Abstract
The osmoregulated betaine transporter BetP is a stable trimer. Structural studies have shown that individual protomers can adopt distinct transport conformations, implying a functional role for the trimeric state in transport, although the role of trimerization in regulation is not yet understood. We designed putative monomeric mutants by molecular-dynamics simulations and in silico alanine-scanning mutagenesis. Several mutants including BetP-W101A/T351A were monomeric in detergent as well as in the membrane, as shown by blue native gel electrophoresis, crosslinking and electron microscopy. This monomeric form retains the ability to accumulate betaine, but is no longer regulated by hyperosmotic shock.
Keywords: betaine transport, conformational changes, electron microscopy, oligomerization, osmoregulation
Introduction
BetP is an efficiently regulated secondary transporter for betaine that is involved in the hyperosmotic stress response (Farwick et al, 1995). BetP performs transport catalysis (betaine uptake) and stress stimulus sensing (perception of stress and intramolecular signal transduction), and adapts the transport activity to the extent of the hyperosmotic stress (Ziegler et al, 2010). One osmotic stress stimulus is an increase in cytoplasmic K+ concentration, which is sensed by the 55-residue-long carboxy-terminal domain of BetP (Peter et al, 1998; Schiller et al, 2004a; Ott et al, 2008), a key component in regulating transport activity. BetP adopts the so-called LeuT-fold (Yamashita et al, 2005), which is shared by several other transporter families (Forrest et al, 2011). However, BetP contains several additional unique features. On the periplasmic side, the amphipathic helix 7 (h7) connects two structurally related repeats, whereas TM2 encloses the transporter core similarly to a stabilizing belt. In addition, the C-terminal domain forms a long α-helix that extends into the cytoplasm. BetP is a trimer in the membrane of two-dimensional crystals, as well as in solubilized form in detergent micelles and in three-dimensional crystals (Ziegler et al, 2004; Ressl et al, 2009). Interactions between protomers in the trimer involve the structural elements mentioned above. Structural data obtained from two-dimensional crystals shows that each protomer in the BetP trimer adopts a distinct conformational state (Tsai et al, 2011), implying coupling between protomers during the transport cycle. In the X-ray crystal structure, the C-terminal helix interacts through a cluster of positively charged residues with negatively charged residues in the cytoplasmic loops of adjacent protomers (Ressl et al, 2009). Thus, the structural data indicate a functional role for the trimeric state in enabling cross-talk between protomers, affecting both catalytic and regulatory cycles.
Here, we report structural and biochemical data that demonstrate a functional role for the BetP trimer in both transport and regulation. To design a monomeric mutant protein, we first identified key interfacial residues by molecular-dynamics simulations and in silico alanine-scanning mutagenesis calculations. We show that double and triple mutants of key positions yielded the monomeric form of BetP in detergent solution, in proteoliposomes and in Escherichia coli cells. Combined with uptake measurements in proteoliposomes and cells, our data support the crucial role of the trimer during both transport and transport regulation. Thus, BetP requires the trimeric state to properly respond to osmotic stress.
Results
Molecular-dynamics simulations
To investigate the molecular interactions responsible for maintaining the trimeric state of BetP, we carried out 30- to 50-ns-long molecular-dynamics simulations of BetP trimers in a hydrated POPG bilayer (Fig 1A). The trimeric protein is stable in these simulations. Thus, for three repeat simulations with different initial velocities, the average root-mean-square deviation of the Cα atoms in the transmembrane helices from the starting structure are low: 1.6±0.1, 1.7±0.1 and 1.6±0.1 Å. The individual protomers in the trimer are similarly stable, as is the monomeric protein in separate simulations (supplementary Fig S1 online). Furthermore, in the latter simulations, no significant destabilization of the helices at the interfacial region was observed (supplementary Fig S1 online), suggesting that a stable monomeric construct of BetP is possible.
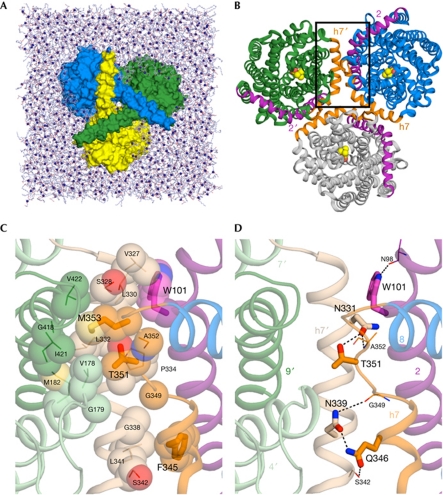
Figure 1.
The structure of BetP and the simulation system. (A) The BetP trimer (surface representation) in a POPG lipid bilayer (sticks, with P atoms as spheres), viewed from the cytoplasm, showing the way in which the carboxy-terminal helices crossover to adjacent protomers. Water molecules and ions are omitted for clarity. (B) View of the BetP trimer from the periplasm. Helices at the trimer interface are shown in purple (TM2) or orange (h7); those belonging to the upper-left protomer are labelled 2′ and h7′, respectively. Bound betaine molecules are shown (spheres). (C,D) Close-up view of the protomer interface in a snapshot of a molecular-dynamics simulation, in the region marked with a box in (B), highlighting hydrophobic (C) or polar (D) interactions. Helices of two adjacent protomers (green and blue in B) are shown: h7 (beige), TM9 (dark green), TM4 and TM7 (pale green) from one protomer (labelled h7′, 4′, 7′ and 9′), and h7/L7-8 (orange), TM2 (purple) and TM8 (blue) from the second protomer. Side chains of hot-spot residues identified using in silico alanine scanning are shown (thick sticks, large labels), along with their primary interaction partners (thin sticks), and relevant inter-protomer hydrogen bonds are indicated (dashed lines). Residues involved in hydrophobic interactions are shown as spheres (C).
In silico alanine scanning
We then calculated the binding free energy on in silico mutation of the interfacial residues to alanine, in the X-ray structure and in several snapshots from the molecular-dynamics trajectories (supplementary Table S1 online). Twenty-three residues were identified as interfacial, nine of which increased the binding free energy (ΔΔGbind) of the protomers by more than 1 kcal/mol on replacement by alanine. Six of these nine residues (bold in supplementary Table S1 online) are located in the horizontal h7 (residues 326–350) or in the loop connecting h7 to TM8 (L7–8; 351–356), indicating that this is the most crucial interfacial region. Indeed, these residues form extensive interactions with adjacent protomers (Fig 1B–D). Between two protomers A and B, e.g. T351A forms hydrogen bonds with N331B or N339B and van der Waals interactions with V178B, G179B, M182B, L332B or G335B. Similarly, F345A forms hydrophobic interactions with the side chains of residues L341B, S342B or G338B.
Several residues from TM2 were also found to be energetically crucial for forming the interface (supplementary Table S1 online). The residue from TM2 most strongly affected by in silico mutation was W101, which formed hydrophobic contacts with residues of h7 from another protomer during the simulations (V327, L330 and occasionally L333 and P334; Fig 1C). Finally, two residues of the C-terminal helix, E552 and R568, were significantly affected by in silico alanine mutation (supplementary Table S1 online). However, the C-terminally truncated mutant of BetP was found to be trimeric in detergent solution, similarly to the wild-type protein (Tsai et al, 2011), indicating that the interactions between the C-terminal helices are not essential for trimer formation.
Disruption of the BetP trimer
On the basis of the in silico alanine scanning, several residues were selected for replacement in an attempt to create a monomeric form of BetP. Only residues from h7, L7–8 or TM2 were considered. We first selected W101, for which the effect of in silico mutation was the largest (supplementary Table S1 online). We also considered T351 and F345, because ΔΔGbind was also large for these residues, and because together they are evenly distributed along the length of the interface (Fig 1C,D).
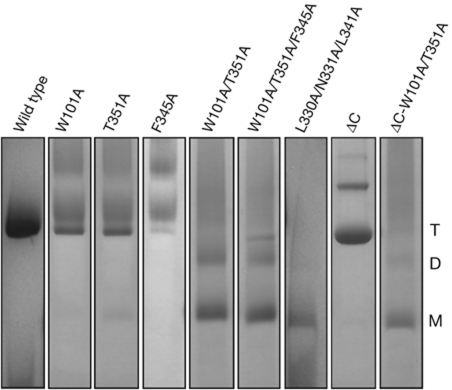
W101, T351 and F345 were replaced by alanine both in the wild type and in a truncated form of BetP that lacks the last 45 residues of its C-terminal domain (BetPΔC). As shown by blue native–polyacrylamide gel electrophoresis (BN-PAGE; Fig 2; supplementary Fig S2 online), although wild-type BetP exclusively forms trimers and dimers of trimers in detergent solution, the mutants BetP-W101A/T351A and BetP-W101A/F345A/T351A predominantly exist as monomers and, to a lesser extent, dimers. The mutant BetP-L330A/N331A/L341A, in which the interaction partners of residues W101, F345 and T351 (Fig 1C,D) have been replaced by alanine, was also mainly monomeric in BN-PAGE (Fig 2). Similarly to wild-type BetP, the truncated protein BetPΔC was found to be trimeric by BN-PAGE, confirming that the C-terminal domain is not crucial for trimer formation (Fig 2). When the same mutations that disrupt the wild-type trimer were introduced into BetPΔC, they again resulted in monomers (Fig 2; supplementary Fig S2 online).
Figure 2.
Mobility of wild-type BetP and mutants in BN–PAGE. Gel lanes are shown separately for viewing purpose; the complete gel and the molecular marker are shown in supplementary Fig S2 online. BN–PAGE, blue native polyacrylamide gel electrophoresis; D, dimer; M, monomer; T, trimer.
The oligomerization state of wild-type BetP and mutants was further evaluated by size-exclusion chromatography (supplementary Fig S3 online). In the chromatographic profiles, wild-type BetP and the mutant BetP-T351A elute at similar volumes, whereas the mutant BetP-W101A/T351A appears in a 2 ml delayed peak, consistent with the smaller size expected for the monomer.
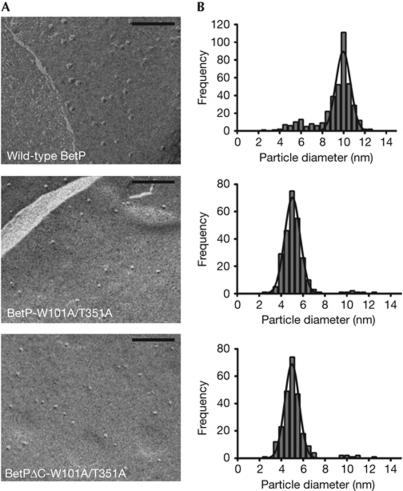
Wild-type BetP and the mutants BetP-T351A, BetP-W101A/T351A and BetPΔC-W101A/T351A were then reconstituted into E. coli polar lipid liposomes, and their oligomeric state in the membrane was analysed by freeze-fracture electron microscopy (Fig 3; supplementary Fig S4 online). The mean particle diameters and areas are shown in Table 1, and their size distribution is shown in Fig 3B and supplementary Fig S4B online. Wild-type BetP and BetP-T351A are mainly trimeric in the membrane of proteoliposomes, whereas the double and triple mutants are mainly monomeric (approximately 95% monomers and approximately 5% a mixture of trimers and dimers).
Figure 3.
Results of freeze-fracture electron microscopy. (A) Electron micrographs of membrane-reconstituted wild-type BetP and mutants. Scale bar, 100 nm. (B) Distribution, that is, number of particles counted as a function of particle size. The line in each plot corresponds to a fit to a Gaussian function.
Table 1. Freeze-fracture particle analysis of BetP wild-type, BetP-W101A/T351A and BetPΔC-W101A/T351A.
| Density (particles/μm2) | Diameter (nm)* | Area (nm2)‡ | |
|---|---|---|---|
| BetP WT | 368.0±15.0 | 10.0±0.8 | 78.5±0.5 |
| BetP-W101A/T351A | 331.0±19.0 | 5.1±0.7 | 20.4±0.4 |
| BetPΔC-W101A/T351A | 295.0±12.0 | 5.0±0.7 | 19.6±0.4 |
| *Diameters were corrected for the thickness of the platinum–carbon film by subtracting 2.5±0.8 nm from the mean diameters obtained from the frequency histograms; ‡Cross-sectional area was obtained by assuming a circular geometry for the particles. WT, wild type. | |||
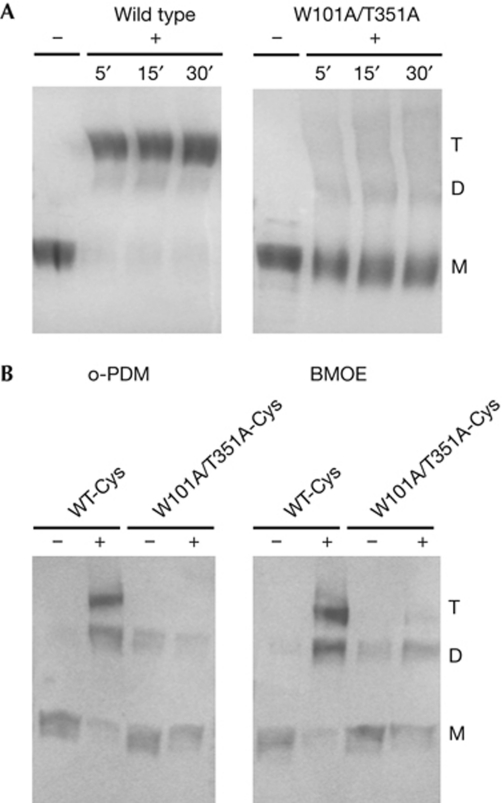
We tested the effect of the nonspecific crosslinking agent glutaraldehyde on wild-type BetP and on the monomeric mutant BetP-W101A/T351A in detergent solution (Fig 4A). Although the wild-type protein is trimeric in the presence of the crosslinking agent, the BetP-W101A/T351A mutant shows only monomers.
Figure 4.
Crosslinking of BetP. (A) Glutaraldehyde crosslinking of detergent solubilized wild-type BetP and BetP-W101A/T351A, shown in a Coomassie-stained 10% SDS–PAGE. Samples were incubated with 0.13% glutaraldehyde (+) for the indicated times, up to 30 min. The control is indicated by −. (B) Western blot of BetP-D97C/S328C (WT-Cys) and BetP-D97C/W101A/S328C/T351A (W101A/T351A-Cys) in membrane vesicles of Escherichia coli cells. + indicates incubation with 5 mM of o-phenylenedimaleimide (o-PDM) or bis-maleimidoethane (BMOE). D, dimer; M, monomer; PAGE, polyacrylamide gel electrophoresis; T, trimer; WT, wild type.
Furthermore, we analysed the oligomeric state of double-cysteine mutants in the membrane of E. coli cells by site-directed intermolecular crosslinking (Fig 4B). Two cysteine residues (D97C and S328C, supplementary Fig S5 online) were introduced into the monomeric BetP-W101A/T351A mutant (BetP W101A/T351A-Cys) and into wild-type BetP (BetP WT-Cys). E. coli membrane vesicles containing either of the double-cysteine mutants were incubated in the presence of the homo-bifunctional crosslinkers o-phenylenedimaleimide and bis-maleimidoethane, which form rigid spacers of approximately 6 and approximately 8 Å, respectively. BetP WT-Cys in the presence of crosslinking agents resulted in a trimeric product, whereas almost no crosslinking could be observed for the monomeric BetP W101A/T351A-Cys (Fig 4B), indicating that in the native E. coli membranes the monomers are too far apart to crosslink.
Betaine transport by monomeric BetP mutants
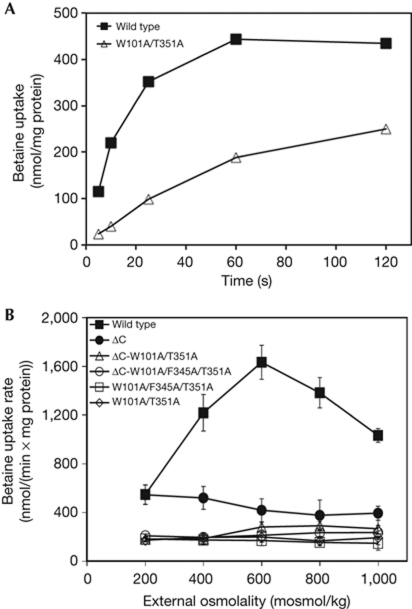
We assayed sodium-driven transport of [14C]-betaine by the monomeric mutants in E. coli polar lipid proteoliposomes (Fig 5). The monomeric mutant BetP-W101A/T351A accumulates betaine up to approximately 250 nmol/mg protein, which is lower than the approximately 435 nmol/mg protein maximum for wild-type BetP (Fig 5A). Wild-type BetP is activated on an increase of external osmolality in the presence of internal K+ (Peter et al, 1998; Fig 5B). By contrast, the mutants BetP-W101A/T351A and BetP-W101A/F345A/T351A have lost the ability to become activated in response to an increase of the external osmolality (Fig 5B).
Figure 5.
Measurement of [14C]-betaine uptake rates in proteoliposomes. (A) Representative uptake of [14C]-betaine in proteoliposomes with wild-type BetP or the mutant BetP-W101A/T351A at 400 mosml/kg. (B) The wild-type BetP activation profile is compared with those of BetPΔC, BetP-W101A/T351A, BetP-W101A/F345A/T351A, BetPΔC-W101A/T351A and BetPΔC-W101A/F345A/T351A. Each value represents the average of three independent measurements. Error bars indicate the standard deviation.
As mentioned above, the C-terminal domain of BetP is a crucial element of activity regulation. However, truncation of the C-terminal domain does not affect trimerization (Ott et al, 2008; Tsai et al, 2011; Fig 2). BetPΔC is not activated in response to osmotic shock (Fig 5B), in agreement with previous studies (Schiller et al, 2004a). Similarly, the monomeric mutants BetPΔC-W101A/T351A and BetPΔC-W101A/F345A/T351A also did not respond to increased osmolality (Fig 5B). To confirm that the loss of regulatory properties observed for the monomeric BetP mutants is not due to solubilization and reconstitution, the same functional measurements were recorded in MKH13 E. coli cells expressing monomeric BetP. Transport activation measurements were fully consistent with those in proteoliposomes. Cells expressing monomeric mutants of BetP accumulate betaine (supplementary Fig S6A online), but did not show an increased rate of uptake after an increase in osmolality (supplementary Fig S6B online). BetP-T351A, which is mainly trimeric with approximately 18% dimers (supplementary Fig S4 online), shows reduced activity at higher osmolalities. By contrast, the monomeric mutants BetP-L330A/N331A/L341A, BetPΔC-W101A/T351A and BetPΔC-W101A/F345A/T351A were not regulated by osmotic shock (supplementary Fig S6B and S7 online). We note that although the transport activity of the monomeric mutants of BetP is low, it is still significant, as shown by comparison of their activation profiles to that of an inactive mutant W377A (supplementary Fig S6B online, Ressl et al, 2009). Moreover, the Km values of wild-type BetP and BetP-W101A/T351A were similar (Table 2, supplementary Fig S6C and D online).
Table 2. Kinetics parameters of WT BetP and BetP-W101A/T351A.
| Km (μM) | Vmax (nmol/min × mg cdw) | |
|---|---|---|
| WT BetP | 13.2±5.0 | 185.7±10.2 |
| BetP-W101A/T351A | 7.7±2.7 | 23.6±1.7 |
| cdw, cell dry weight; WT, wild-type. | ||
Taken together, our results indicate that the functional unit for transport of BetP is the monomer, and that disrupting either of the inter-protomer contact networks observed in the structure (Fig 1)—e.g., by deletion of the C-terminal domain or by preventing trimerization—abolishes the ability of BetP to be activated by increased osmolality.
Discussion
The protomer of a secondary transporter is generally assumed to be the functional transporting unit, whereas oligomerization is often a structure-stabilizing process (Van Dort et al, 2001). The possibility of a functional role for an oligomeric state has only been addressed for a few transporters because obtaining and investigating the monomeric form of an otherwise stable oligomer is difficult. Recently, the successful monomerization of the Cl−/H+ exchanger CLC-ec1 from E. coli (Robertson et al, 2010) provided structural and functional evidence that the monomer is the functional unit for transport. For the sodium/proton antiporter NhaA, the functional unit has been shown to be the monomer, while dimerization was found to be beneficial under stress conditions (Rimon et al, 2007; Herz et al, 2009). A dimeric state was assigned as the functional unit of the small multidrug transporter EmrE on the basis of negative-dominance studies on a genetically fused protomer tandem (Steiner-Mordoch et al, 2008). In the case of the multidrug transporter AcrB, the trimeric form apparently facilitates functional cooperativity of the protomers (Murakami et al, 2004, 2006; Eicher et al, 2009).
BetP was shown to be a stable trimer in the membrane of two-dimensional crystals (Tsai et al, 2011) and in three-dimensional crystals in the presence of detergent (Ressl et al, 2009). Specific functional roles for the BetP trimer could be inferred by the various inter-protomer contacts found in the three-dimensional structural data. Moreover, in two-dimensional crystals, individual protomers within one trimer were found to adopt distinct conformations, which correspond to specific states in the alternating-access cycle (Tsai et al, 2011). As this conformational asymmetry has been observed both in the presence and absence of the C-terminal domain (that is, the key requisite for regulation), it has been interpreted as reflecting functional coupling between the protomers during the catalytic cycle rather than during regulation, although the mechanism and functional consequences of this coupling are unknown (Tsai et al, 2011).
Here, we have shown that the core ‘transporting unit’ is the BetP protomer. One possible mechanism of coupling between such independent units would be cooperativity in their substrate binding, which should be reflected by lower affinities in the monomeric mutant compared with those in the trimer. However, the monomeric and trimeric forms have similar Km values (Table 2). Instead, the catalytic turnover rate and the resultant accumulation of betaine were found to be slower in the monomer and in partly dimeric T351A (supplementary Fig S4 online; supplementary Table S2 online) compared with the trimeric versions of the protein—BetP-WT, BetP-W101A and BetP-F345A (Table 2)—supporting the hypothesis that there is conformational coupling in the catalytic cycle when BetP is trimeric. In addition to the role of the trimer in transport, the crystal structure of BetP also indicates a role for the trimeric state in the mechanism for regulation. Specifically, the structure shows that the C-terminal domain can interact with cytoplasmic loops in adjacent protomers; these loops are directly connected to helices that undergo essential conformational changes during substrate translocation (Ressl et al, 2009). Thus, interaction with the C-terminal domain probably restricts the flexibility of the cytoplasmic loops, which might result in transport inactivation in that particular protomer. Biochemical studies also support the presence of this cytoplasmic interaction network, although those data cannot distinguish between inter-protomer and intra-protomer interactions (Ott et al, 2008).
Our findings show that monomeric BetP is not regulated, even when the C-terminal domain is present, suggesting that the C-terminal domain is not interacting with the cytoplasmic loops of the same protomer. As a trimer lacking the C-terminal domains is also not regulated, these data show that regulation of activity can occur only if three monomers with intact C-terminal domains assemble together. Apparently, the long C-terminal domain itself does not have an essential role in trimerization, and the key interactions are instead located on the periplasmic side. However, these periplasmic interactions seem to be more than simply a prerequisite for enabling the C-terminal domain to interact with the cytoplasmic loops. The partly dimeric mutant BetP-T351A also shows a significantly reduced regulation profile and a reduced Vmax compared to the wild-type protein (Table 2; supplementary Table S2 online). The implication being that even without disrupting the trimer or the cytoplasmic interaction network, partial destabilization of the periplasmic interaction at L7 (which connects h7 to TM8) is sufficient to alter the regulatory cross-talk in BetP. However, at some point, the catalytic and regulatory cycles begin to overlap. Although the monomer is the minimal functional unit for the catalytic cycle, regulation of BetP requires it to be trimeric.
Methods
Molecular-dynamics simulations. Molecular-dynamics simulations were carried out on the X-ray structure of BetP (Protein Data Bank entry 2WIT; Ressl et al, 2009). The protein was inserted into a POPG bilayer according to Faraldo-Gómez et al (2002). Three independent simulations of either 30 or 50 ns in length were run for the trimer and monomer systems, respectively, using different initial velocities. Further details are provided in the supplementary information online.
In silico alanine scanning. Mutations destabilizing protomer–protomer interactions in BetP were identified by in silico alanine scanning using the Robetta server (Kortemme & Baker, 2002, 2004). Calculations were performed on either the X-ray structure (Ressl et al, 2009) or on 27 snapshots extracted from three independent molecular-dynamics trajectories of BetP trimers at equal (10 ns) time intervals.
Reconstitution of BetP. Functional reconstitution was performed as described previously (Ott et al, 2008).
BN–PAGE. BN–PAGE was carried out as described by Schägger and group (Wittig et al, 2006) with 4–12% polyacrylamide Tris Glycine gels (Invitrogen).
Size-exclusion chromatography. Protein in 50 mM Tris–HCl (pH 7.5), 200 mM NaCl, 8.7% glycerol and 0.05% DDM was loaded onto a Superdex 200 10/30 (GE Healthcare) size-exclusion column previously equilibrated with 20 mM Tris–HCl (pH 7.5), 200 mM NaCl and 0.05% DDM. All runs were performed at a flow rate of 0.2 ml/min.
Freeze-fracture electron microscopy. Freeze-fracture replicas were prepared from proteoliposomes subjected to ultra-rapid cooling into liquid ethane. Frozen samples were fractured at the same time in a Bal-Tec BAF060 instrument at 1.5 × 10−7 torr. Replicas were exanimate under a Philips EM208 S electron microscope.
Crosslinking. We performed crosslinking with glutaraldehyde on purified proteins in buffer 150 mM KPi (pH 7.5), 200 mM NaCl and 0.05% DDM. A volume of 100 μl of protein 0.25 mg/ml was treated with 0.13% glutaraldehyde; the reaction was terminated by the addition of 10 μl of 1 M Tris–HCl (pH 8.0). Site-directed intermolecular crosslinking was conducted in situ on membrane vesicles of E. coli cells. A measure of 300 μg of protein was incubated for 30 min at 25 °C and gently shaken with 5 mM of a freshly prepared crosslinkers—o-phenylenedimaleimide or bis-maleimidoethane—dissolved in DMSO. The reaction was terminated by addition of DTT at a final concentration of 20 mM.
Transport assays. Uptake of [14C]-betaine (Moravek Biochemicals and Radiochemicals, USA) in E. coli cells and proteoliposomes was performed as described previously (Ott et al, 2008). The betaine uptake rate was calculated according to Schiller et al (2006). The kinetic constants were derived by Michaelis–Menten curve fitting of the uptake rates compared with the substrate concentration with GraphPad Prism version 5.0c for Mac OS X, GraphPad Software (Motulsky, 1999).
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Acknowledgments
This work was supported by the DFG (German Research Foundation), and Collaborative Research Center 807 ‘Transport and Communication across Biological Membranes’ (L.R.F. and C.Z.).
Footnotes
The authors declare that they have no conflict of interest.
References
- Eicher T, Brandstätter L, Pos KM (2009) Structural and functional aspects of the multidrug efflux pump AcrB. Biol Chem 390: 693–699 [DOI] [PubMed] [Google Scholar]
- Faraldo-Gómez JD, Smith GR, Sansom MS (2002) Setting up and optimization of membrane protein simulations. Eur Biophys J 31: 217–227 [DOI] [PubMed] [Google Scholar]
- Farwick M, Siewe RM, Kramer R (1995) Glycine betaine uptake after hyperosmotic shift in Corynebacterium glutamicum. J Bacteriol 177: 4690–4695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest L, Krämer R, Ziegler C (2011) The structural basis of secondary active transport mechanisms. Biochim Biophys Acta 1807: 167–188 [DOI] [PubMed] [Google Scholar]
- Herz K, Rimon A, Jeschke G, Padan E (2009) β-Sheet-dependent dimerization is essential for the stability of NhaA Na+/H+ antiporter. J Biol Chem 284: 6337–6347 [DOI] [PubMed] [Google Scholar]
- Kortemme T, Baker D (2002) A simple physical model for binding energy hot spots in protein–protein complexes. Proc Natl Acad Sci USA 99: 14116–14121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortemme T, Baker D (2004) Computational design of protein–protein interactions. Curr Opin Chem Biol 8: 91–97 [DOI] [PubMed] [Google Scholar]
- Motulsky H (1999) Analyzing Data with GraphPad Prism. GraphPad Software Inc., San Diego, CA, USA. www.graphpad.com [Google Scholar]
- Murakami S, Tamura N, Saito A, Hirata T, Yamaguchi A (2004) Extramembrane central pore of multidrug exporter AcrB in Escherichia coli plays an important role in drug transport. J Biol Chem 279: 3743–3748 [DOI] [PubMed] [Google Scholar]
- Murakami S, Nakashima R, Yamashita E, Matsumoto T, Yamaguchi A (2006) Crystal structures of a multidrug transporter reveal a functionally rotating mechanism. Nature 443: 173–179 [DOI] [PubMed] [Google Scholar]
- Ott V, Koch J, Späte K, Morbach S, Kramer R (2008) Regulatory properties and Interaction of the C- and N-terminal domains of BetP, an osmoregulated Betaine transporter from Corynebacterium glutamicum. Biochemistry 47: 12208–12218 [DOI] [PubMed] [Google Scholar]
- Peter H, Burkovski A, Krämer R (1998) Osmosensing by N- and C-terminal extensions of the glycine betaine uptake system BetP of Corynebacterium glutamicum. J Biol Chem 273: 2567–2574 [DOI] [PubMed] [Google Scholar]
- Ressl S, Terwisscha van Scheltinga A, Vonrhein C, Ott V, Ziegler C (2009) Molecular basis of transport and regulation in the Na+/betaine symporter BetP. Nature 458: 47–52 [DOI] [PubMed] [Google Scholar]
- Rimon A, Tzubery T, Padan E (2007) Monomers of the NhaA Na+/H+ antiporter of Escherichia coli are fully functional yet dimers are beneficial under extreme stress conditions at alkaline pH in the presence of Na+ or Li+. J Biol Chem 282: 26810–26821 [DOI] [PubMed] [Google Scholar]
- Robertson J, Kolmakova-Partensky L, Miller C (2010) Design, function and structure of a monomeric ClC transporter. Nature 468: 844–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D, Rübenhagen R, Krämer R, Morbach S (2004a) The C-terminal domain of the betaine carrier BetP of Corynebacterium glutamicum is directly involved in sensing K+ as an osmotic stimulus. Biochemistry 43: 5583–5591 [DOI] [PubMed] [Google Scholar]
- Schiller D, Ott V, Krämer R, Morbach S (2006) Influence of membrane composition on osmosensing process of the betaine carrier BetP from Corynebacterium glutamicum. J Biol Chem 281: 7737–7746 [DOI] [PubMed] [Google Scholar]
- Steiner-Mordoch S, Soskine M, Solomon D, Rotem D, Gold A, Yechieli M, Adam Y, Schuldiner S (2008) Parallel topology of genetically fused EmrE homodimers. EMBO J 27: 17–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai CJ, Khafizov KF, Hakulinen J, Forrest LR, Krämer R, Kühlbrandt W, Ziegler C (2011) Structural asymmetry in a trimeric Na(+)/betaine symporter BetP from Corynebacterium glutamicum. J Mol Biol 407: 368–381 [DOI] [PubMed] [Google Scholar]
- Van Dort H, Knowles D, Chasis J, Lee G, Mohandas N, Low P (2001) Analysis of integral membrane protein contributions to the deformability and stability of the human erythrocyte membrane. J Biol Chem 276: 46968–46974 [DOI] [PubMed] [Google Scholar]
- Wittig I, Braun HP, Schägger H (2006) Blue native PAGE. Nat Protoc 1: 418–428 [DOI] [PubMed] [Google Scholar]
- Yamashita A, Singh SK, Kawate T, Jin Y, Gouaux E (2005) Crystal structure of a bacterial homologue of Na+/Cl− dependent neurotransmitter transporters. Nature 437: 215–223 [DOI] [PubMed] [Google Scholar]
- Ziegler C, Morbach S, Schiller D, Krämer R, Tziatzios C, Schubert D, Kühlbrandt W (2004) Projection structure and oligomeric state of the osmoregulated sodium/glycine betaine symporter BetP of Corynebacterium glutamicum. J Mol Biol 337: 1137–1147 [DOI] [PubMed] [Google Scholar]
- Ziegler C, Bremer E, Krämer R (2010) The BCCT family of carriers: from physiology to crystal structure. Mol Microbiol 78: 13–34 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.