardized to the age distribution of the study population.
All nutrients except calories and alcohol are adjusted for total energy intake.
The adduct levels were strongly correlated with each other. The correlation was 0.69 between AA-Hb and GA-Hb, 0.92 between AA-Hb and sum of AA+GA-Hb, and 0.91 between GA-Hb and AA+GA-Hb (all p<0.0001). Adduct levels were not correlated with the length of storage of the blood samples, and mean adduct levels were similar for blood samples collected in 1998 versus 1999 and 2000.
Reproducibility of acrylamide adduct measures
Forty-five women in the validation group provided two blood samples at least 10 months apart. These samples were used to determine the stability of acrylamide and glycidamide adducts over time. The average age in the reproducibility subgroup was 44, and average acrylamide intake from the 1999 FFQ was 19.7 mcg/day (0.28 mcg/kg/d). Median time between blood samples was 23 months, with a range of 10 to 32 months. The intraclass correlation coefficients were 0.78 for AA-Hb, 0.80 for GA-Hb, and 0.77 for AA+GA-Hb.
Validation of FFQ acrylamide
Table 3 shows the correlation between FFQ acrylamide and Hb adduct levels. Adjusting only for laboratory batch, the correlation was 0.19 (95% CI: 0.08–0.30) for AA-Hb, 0.24 (0.13–0.35) for GA-Hb, and 0.24 (0.12–0.34) for AA+GA-Hb. Adjustment for energy intake improved the correlations, while adjustment for age had no effect. Correlations adjusted for age and energy intake were 0.26 (0.14–0.36) for AA-Hb, 0.31 (0.20–0.41) for GA-Hb, and 0.31 (0.20–0.41) for AA+GA-Hb. We further adjusted correlations for BMI and alcohol intake, as both were associated with adduct levels independent of acrylamide intake. Adjusting for such factors reduces variation in adduct levels that is unrelated to intake. This gave correlations of 0.27 (0.16–0.38) for AA-Hb, 0.33 (0.22–0.43), and 0.32 (0.21–0.42).
Table 3.
Pearson correlations (95% CI) between 1999 FFQ acrylamide and hemoglobin adducts of acrylamide and glycidamide
| Crude acrylamide intake* |
Calorie- adjusted acrylamide |
Calorie- and age- adjusted |
Also adj. for BMI and alcohol |
De- attenuated** |
|
|---|---|---|---|---|---|
| AA-Hb | 0.19a | 0.26 | 0.26 | 0.27 | 0.29 |
| 0.08 – 0.30 | 0.14 – 0.36 | 0.14 – 0.36 | 0.16 – 0.38 | 0.17 – 0.40 | |
| GA-Hb | 0.24 | 0.31 | 0.31 | 0.33 | 0.35 |
| 0.13 – 0.35 | 0.20 – 0.41 | 0.20 – 0.41 | 0.22 – 0.43 | 0.24 – 0.46 | |
| AA-Hb + GA-Hb | 0.24 | 0.31 | 0.31 | 0.32 | 0.34 |
| 0.12 – 0.34 | 0.20 – 0.41 | 0.20 – 0.41 | 0.21 – 0.42 | 0.23 – 0.45 |
Note: p-values for all correlations are significant at p<0.0001, except as noted.
p=0.001
All correlations are adjusted for laboratory batch
De-attenuated correlations are corrected for random within-person measurement error in the adduct measurement, using data from the reproducibility substudy.
Because we had only one blood measurement per person in the full group, the observed correlations were attenuated due to random within-person variation in the blood measures. Intraclass correlation coefficients from the reproducibility analysis were used to de-attenuate these correlations, and corrected confidence intervals were calculated using the method of Rosner and Willett.(26) Because of the high stability of adduct levels over time, the de-attenuation only slightly improved the correlations. Corrected correlations were 0.29 (95% CI: 0.17–0.40) for AA-Hb, 0.35 (0.24–0.46) for GA-Hb, and 0.34 (0.23–0.45) for AA+GA-Hb.
We conducted several analyses to investigate whether misclassification of smoking status might be affecting our results. Five women in our validation sample reported that they quit smoking between the 1997 and 1999 questionnaires. It is possible these women quit smoking shortly enough before giving a blood sample that their adduct levels still reflected cigarette exposure. However, excluding these women did not affect our correlation results. Additionally, excluding one non-smoking woman with an unusually high AA-Hb value (148 pmol/g) had no effect.
Cross-tabulation of quartiles of FFQ acrylamide and quartiles of AA+GA-Hb are shown in Table 4. Based on the FFQ classification, 47% of those in the lowest quartile of acrylamide intake were in the lowest quartile of AA+GA-Hb. Of those in the highest quartile of FFQ acrylamide, 30% were in the highest quartile of AA+GA-Hb. Overall, 31% of participants were classified in the same quartile for both FFQ acrylamide and acrylamide adducts. For AA-Hb alone, 29% of participants were classified in the same quartile, and for GA-Hb, 31% were classified in the same quartile.
Table 4.
Cross-tabulation of FFQ acrylamide quartile and AA+GA-Hb adduct quartile
| Quartile of GA+AA-Hb | ||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | Total | ||
| Quartile of FFQ AA | 1 | 35 | 16 | 15 | 8 | 74 |
| cell % | 12% | 5% | 5% | 3% | 25% | |
| row % | 47% | 22% | 20% | 11% | ||
| col % | 45% | 22% | 20% | 11% | ||
| 2 | 21 | 19 | 17 | 17 | 74 | |
| cell % | 7% | 6% | 6% | 6% | 25% | |
| row % | 28% | 26% | 23% | 23% | ||
| col % | 27% | 26% | 23% | 24% | ||
| 3 | 12 | 22 | 16 | 24 | 74 | |
| cell % | 4% | 7% | 5% | 8% | 25% | |
| row % | 16% | 30% | 22% | 32% | ||
| col % | 16% | 30% | 22% | 34% | ||
| 4 | 9 | 17 | 26 | 22 | 74 | |
| cell % | 3% | 6% | 9% | 7% | 25% | |
| row % | 12% | 23% | 35% | 30% | ||
| col % | 12% | 23% | 35% | 31% | ||
| Total | 77 | 74 | 74 | 71 | 296 | |
| 26% | 25% | 25% | 24% | 100% | ||
Quartile of GA+AA-Hb are adjusted for laboratory batch.
FFQ AA is adjusted for total energy intake
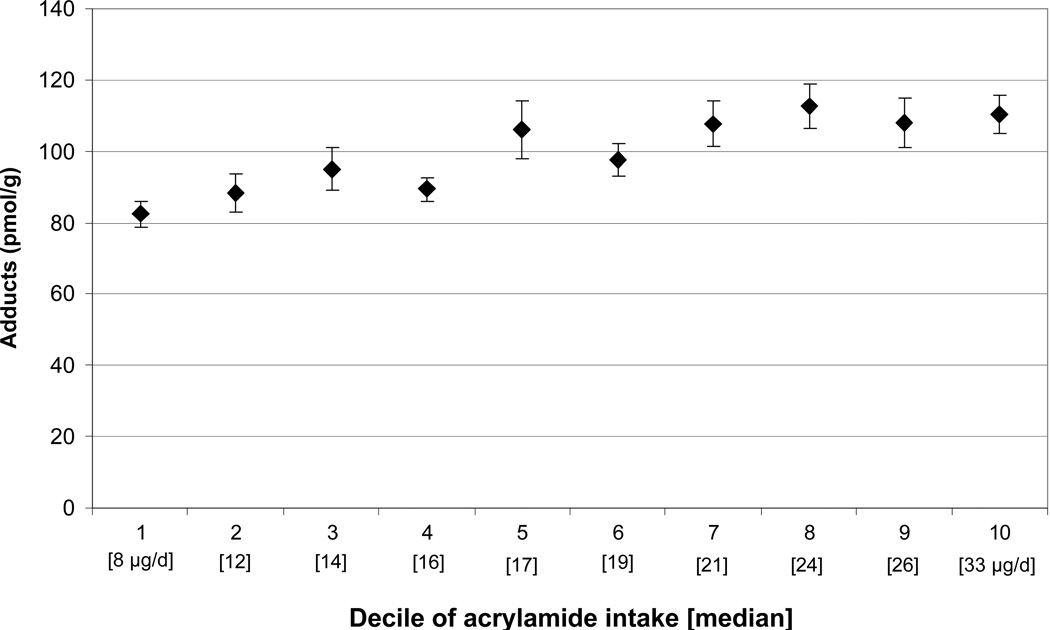
Figure 2 shows mean adduct levels according to decile of FFQ acrylamide intake. The mean sum of AA+GA-Hb adducts was 82.4 pmol/g (SE±3.8) for the lowest decile of intake and 110.2 (±5.5) for the highest decile of intake.
Figure 2.
Mean (±SE) AA+GA-Hb adducts by decile of FFQ acrylamide intake.
We also conducted several stratified correlation analyses to see if the association between FFQ acrylamide and Hb adducts varied among different groups. There was no appreciable difference in correlations among women with longer and shorter times between the blood draw and the FFQ. There was also no difference between women with higher or lower BMIs or between women who did and did not consume alcohol (data not shown).
We performed stepwise regression to determine which of the top 20 acrylamide-contributing foods from the FFQ were most predictive of Hb adducts (Table 5). All models included terms for laboratory batch, age, BMI, alcohol intake, and total energy intake. Potato chips, sweet potatoes/yams, caffeinated coffee, cold breakfast cereal, French fries, prune juice, popcorn, and decaffeinated coffee were the most predictive of the sum of acrylamide and glycidamide adducts. The partial correlation between adduct levels and foods in the model ranged from 0.09 to 0.21.
Table 5.
Stepwise regression predicting Hb adducts from top 20 acrylamide-contributing foods
| Adduct | Independent Variable |
Partial r |
Model R2 |
p- value |
|---|---|---|---|---|
| Total AA + GA | Potato chips | 0.21 | 0.09 | 0.0003 |
| Sweet potatoes | 0.16 | 0.11 | 0.006 | |
| Coffee | 0.13 | 0.13 | 0.03 | |
| Breakfast cereal | 0.12 | 0.14 | 0.03 | |
| French fries | 0.11 | 0.16 | 0.04 | |
| Prune juice | 0.11 | 0.17 | 0.06 | |
| Popcorn | 0.10 | 0.18 | 0.06 | |
| Decaf coffee | 0.09 | 0.19 | 0.12 | |
| AA-Hb | Potato chips | 0.19 | 0.16 | 0.001 |
| French fries | 0.12 | 0.17 | 0.03 | |
| Breakfast cereal | 0.12 | 0.19 | 0.03 | |
| Coffee | 0.10 | 0.19 | 0.08 | |
| Popcorn | 0.10 | 0.20 | 0.08 | |
| Decaf coffee | 0.09 | 0.21 | 0.11 | |
| Prune juice | 0.08 | 0.22 | 0.12 | |
| Sweet potatoes | 0.08 | 0.23 | 0.14 | |
| GA-Hb | Sweet potatoes | 0.21 | 0.09 | 0.001 |
| Potato chips | 0.20 | 0.13 | 0.0004 | |
| Coffee | 0.14 | 0.15 | 0.01 | |
| Dark bread | 0.12 | 0.16 | 0.04 | |
| Popcorn | 0.11 | 0.17 | 0.06 | |
| Prune juice | 0.11 | 0.18 | 0.06 | |
| Breakfast cereal | 0.11 | 0.19 | 0.05 | |
| French fries | 0.08 | 0.20 | 0.14 | |
All models are adjusted for laboratory batch, age, BMI, alcohol intake, and total energy intake.
Discussion
Validation of FFQ acrylamide intake
We found moderate correlations between acrylamide intake assessed by FFQ and adduct levels of acrylamide and glycidamide. FFQ intake correlated slightly better with the sum of acrylamide and glycidamide adducts and with glycidamide adducts alone than with acrylamide adducts alone; however, the FFQ was significantly correlated with all three adduct measures.
In validating food frequency questionnaires against biomarkers of diet it is important to use a biomarker that reflects differences in dietary intakes rather than primarily reflecting differences in metabolism or homeostatic control mechanisms.(25) Hemoglobin adducts of acrylamide and glycidamide are commonly used biomarkers of acrylamide exposure in animal and human studies. Adduct levels show dose-response relationships with occupational acrylamide exposure and with tobacco use.(27–30) Less work has been done with adduct levels at low exposure levels; however, it appears that dose-response relationships do exist between diet-level exposures and adduct levels in animal(31–33) and human studies (Abramsson-Zetteberg et al., to be published).
Our findings are limited to women and to nonsmokers. In addition, we have only one blood measurement for each participant, representing approximately the past 3–6 months of exposure. Because we are interested in long-term intake of acrylamide and its relation to cancer risk, it would be beneficial to have several blood measures over time, and to see how multiple blood measures correlate with multiple FFQs. However, the high with-in person correlation over time over a two-year period suggests that even a single measurement is a relatively good indicator of long term intake.
Strengths of our FFQ measure of acrylamide intake include a detailed acrylamide database which includes the acrylamide content of 42 food items, twice as many as in most previous studies. In addition, the information we have on specific brands of breakfast cereals allowed us to estimate intake more accurately. This is especially important in the U.S. population where breakfast cereal is one of the major contributors to acrylamide intake.(20)
Another strength of the study is the measurement of both acrylamide and glycidamide adducts to hemoglobin. Glycidamide is thought to be the metabolite responsible for acrylamide's carcinogenic effects.(34,35) The degree of metabolism of acrylamide to glycidamide varies between people and with the level of acrylamide consumption.(24,36,37) As a result, a combined measure of acrylamide and glycidamide would seem to be the best measure of dietary acrylamide intake. Indeed, we found somewhat stronger correlations between the sum of acrylamide and glycidamide adducts and the FFQ than for acrylamide adducts alone.
Adduct levels and FFQ-calculated acrylamide intakes are not expected to be perfectly correlated. As discussed above, FFQs group multiple foods with varying acrylamide content into single items. Even within single foods, acrylamide content varies widely depending on a variety of factors. In addition, FFQs will always have some measurement error due to the limited food list, imperfect recall by participants, and variations in serving sizes. However, even without measurement error, FFQs and hemoglobin adducts would not be perfectly correlated because they measure different aspects of acrylamide exposure. FFQs estimate intake of acrylamide over the previous year, whereas hemoglobin adducts account for exposure, absorption, and metabolism of acrylamide over the previous four months. In addition, hemoglobin adducts are not specific to dietary acrylamide exposure. Currently it is assumed that adduct levels in non-smoking, non-occupationally exposed people reflects food acrylamide only; however, this is not known for sure. Our results therefore provide a lower bound estimate of the validity of our FFQ.
Other studies of FFQ acrylamide and adduct levels have shown mixed results. Wirfalt, et al.(13), found correlations of 0.36 in smokers and 0.43 in nonsmokers in the Malmo Diet and Cancer Cohort. These correlations are likely somewhat overstated because some participants were selected based on high or low consumption of acrylamide-rich foods, introducing artificially large between-person variation in intake. Kütting et al.(15) found lower (0.17–0.18), but statistically significant, correlations between acrylamide adducts and FFQ acrylamide in a study of 828 non-smokers in Germany. However, these results were not adjusted for caloric intake and are quite similar to our unadjusted results. In a study of 50 people in Norway, Bjellaas et al.(14) report finding no correlation between acrylamide adducts and acrylamide intake. However, details on the calculation of acrylamide intake were not given, and age, sex, and caloric intake were not adjusted for in the correlation analysis.
The studies which adjusted for energy intake found stronger correlations between FFQ intakes and adduct levels, which may explain some of the differences in findings. Energy adjustment has the effect of putting FFQ measurements in terms of diet composition, or relative intake of food and nutrients, rather than in terms of absolute intake amounts. Also, adjusting for total energy intake has been shown to improve FFQ performance by reducing within-person measurement error.(25) Another possible reason for differences in findings is the variation in acrylamide adduct levels across study populations. Adduct levels were somewhat lower in the studies of Kütting et al. and Bjellaas et al. than in the other three reports. This may reflect differences in acrylamide intake and differences in adduct assays.
Reproducibility of adduct levels
The intraclass correlation coefficient for acrylamide and glycidamide adduct levels was high, suggesting that adduct levels, and likely dietary intakes, are fairly consistent over several years. There was no indication that adduct levels varied with the length of storage of blood samples, suggesting that stored blood from cohort studies is well suited to acrylamide and glycidamide adduct measurements.
In conclusion, our validation results suggest that FFQs may provide useful estimates of acrylamide intake, but the observed correlation with adducts is at the lower end of acceptable validity, probably due largely to the difficulty in capturing the details of heating of foods. Therefore, the level of measurement error present will prevent us from detecting small associations between acrylamide intake and disease risk. Our reproducibility results suggest that Hb adducts of acrylamide and glycidamide have high within-person consistency over time and are correlated with known dietary sources of acrylamide. This suggests that a single measure of these adducts can serve as an informative biomarker of dietary acrylamide exposure in epidemiologic studies. Given the moderate correlation between FFQ acrylamide and Hb adducts, future studies combining both types of exposure measures may provide the most compelling evidence on the possible effects of dietary acrylamide.
Acknowledgments
Grant support: This work was supported by a grant from the National Cancer Institute, CA050385. KMW is partially supported by NCI/NIH Training Grant T32 CA09001.
Footnotes
Conflicts of interest: None of the authors has any personal or financial conflict of interest.
References
- 1.IARC. Monographs on the evaluation of carcinogenic risks to humans. Vol 60. 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.International Programme on Chemical Safety. Acrylamide (EHC 49, 1985) Available at: www.inchem.org. [Google Scholar]
- 3.Tareke E, Rydberg P, Karlsson P, Eriksson S, Tornqvist M. Analysis of acrylamide, a carcinogen formed in heated foodstuffs. J. Agric. Food Chem. 2002 Aug 14;50(17):4998–5006. doi: 10.1021/jf020302f. [DOI] [PubMed] [Google Scholar]
- 4.Mucci LA, Dickman PW, Steineck G, Adami HO, Augustsson K. Dietary acrylamide and cancer of the large bowel, kidney, and bladder: absence of an association in a population-based study in Sweden. Br. J. Cancer. 2003 Jan 13;88(1):84–89. doi: 10.1038/sj.bjc.6600726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mucci LA, Lindblad P, Steineck G, Adami HO. Dietary acrylamide and risk of renal cell cancer. Int. J. Cancer. 2004 May 1;109(5):774–776. doi: 10.1002/ijc.20011. [DOI] [PubMed] [Google Scholar]
- 6.Mucci LA, Sandin S, Balter K, Adami HO, Magnusson C, Weiderpass E. Acrylamide intake and breast cancer risk in Swedish women. JAMA. 2005 Mar 16;293(11):1326–1327. doi: 10.1001/jama.293.11.1326. [DOI] [PubMed] [Google Scholar]
- 7.Mucci LA, Adami HO, Wolk A. Prospective study of dietary acrylamide and risk of colorectal cancer among women. Int. J. Cancer. 2006 Jan 1;118(1):169–173. doi: 10.1002/ijc.21309. [DOI] [PubMed] [Google Scholar]
- 8.Pelucchi C, Franceschi S, Levi F, Trichopoulos D, Bosetti C, Negri E, et al. Fried potatoes and human cancer. Int. J. Cancer. 2003 Jul 1;105(4):558–560. doi: 10.1002/ijc.11118. [DOI] [PubMed] [Google Scholar]
- 9.Pelucchi C, Galeone C, Levi F, Negri E, Franceschi S, Talamini R, q, et al. Dietary acrylamide and human cancer. Int. J. Cancer. 2006 Jan 15;118(2):467–471. doi: 10.1002/ijc.21336. [DOI] [PubMed] [Google Scholar]
- 10.Pelucchi C, Galeone C, Dal Maso L, Talamini R, Montella M, Ramazzotti V, et al. Dietary acrylamide and renal cell cancer. Int. J. Cancer. 2007 Mar 15;120(6):1376–1377. doi: 10.1002/ijc.22457. [DOI] [PubMed] [Google Scholar]
- 11.Hogervorst JG, Schouten LJ, Konings EJ, Goldbohm RA, van den Brandt PA. A prospective study of dietary acrylamide intake and the risk of endometrial, ovarian, and breast cancer. Cancer Epidemiol. Biomarkers Prev. 2007 Nov;16(11):2304–2313. doi: 10.1158/1055-9965.EPI-07-0581. [DOI] [PubMed] [Google Scholar]
- 12.Stadler RH, Scholz G. Acrylamide: an update on current knowledge in analysis, levels in food, mechanisms of formation, and potential strategies of control. Nutr. Rev. 2004 Dec;62(12):449–467. doi: 10.1111/j.1753-4887.2004.tb00018.x. [DOI] [PubMed] [Google Scholar]
- 13.Wirfalt E, Paulsson B, Tornqvist M, Axmon A, Hagmar L. Associations between estimated acrylamide intakes, and hemoglobin AA adducts in a sample from the Malmo Diet and Cancer cohort. Eur. J. Clin. Nutr. 2007 Mar 14; doi: 10.1038/sj.ejcn.1602704. [DOI] [PubMed] [Google Scholar]
- 14.Bjellaas T, Olesen PT, Frandsen H, Haugen M, Stolen LH, Paulsen JE, et al. Comparison of estimated dietary intake of acrylamide with hemoglobin adducts of acrylamide and glycidamide. Toxicol. Sci. 2007 Jul;98(1):110–117. doi: 10.1093/toxsci/kfm091. [DOI] [PubMed] [Google Scholar]
- 15.Kutting B, Uter W, Drexler H. The association between self-reported acrylamide intake and hemoglobin adducts as biomarkers of exposure. Cancer Causes Control. 2007 Nov 6; doi: 10.1007/s10552-007-9090-9. [DOI] [PubMed] [Google Scholar]
- 16.Bergmark E, Calleman CJ, He F, Costa LG. Determination of hemoglobin adducts in humans occupationally exposed to acrylamide. Toxicol. Appl. Pharmacol. 1993 May;120(1):45–54. doi: 10.1006/taap.1993.1085. [DOI] [PubMed] [Google Scholar]
- 17.Tornqvist M, Fred C, Haglund J, Helleberg H, Paulsson B, Rydberg P. Protein adducts: quantitative and qualitative aspects of their formation, analysis and applications. J. Chromatogr. B. Analyt Technol. Biomed. Life. Sci. 2002 Oct 5;778(1–2):279–308. doi: 10.1016/s1570-0232(02)00172-1. [DOI] [PubMed] [Google Scholar]
- 18.U.S. Food and Drug Administration. Survey Data on Acrylamide in Food: Individual Food Products, 2002–2004. 2006 Available at: http://www.cfsan.fda.gov/~dms/acrydata.html.
- 19.U.S. Food and Drug Administration. Survey Data on Acrylamide in Food: Total Diet Study Results, 2003–2006. 2006
- 20.U.S. Food and Drug Administration. The 2006 Exposure Assesment for Acrylamide. 2006 Available at: http://www.cfsan.fda.gov/~dms/acryexpo.html.
- 21.Rosen J, Nyman A, Hellenas KE. Retention studies of acrylamide for the design of a robust liquid chromatography-tandem mass spectrometry method for food analysis. J. Chromatogr. A. 2007 Nov 16;1172(1):19–24. doi: 10.1016/j.chroma.2007.09.050. [DOI] [PubMed] [Google Scholar]
- 22.Wenzl T, Karasek L, Rosen J, Hellenaes KE, Crews C, Castle L, et al. Collaborative trial validation study of two methods, one based on high performance liquid chromatography-tandem mass spectrometry and on gas chromatography-mass spectrometry for the determination of acrylamide in bakery and potato products. J. Chromatogr. A. 2006 Nov 3;1132(1–2):211–218. doi: 10.1016/j.chroma.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 23.Vesper HW, Ospina M, Meyers T, Ingham L, Smith A, Gray JG, et al. Automated method for measuring globin adducts of acrylamide and glycidamide at optimized Edman reaction conditions. Rapid Commun. Mass Spectrom. 2006;20(6):959–964. doi: 10.1002/rcm.2396. [DOI] [PubMed] [Google Scholar]
- 24.Vesper HW, Bernert JT, Ospina M, Meyers T, Ingham L, Smith A, et al. Assessment of the relation between biomarkers for smoking and biomarkers for acrylamide exposure in humans. Cancer Epidemiol. Biomarkers Prev. 2007 Nov;16(11):2471–2478. doi: 10.1158/1055-9965.EPI-06-1058. [DOI] [PubMed] [Google Scholar]
- 25.Willett WC. Nutritional Epidemiology. New York: Oxford University Press; 1998. [Google Scholar]
- 26.Rosner B, Willett WC. Interval estimates for correlation coefficients corrected for within-person variation: implications for study design and hypothesis testing. Am. J. Epidemiol. 1988 Feb;127(2):377–386. doi: 10.1093/oxfordjournals.aje.a114811. [DOI] [PubMed] [Google Scholar]
- 27.Bergmark E. Hemoglobin adducts of acrylamide and acrylonitrile in laboratory workers, smokers and nonsmokers. Chem. Res. Toxicol. 1997 Jan;10(1):78–84. doi: 10.1021/tx960113p. [DOI] [PubMed] [Google Scholar]
- 28.Hagmar L, Wirfalt E, Paulsson B, Tornqvist M. Differences in hemoglobin adduct levels of acrylamide in the general population with respect to dietary intake, smoking habits and gender. Mutat. Res. 2005 Feb 7;580(1–2):157–165. doi: 10.1016/j.mrgentox.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 29.Schettgen T, Rossbach B, Kutting B, Letzel S, Drexler H, Angerer J. Determination of haemoglobin adducts of acrylamide and glycidamide in smoking and non-smoking persons of the general population. Int. J. Hyg. Environ. Health. 2004 Dec;207(6):531–539. doi: 10.1078/1438-4639-00324. [DOI] [PubMed] [Google Scholar]
- 30.Schettgen T, Weiss T, Drexler H, Angerer J. A first approach to estimate the internal exposure to acrylamide in smoking and non-smoking adults from Germany. Int. J. Hyg. Environ. Health. 2003 Jan;206(1):9–14. doi: 10.1078/1438-4639-00195. [DOI] [PubMed] [Google Scholar]
- 31.Aureli F, Di Pasquale M, Lucchetti D, Aureli P, Coni E. An absorption study of dietary administered acrylamide in swine. Food Chem. Toxicol. 2007 Jul;45(7):1202–1209. doi: 10.1016/j.fct.2006.12.029. [DOI] [PubMed] [Google Scholar]
- 32.Doerge DR, Young JF, McDaniel LP, Twaddle NC, Churchwell MI. Toxicokinetics of acrylamide and glycidamide in Fischer 344 rats. Toxicol. Appl. Pharmacol. 2005 Nov 1;208(3):199–209. doi: 10.1016/j.taap.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 33.Doerge DR, Young JF, McDaniel LP, Twaddle NC, Churchwell MI. Toxicokinetics of acrylamide and glycidamide in B6C3F1 mice. Toxicol. Appl. Pharmacol. 2005 Feb 1;202(3):258–267. doi: 10.1016/j.taap.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 34.Besaratinia A, Pfeifer GP. Genotoxicity of acrylamide and glycidamide. J. Natl. Cancer Inst. 2004 Jul 7;96(13):1023–1029. doi: 10.1093/jnci/djh186. [DOI] [PubMed] [Google Scholar]
- 35.Manjanatha MG, Aidoo A, Shelton SD, Bishop ME, McDaniel LP, Lyn-Cook LE, et al. Genotoxicity of acrylamide and its metabolite glycidamide administered in drinking water to male and female Big Blue mice. Environ. Mol. Mutagen. 2006 Jan;47(1):6–17. doi: 10.1002/em.20157. [DOI] [PubMed] [Google Scholar]
- 36.Calleman CJ, Bergmark E, Stern LG, Costa LG. A nonlinear dosimetric model for hemoglobin adduct formation by the neurotoxic agent acrylamide and its genotoxic metabolite glycidamide. Environ. Health Perspect. 1993 Mar;99:221–223. doi: 10.1289/ehp.9399221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vesper HW, Licea-Perez H, Meyers T, Ospina M, Myers GL. Pilot study on the impact of potato chips consumption on biomarkers of acrylamide exposure. Adv. Exp. Med. Biol. 2005;561:89–96. doi: 10.1007/0-387-24980-X_7. [DOI] [PubMed] [Google Scholar]