Abstract
Phosphorylase kinase (PhK), an (αβγδ)4 complex, stimulates energy production from glycogen in the cascade activation of glycogenolysis. Its large homologous α and β subunits regulate the activity of the catalytic γ subunit and account for 81% of PhK’s mass. Both subunits are thought to be multi-domain structures, and recent predictions based on their sequences suggest the presence of potentially functional glucoamylase (GH15)-like domains near their amino-termini. We present the first experimental evidence for such a domain in PhK, by demonstrating that the glucoamylase inhibitor acarbose binds PhK, perturbs its structure, and stimulates its kinase activity.
Phosphorylase kinase (PhK), a 1.3 MDa (αβγδ)4 complex, regulates energy production via carbohydrate metabolism through its Ca2+-dependent phosphorylation and activation of glycogen phosphorylase in the cascade activation of glycogenolysis (reviewed in 1). Through allosteric and covalent modification sites on its α, β and δ (endogenous calmodulin) regulatory subunits, PhK integrates neural (Ca2+), hormonal (cAMP and Ca2+) and metabolic (ADP) signals, resulting in large increases in the activity of its catalytic γ subunit and the tight control of glycogenolysis and subsequent energy production. Despite being the first protein kinase to be discovered, little is known about the structure of the PhK complex. High resolution crystal structures are available only for the catalytic domain of the γ subunit and, of course, calmodulin, the δ subunit; but together, these account for only 15% of PhK’s mass. Virtually nothing is known about the structures of PhK’s large regulatory α (138.4 kDa) and β subunits (125.2 kDa), which account for 81% of its mass.
The α and β subunits are homologous and are generally considered to be products of an early gene duplication event (2); however, they do posses distinct regions near their N- and C-termini that are phosphorylated by cAMP-dependent protein kinase or autophosphorylated by the γ subunit within the complex (1). Aside from each other, the intact full-length α and β subunits have no apparent homologs found in current data bases, although bioinformatics approaches indicate that both subunits do contain domains with structural similarities to known enzymes. Using several bioinformatics approaches, Carrière et al. (3) predicted that α and β each contain four distinct domains (A, B, C and D), and that the C-terminal D domains of these subunits are related to calcinuerin B proteins. Domain A was argued to have significant similarities with the α/β barrel glucoamylase GH15 family of proteins, in agreement with an earlier prediction for this domain by Pallen (4).
Callebaut and coworkers carried out 3D modeling of the GH15-like domains of α and β using as a template the crystal structure of the Clostridium thermosaccharolyticum glucoamylase GH15 domain complexed with acarbose (5), a pseudotetrasccharide transition state analog inhibitor of α-glucosidases (6,7). Comparison of the active site structure of GH15 with the predicted GH15-like domain of α showed that almost all the residues that contact acarbose in GH15 are conserved in α, including the catalytic glutamate residues involved in hydrolysis of the glycosidic bond of the polysaccharide (5). Analysis of the β subunit’s GH15-like domain indicated only partial conservation of the residues involved in ligand contacts and catalysis in GH15. In the same study, it was demonstrated that many mutations in the α subunit known to cause X-linked liver glycogenoses map directly within the GH15-like glycoside binding site, suggesting an important functional role for this domain. As a first step in determining the validity of the predictions of glucoamylase-like domains in PhK’s α and β subunits, we determined whether PhK was directly affected by acarbose. We show herein that acarbose binds to PhK with relatively high affinity, and that in doing so, it perturbs the structure of the kinase and brings about its activation.
Substrate-induced changes in the intrinsic fluorescence of Clostridium thermosaccharolyticum glucoamylase have been used successfully to monitor the binding of a series of malto-oligosaccharide substrates (8). In the glucoamylase active site, both maltose and acarbose form contacts with, or are proximal to, four tryptophan residues (6), several of which are conserved among the GH15 family of enzymes and the GH15-like domains of the PhK α and β subunits (5). To determine whether PhK behaves similarly to glucoamylase, the concentration-dependent binding of acarbose by PhK was followed by measuring the intrinsic fluorescence of the complex at both pH 6.8 (nonactivating for PhK) and pH 8.2 (activating). Plots of the differences in relative fluorescence measured for the kinase in the presence and absence of increasing concentrations of acarbose were hyperbolic (Fig. 1), and fitted by non-linear least square methods (described under Supporting Information). Apparent KD values calculated for binding of the oligosaccharide were nearly equivalent, 3.7μM (pH 6.8) and 5.6 μM (pH 8.2), indicating that nonactivated and pH-activated forms of PhK have similar affinities for acarbose.
FIGURE 1.
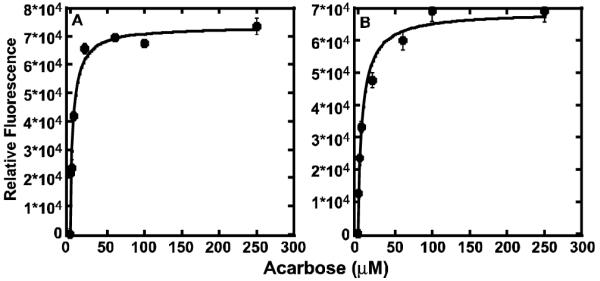
Concentration-dependent binding of acarbose by PhK at pH 6.8 (A) and pH 8.2 (B). Fluorescence emission spectra of PhK were collected from 295 to 405 nm using an excitation wavelength of 280 nm. Each point represents the difference in relative peak intensities measured for PhK (100 μg/ml) ± acarbose. No fluorescence signals were detected for negative PhK control samples at acarbose concentrations 10-fold greater than the maximal concentration used in the protein assays. All measurements were carried out in duplicate.
We next examined whether acarbose influences the catalytic activity of PhK. Activity measurements were performed using as substrate a well-characterized synthetic tetradecapeptide corresponding to the phosphorylatable N-terminus of glycogen phosphorylase, the natural substrate of PhK. Phosphorylase was not used as substrate to avoid the possibility of acarbose binding to its glycogen storage site and altering its ability to serve as a substrate for PhK, thus only indirectly influencing PhK’s activity. With three different preparations of PhK, we found that 250 μM acarbose increased the rate of phosphorylation of the peptide by 2- to 3-fold at pH 6.8 (Figure S1 of Supporting Information).
An alternative activity assay to measure the effect of acarbose is autophosphorylation of PhK’s α and β subunits; however, even though any effect of acarbose would by definition be directly on PhK, the acarbose could theoretically affect the catalytic activity of the γ subunit, or the ability of the α and/or β subunits to serve as substrates, or a combination of both. The extent of autophosphorylation of PhK’s α and β subunits ± 250 μM acarbose was followed over time at pH 6.8 and 8.2. At the lower pH value, acarbose stimulated phosphorylation of α by 3-fold (Fig. 2A) and β by nearly 2-fold (Fig. 2C), similar to its stimulation of synthetic peptide phosphorylation at this pH value. As expected, the stimulation by acarbose at pH 8.2 was considerably less and was similar for both subunits (1.2-fold; Figs. 2B&D).
Figure 2.
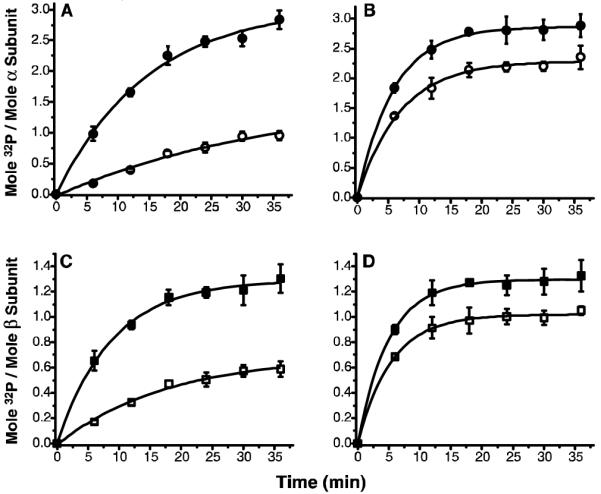
Time- and pH-dependent autophosphorylation of PhK ± acarbose. Total phosphate incorporation into the α and β subunits of PhK in the absence (open symbols) and presence (closed symbols) of acarbose (250 μM) at pH 6.8 (A&C) and pH 8.2 (B&D) was measured on P81 filters. In parallel, at indicated intervals, aliquots of the reaction mixture were run on 7.5%T SDS-PAGE. The 32P incorporation into each subunit was quantified on a Typhoon 9410 Phosphor Imager (Amersham Biosciences, Piscataway, NJ) following autoradiography of the gels. An asymptotic function y=a−bcx was applied for exponential fitting (p<0.05) of each data set using Origin 7.5 software (OriginLab, Northampton, MA).
To test for acarbose-induced changes in PhK’s α and β subunits, we used chemical crosslinkers as conformational probes, specifically employing crosslinkers known to be selective for those subunits. No changes in cross-linking were observed for crosslinkers that primarily form α-conjugates (9,10); however, acarbose perturbed crosslinking of PhK by N-[γ-maleimidobutyryloxy] succinimide ester (GMBS), an affinity-based crosslinker that selectively targets β and γ subunits in the (αβγδ)4 complex (11). The composition of all conjugates was determined by their apparent mass and cross reactivity against anti-α, anti-β and anti-γ subunit-specific monoclonal antibodies (mAbs) (Fig. 3B), as previously described (12,13); no conjugates containing the intrinsic calmodulin subunit (δ) were detected in Western blots using an anti-calmodulin mAb (data not shown). In the absence of acarbose, GMBS formed small amounts of a βγγ heterotrimer (massExp = 213 kDa: 0.74% error), as well as a large conjugate with an apparent mass exceeding 300 kDa and containing α, β and γ subunits (Fig. 3, lanes 2). Acarbose (100 μM) dramtically increased the formation of both complexes, as well as promoting the formation of two new species: βγγi (massExp = 210 kDa: 2.1% error) and βi (massExp = 118 kDa: 5.7 % error) (Fig. 3, lanes 3). These two new species undoubtedly represent intrasubunit crosslinked forms of βγγ and β.
FIGURE 3.
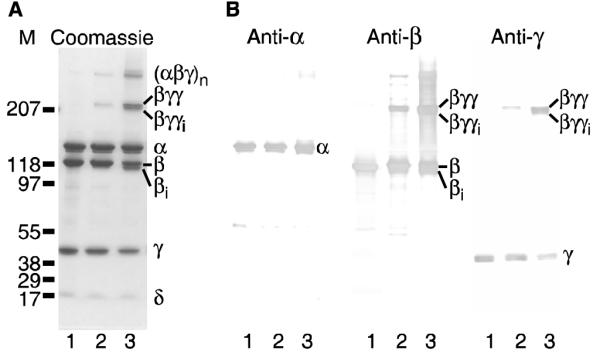
GMBS crosslinking of the PhK complex ± acarbose. (A) PhK (lane 1) was crosslinked by GMBS in the absence (lane 2) and presence (lane 3) of acarbose (100 μM) and resolved by SDS-PAGE. (B) Parallel samples were transferred to PVDF membranes and probed with mAbs against all the PhK subunits. No conjugate crossreacted with the anti-CaM (δ) mAb (data not shown). Subscript ‘i’ denotes intramolecular crosslinking of the indicated subunit.
The sum of the data in this study clearly demonstrates that PhK binds acarbose with relatively high affinity and that this oligosaccharide perturbs the structure of the PhK complex and activates it, providing the first experimental evidence supporting the existence of the predicted glucoamylase-like domains in PhK’s large regulatory α and β subunits (3-5). It is unclear from this study which subunit, or subunits, bind the acarbose. The autophosphorylation of both is stimulated, and even though the crosslinking of the β subunit is altered, this could be through an indirect effect, as the α and β subunits are most certainly structurally coupled in the complex (14). The activation by saturating acarbose is less than that observed with other allosteric activators of PhK, which may be related to the inability of acarbose to enhance formation of α-γ conjugates with a variety of crosslinkers, a property routinely observed with other activators (15). Although acarbose is most similar to glycogen, activation by the latter is poorly characterized, including the subunit(s) to which it binds. It remains to be determined whether the predicted glucoamylase-like domains supported by the work herein are catalytic, and if so, whether this potential second function of PhK is affected by its state of activation. Our results demonstrate that acarbose will be a valuable tool, however, in evaluating this proposed new function.
Supplementary Material
ACKNOWLEDGMENT
Thiswork was supported by National Institutes of Health Grant DK032953 to G.M.C.
Footnotes
SUPPORTING INFORMATION PARAGRAPH
Details of chemical crosslinking, experimental procedures and Figure S1. This material is available free of charge via the internet at http://pubs.acs.org.
REFERENCES
- 1.Brushia RJ, Walsh DA. Phosphorylase kinase: the complexity of its regulation is reflected in the complexity of its structure. Front. Biosci. 1999;4:d618–41. doi: 10.2741/brushia. [DOI] [PubMed] [Google Scholar]
- 2.Kilimann MW, Zander NF, Kuhn CC, Crabb JW, Meyer HE, Heilmeyer LM., Jr. The alpha and beta subunits of phosphorylase kinase are homologous: cDNA cloning and primary structure of the beta subunit. Proc. Natl. Acad. Sci. U.S.A. 1988;85:9381–9385. doi: 10.1073/pnas.85.24.9381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carriere C, Mornon JP, Venien-Bryan C, Boisset N, Callebaut I. Calcineurin B-like domains in the large regulatory α/β subunits of phosphorylase kinase. Proteins. 2008;71:1597–1606. doi: 10.1002/prot.22006. [DOI] [PubMed] [Google Scholar]
- 4.Pallen MJ. Glucoamylase-like domains in the α- and β-subunits of phosphorylase kinase. Protein Sci. 2003;12:1804–1807. doi: 10.1110/ps.0371103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carriere C, Jonic S, Mornon JP, Callebaut I. 3D mapping of glycogenosis-causing mutations in the large regulatory alpha subunit of phosphorylase kinase. Biochim. Biophys. Acta. 2008;1782:664–670. doi: 10.1016/j.bbadis.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 6.Aleshin AE, Feng P-H, Honzatko RB, Reilly PJ. Crystal structure and evolution of a prokaryotic glucoamylase. J. Mol. Boil. 327:61–73. doi: 10.1016/s0022-2836(03)00084-6. [DOI] [PubMed] [Google Scholar]
- 7.Svensson B, Sierks MR. Roles of the aromatic side chains in the binding of substrates, inhibitors and cyclomalto-oligosaccharides to the glucoamylase from Aspergillus niger probed by perturbation difference spectroscopy, chemical modification and mutagenesis. Carbohydr. Res. 1992;227:29–44. doi: 10.1016/0008-6215(92)85059-9. [DOI] [PubMed] [Google Scholar]
- 8.Ohnishi H, Matsumoto H, Sakai H, Takahisa O. Functional roles of Trp337 and Glu632 in Clostridium glucoamylase, as determined by chemical modification, mutagenesis and the stopped-flow method. J. Biol. Chem. 1994;269:3503–3510. [PubMed] [Google Scholar]
- 9.Nadeau OW, Traxler KW, Fee LR, Baldwin BA, Carlson GM. Activators of phosphorylase kinase alter the cross-linking of its catalytic subunit to the C-terminal one-sixth of its regulatory α subunit. Biochemistry. 1999;38:2551–2559. doi: 10.1021/bi982060b. [DOI] [PubMed] [Google Scholar]
- 10.Rice NA, Nadeau OW, Yang Q, Carlson GM. The calmodulin-binding domain of the catalytic γ subunit of phosphorylase kinase interacts with its inhibitory α subunit: evidence for a Ca2+ sensitive network of quaternary interactions. J. Biol. Chem. 2002;277:14681–14687. doi: 10.1074/jbc.M201229200. [DOI] [PubMed] [Google Scholar]
- 11.Nadeau OW, Anderson DW, Yang Q, Artigues A, Paschall JE, Wyckoff GJ, McClintock JL, Carlson GM. Evidence for the location of the allosteric activation switch in the multisubunit phosphorylase kinase complex from mass spectrometric identification of chemically crosslinked peptides. J. Mol. Biol. 2007;365:1429–1445. doi: 10.1016/j.jmb.2006.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilkinson DA, Marion TN, Tillman DM, Norcum MT, Hainfeld JF, Seyer JM, Carlson GM. An epitope proximal to the carboxyl terminus of the α-subunit is located near the lobe tips of the phosphorylase kinase hexadecamer. J. Mol. Biol. 1994;235:974–982. doi: 10.1006/jmbi.1994.1051. [DOI] [PubMed] [Google Scholar]
- 13.Wilkinson DA, Norcum MT, Fizgerald TJ, Marion TN, Tillman DM, Carlson GM. Proximal regions of the catalytic γ and regulatory β subunits on the interior lobe face of phosphorylase kinase are structurally coupled to each other and with enzyme activation. J. Mol. Biol. 1997;265:319–29. doi: 10.1006/jmbi.1996.0739. [DOI] [PubMed] [Google Scholar]
- 14.Nadeau OW, Traxler KW, Carlson GM. Zero-length crosslinking of the β subunit of phosphorylase kinase to the N-terminal half of its regulatory α subunit. Biochem. Biophys. Res. Commun. 1998;251:637–641. doi: 10.1006/bbrc.1998.9507. [DOI] [PubMed] [Google Scholar]
- 15.Nadeau OW, Sacks DB, Carlson GM. Differential affinity cross-linking of phosphorylase kinase conformers by the geometric isomers of phenylenedimaleimide. J. Biol. Chem. 1997;272:26196–26201. doi: 10.1074/jbc.272.42.26196. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


