Abstract
Trypanosoma brucei imports all mitochondrial transfer RNAs (tRNAs) from the cytosol. By using cell lines that allow independent tetracycline-inducible RNA interference and isopropyl-β-D-thiogalactopyranoside-inducible expression of a tagged tRNA, we show that ablation of Tim17 and mitochondrial heat-shock protein 70, components of the inner-membrane protein translocation machinery, strongly inhibits import of newly synthesized tRNAs. These findings, together with previous results in yeast and plants, suggest that the requirement for mitochondrial protein-import factors might be a conserved feature of mitochondrial tRNA import in all systems.
Keywords: F1–ATP synthase, mitochondrial protein import, mitochondrial tRNA import, parasitic protozoa
Introduction
Mitochondrial genomes of most species lack a variable number of apparently essential mitochondrial transfer RNA (tRNA) genes, which is compensated for by the import of the corresponding cytosolic tRNAs (Salinas et al, 2008). In Saccharomyces cerevisiae, the cytosolic tRNALys is first targeted to mitochondria by the glycolytic enzyme enolase. Subsequently, the tRNA interacts with the precursor of mitochondrial tRNALys synthetase, with which it is co-imported across the protein import pathway (Tarassov et al, 2007). Studies in plants indicate that outer-membrane translocation of tRNAs requires the voltage-dependent anion channel (VDAC), as well as the Tom40 and Tom20 components of the TOM complex, the protein translocase of the outer membrane (Salinas et al, 2006). However, unlike in yeast, tRNAs are not co-imported with proteins. One of the most extreme examples of mitochondrial tRNA import are the trypanosomatids—such as Trypanosoma brucei and Leishmania spp.—the mitochondria of which have lost all tRNA genes and which consequently need to import the entire set of mitochondrial tRNAs. In a controversial study in L. tropica, an inner-membrane protein complex termed RIC—one component of which is the α-subunit of the F1–ATP synthase complex (F1α)—has been implicated in tRNA import (Goswami et al, 2006; Schekman, 2010). In T. brucei, it was shown that binding to cytosolic translation elongation factor 1α (eEF1α) is essential for in vivo import of tRNAs (Bouzaidi-Tiali et al, 2007). Conversely, trypanosomal VDAC is dispensable for normal growth and therefore not required for tRNA import (Pusnik et al, 2009). Thus, all presently known tRNA import factors are well-known house-keeping proteins performing a second function.
Here, we report a new in vivo system for T. brucei, allowing tetracycline (Tet)-inducible RNA interference (RNAi) in combination with isopropyl-β-D-thiogalactopyranoside (IPTG)-inducible expression of a tagged imported tRNA. This system allows monitoring of the fate of a newly synthesized tRNA in cell lines undergoing RNAi, and provides a sensitive assay to test whether a protein is required for tRNA import in vivo. By using this system, we provide evidence that components of the protein translocase of the inner membrane are required for mitochondrial tRNA import in vivo.
Results
A double-inducible in vivo system to study tRNA import
tRNAs have a long half-life. Thus, to devise a system that allows the quantification of tRNA import in cell lines undergoing RNAi, we had to overcome the problem that the ablation of a putative tRNA import factor will primarily affect newly synthesized tRNAs, but not the larger tRNA population that was imported before the induction of RNAi (Bouzaidi-Tiali et al, 2007). The problem was solved by the establishment of a cell line in which RNAi is under the control of the Tet repressor, whereas expression of the tagged tRNA is regulated by the lac repressor (supplementary Fig S1A online). This system can be used to follow the fate of a newly synthesized tRNA in any RNAi cell line. Mitochondrial translation and therefore mitochondrial tRNA import are essential for T. brucei, as a consequence ablation of candidate tRNA import factors is expected to impair growth. To detect import inhibition that is caused by the lack of the ablated protein, the import of the newly synthesized tRNA needs to be analysed at the time of—or shortly after—the appearance of the growth phenotype, which can vary between cell lines. In our system, induction of RNAi and expression of the tagged tRNA are controlled independently, so it is possible to compare tRNA import between RNAi cell lines with different kinetics in the appearance of the growth phenotype. Thus, in all of our RNAi cell lines, IPTG was added 16 h before the cells were analysed by cell fractionation.
Ablation of eEF1α affects tRNA import
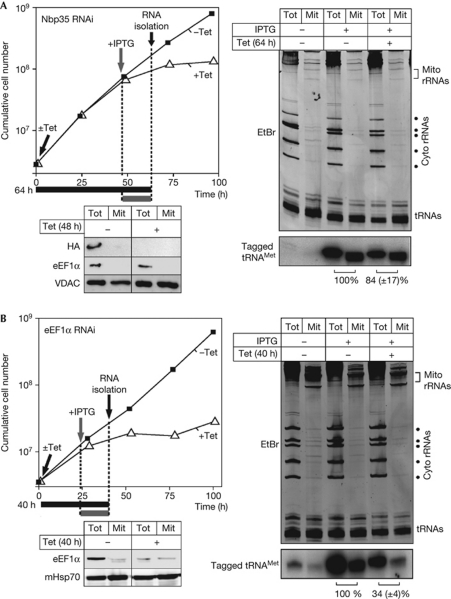
Nbp35 is a protein of the cytosolic FeS-cluster biogenesis pathway that is also required for thiolation of tRNAs (the tagged tRNAMet that is used as an import substrate in the in vivo system is not subject to thiolation). Although TbNbp35 is essential, it is not required for mitochondrial tRNA import and can therefore serve as a negative control (Bruske et al, 2009; Paris et al, 2009). Digitonin extraction was used to isolate mitochondrial RNA from untreated, IPTG-only-, or IPTG- and Tet-treated cultures. The ethidium-bromide stain (Fig 1A, right top panel) shows the quality of the cell fractionation and serves as a loading control. The northern blot analysis in Fig 1A (right bottom panel) indicates that the tagged tRNA is only expressed in the presence of IPTG. Finally, comparing the ratio of tagged tRNA in cytosolic and mitochondrial fractions of Tet-uninduced and Tet-induced TbNbp35 RNAi cell lines shows, as expected, that import of the newly synthesized tRNA is not affected.
Figure 1.
Ablation of eEF1α, but not Nbp35, affects mitochondrial transfer RNA import. (A) Left top panel: growth curve (±Tet) of a representative clonal IPTG- and Tet-inducible Nbp35 RNAi cell line. 1 μg/ml Tet and/or 1 mM IPTG were added before RNA isolation at the time points and for the durations indicated by black and grey arrows and bars, respectively. Left bottom panel: an immunoblot analysis containing total cellular (Tot) and digitonin-purified mitochondrial fractions (Mit) of Tet-uninduced and Tet-induced RNAi cells expressing HA-tagged Nbp35. The blot was probed with anti-sera recognizing the HA tag (HA), eEF1α and VDAC. Right top panel: EtBr stain of total cellular (Tot) and digitonin-extracted mitochondrial RNA fractions (Mit) separated on an 8 M urea and 10% polyacrylamide gel. RNA was isolated from cell cultures, treated as indicated. Right bottom panel: the corresponding northern blot probed for the tagged tRNAMet. Relative import efficiencies of the tagged tRNAMet are indicated at the bottom. The ratio between the signals in the total and the mitochondrial RNA fractions in the culture growing in the absence of Tet was set to 100%. Loading differences between the − and +Tet fractions were corrected for by quantification of the tRNA region on the EtBr stain. Experiments were done in triplicate, the standard error is indicated. (B) As in (A), but analysis was done with an eEF1α RNAi cell line. Cyto, cytosolic; eEF1α, elongation factor 1α; EtBr; ethidium bromide; HA, haemagglutinin; IPTG, isopropyl-β-D-thiogalactopyranoside; mHsp70, mitochondrial heat-shock protein 70; RNAi, RNA interference; rRNA, ribosomal RNA; Tet, tetracycline; tRNA, transfer RNA; VDAC, voltage-dependent anion channel.
The only factor known to be required for mitochondrial tRNA import in T. brucei is cytosolic TbeEF1α (Bouzaidi-Tiali et al, 2007). This protein therefore served as a positive control. Fig 1B shows that import of the newly synthesized tRNA is reduced on induction of RNAi, confirming its role in mitochondrial tRNA import.
F1α and F1β ablation does not affect tRNA import
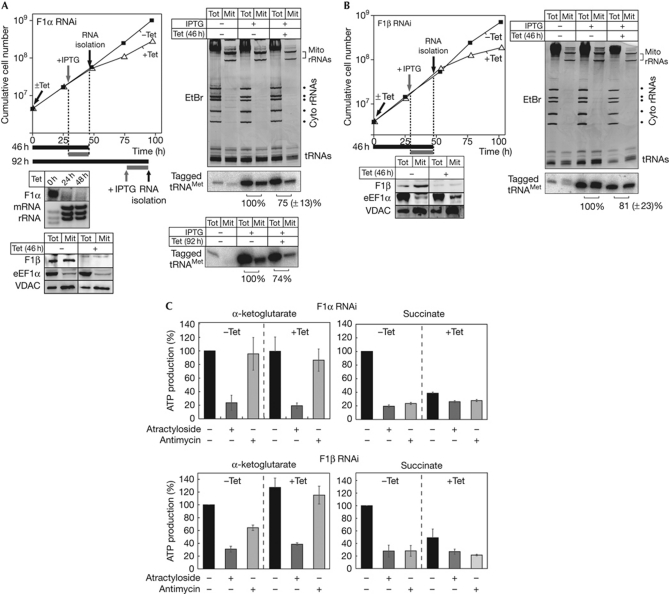
In the first series of experiments, we used our double-inducible system to test whether, as has been claimed in L. tropica, F1α is involved in mitochondrial tRNA import in T. brucei. Fig 2A shows that in T. brucei, import of the newly synthesized tRNA in a Tet-induced TbF1α-RNAi cell line is reduced by 25%. Moreover, a similar reduction (19%) occurred when TbF1β was ablated. This result was expected because—as shown previously (Lefebvre-Legendre et al, 2001) and confirmed in the immunoblot analysis in Fig 2A—ablation of F1α destabilizes F1β.
Figure 2.
Ablation of F1α and F1β does not affect mitochondrial transfer RNA import but impairs oxidative phosphorylation. (A) As in Fig 1, but a F1α-RNAi cell line was analysed. Left middle panel: RNAi-induced ablation of the F1α mRNA was verified by northern blot analysis. The EtBr-stained rRNA region acts as a loading control. Left bottom panel: immunoblot containing total cellular (Tot) and mitochondrial fractions (Mit) of Tet-uninduced and Tet-induced RNAi cells probed with anti-sera against F1β, eEF1a and VDAC. Cells were analysed after 46 and 92 h of Tet induction. (B) As in A but a F1β-RNAi cell line was analysed. (C) In organello, ATP production was determined in digitonin-isolated mitochondria of F1α (top panel) and F1β RNAi cell lines (lower panel). Uninduced cells (−Tet) are shown on the left, and induced cells (+Tet) are shown on the right. The substrate tested is indicated at the top, and the additions of antimycin and atractyloside are shown at the bottom. ATP production in mitochondria isolated from uninduced cells tested without antimycin or atractyloside was set to 100%. The bars represent means expressed as percentages from at least three independent inductions. Standard errors are indicated. Cyto, cytosolic; eEF1α, elongation factor 1α; EtBr; ethidium bromide; IPTG, isopropyl-β-D-thiogalactopyranoside; mRNA, messenger RNA; RNAi, RNA interference; rRNA, ribosomal RNA; Tet, tetracycline; tRNA, transfer RNA; VDAC, voltage-dependent anion channel.
Mitochondrial ATP synthase functions in oxidative phosphorylation. In T. brucei, different modes of mitochondrial ATP production can be measured in an in organello assay (Bochud-Allemann & Schneider, 2002). The results in Fig 2C show that ablation of both TbF1α and TbF1β causes a reduction in oxidative phosphorylation at the time point when import of the newly synthesized tRNA was analysed. Mitochondrial substrate-level phosphorylation was not affected. Thus, the slight reduction of import (21–25%) of the newly synthesized tRNA that is observed in the two cell lines is probably a secondary effect due to lower mitochondrial ATP levels.
T. brucei and L. tropica are closely related. Thus, we expected that their tRNA-import machineries would be similar. Antisense-mediated ablation of L. tropica F1α was reported to lead to complete depletion of mitochondrial tRNAs within 24 h, whereas if F1β was ablated, their level was not affected (Goswami et al, 2006). This is in contrast to the results obtained in T. brucei in which neither ablation of TbF1α nor TbF1β impairs tRNA import. Moreover, dissimilarly to the study in L. tropica, which analysed the steady-state levels of tRNAs, we selectively monitored newly synthesized tRNA, which is the more-sensitive method by which to measure import.
Ablation of Tim17 and mHsp70 inhibits tRNA import
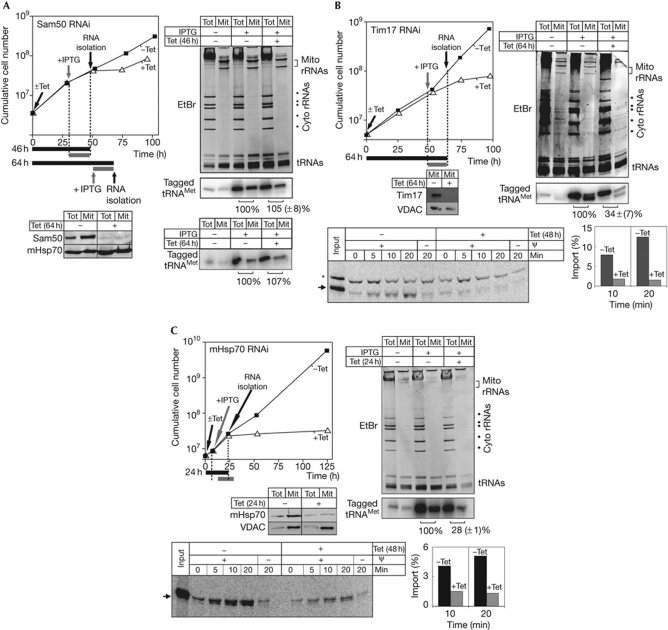
Few components of the mitochondrial protein translocases have been identified in T. brucei (Schneider et al, 2008). They include TbSam50, the core subunit of the SAM complex (Sharma et al, 2010)—which functions to insert β-barrel proteins into the outer membrane—and a single member of the Tim17/22/23 protein family, termed TbTim17 (Gentle et al, 2007; Singha et al, 2008). Finally, the matrix of T. brucei mitochondria contains a highly conserved mitochondrial heat-shock protein 70 (mHsp70; TbmHsp70).
Ablation of Sam50 does not affect the import of the newly synthesized tRNA, even after long Tet induction (Fig 3A). Ablation of TbTim17 and TbmHsp70, however, not only inhibits import of matrix proteins (bottom panels of Fig 3B,C), but also impairs import of the newly synthesized tagged tRNA (right panels Fig 3B,C). As in all experiments, the loading differences between the mitochondrial fractions of the IPTG-only-treated and the IPTG- and Tet-treated samples were corrected before the quantification (see Methods section).
Figure 3.
Ablation of Tim17 and mHsp70, but not of Sam50, affects import of newly synthesized tRNAMet. (A) As in Fig 2, but a Sam50 RNAi cell line was analysed. Left bottom panel: ablation of Sam50 was verified by immunoblot analysis. Cells were analysed after 46 and 64 h of Tet induction. (B) As in A but an IPTG- and Tet-inducible Tim17 RNAi cell line was analysed. Left middle panel: ablation of Tim17 was verified by immunoblot analysis. Left bottom panel: in vitro import of in vitro translated precursor of yeast alcohol dehydrogenase III was assayed in Tet-uninduced and Tet-induced cell lines in the presence and absence of the membrane potential (ψ). The band indicated by the asterisk is a labelled protein that is template-independently synthesized in some batches of reticulocyte lysate. Bottom right: quantification of the efficiency of protein (indicated as percentage of added substrate) import at 10 and 20 min of incubation in uninduced (black bars) and induced (grey bars) RNAi cell lines. (C) As in (B) but a mHsp70 RNAi cell line was analysed. Left middle panel: ablation of the mHsp70 was verified by immunoblot analysis. Left bottom panel: in vitro protein import was assayed as in (B). EtBr; ethidium bromide; IPTG, isopropyl-β-D-thiogalactopyranoside; mHsp70, mitochondrial heat-shock protein 70; Mit, mitochondrial fraction; RNAi, RNA interference; rRNA, ribosomal RNA; Tet, tetracycline; Tot, total cellular fraction; tRNA, transfer RNA; VDAC, voltage-dependent anion channel.
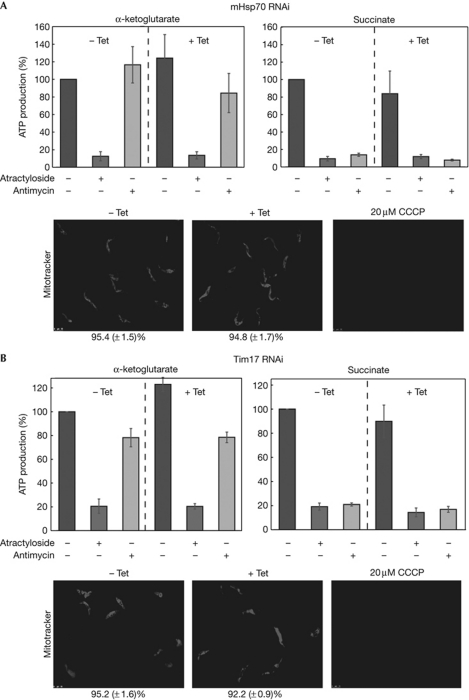
It is important to show that besides tRNA import, mitochondria of induced TbTim17 and TbmHsp70 RNAi are still functional at the time of the analysis. Immunoblot analyses in supplementary Fig S2 online show that the steady-state levels of a panel of imported mitochondrial membrane and matrix proteins are not affected in induced TbTim17 and TbmHsp70 RNAi cell lines. Quantitative analysis of Mitotracker-stained cells indicates that at these early time points the same is true for the membrane potential, as well as for mitochondrial ATP production by substrate-level phosphorylation and oxidative phosphorylation (Fig 4). In summary, these results show that in the TbTim17 and TbmHsp70 RNAi cell lines the steady-state levels of imported proteins are not reduced at the time of the analysis. These experiments, together with the early appearance of the tRNA import phenotype, suggest that TbTim17 and TbmHsp70 are directly involved in tRNA import, although the possibility that inhibition of tRNA import could be caused by the lack of import of a short-lived component of an unknown tRNA import factor cannot be excluded.
Figure 4.
Ablation of Tb-Tim17 and Tb-mHsp70 does not affect mitochondrial ATP production or the membrane potential. Substrate-level phosphorylation and oxidative phosphorylation were determined in digitonin-isolated mitochondria of Tb-Tim17 (A) and Tb-mHsp70 (B) RNAi cell lines. The micrographs show Mitotracker-stained Tet-uninduced and Tet-induced, as well CCCP-treated cells. The percentage of membrane potential positive cells is indicated at the bottom. CCCP, carbonyl cyanide m-chlorophenylhydrazone; mHsp70, mitochondrial heat-shock protein 70; RNAi, RNA interference; Tet, tetracycline.
The involvement of TbTim17 and TbmHsp70 in mitochondrial tRNA import is in contrast to L. tropica, in which RIC—which contains F1α and subunits of the mitochondrial respiratory complexes but lacks Tim17 and mHsp70—was thought to be necessary and sufficient for inner-membrane translocation of tRNAs (Mukherjee et al, 2007). The absence of RIC in T. brucei is also supported by the fact that tRNAs are imported into mitochondria of bloodstream forms of the parasite, which lack respiratory complexes (Cristodero et al, 2010).
Discussion
Our results show that in T. brucei, as shown previously in S. cerevisiae and in plants, there is a connection between tRNA import and mitochondrial protein import. In yeast, the tRNALys is co-imported in complex with a mitochondrial precursor protein (Tarassov et al, 2007). In plants, in vitro import of tRNAs is independent of the addition of cytosolic factors, indicating that the co-import model is not applicable (Delage et al, 2003). However, antibody inhibition assays have shown that as well as VDAC, which probably acts as the tRNA import pore, two components of the plant TOM complex—Tom20 and Tom40—are required for the process (Salinas et al, 2006). In vivo import of tRNAs into T. brucei mitochondria requires TbTim17 and TbmHsp70. As tRNA import into isolated mitochondria works without cytosolic factors, the trypanosomal system seems to be more similar to plants than to S. cerevisiae. However, in vitro import into mitochondria of T. brucei has not been analysed extensively, and it is unclear to what extent it reflects the in vivo situation. For example, there is no consensus on the specificity of the in vitro system (Yermovsky-Kammerer & Hajduk, 1999; Bouzaidi-Tiali et al, 2007). It might therefore be premature to exclude co-import as a mechanism for tRNA import in T. brucei. To distinguish between the direct import and co-import models, further studies are required.
It is both striking and exciting that in yeast, plants and T. brucei—which represent three out of the six eukaryotic supergroups (Simpson & Roger, 2004)—the tRNA import machinery includes components of the mitochondrial-protein import system. Should further experimental work reinforce these findings, the notion that mitochondrial tRNA import has a polyphyletic evolutionary origin might need to be reconsidered.
Methods
Double-inducible expression system. The system is based on the neomycin-resistant T. brucei 427 SiMP single-marker cell line (gift of B. Wickstead and K. Gull, Oxford University, UK). This cell line expresses the T7 RNA polymerase and the Tet repressor, the genes of which have been integrated into the EP-procyclin promoter region. Constitutive expression of the lac repressor was achieved by transfection of the SiMP cell line with pHD359 (gift of C. Clayton, Zentrum für Molekulare Biologie der Universität Heidelberg; ZMBH), which encodes the lac-repressor gene and integrates into the tubulin locus. To construct the IPTG-inducible tRNA expression cassette, we used a derivative of pLew100 in which the 2,296 base-pair (bp) KpnI/BamHI fragment was replaced by a KpnI/BamHI fragment, consisting of the 30 bp lac operator (5′TGTGGAATTGTGAGCGCTCACAATTCCACA3′), followed by a variant tRNAMet gene and 77 bp 3′-flanking region of the wild-type initiator tRNAMet. The variant tRNAMet gene was identical to the one used previously (Bouzaidi-Tiali et al, 2007) and can be detected by specific oligonucleotide hybridization (Crausaz-Esseiva et al, 2004). Subsequent transfection with pLew100-derived stem–loop RNAi constructs (Bochud-Allemann & Schneider, 2002) allows Tet-inducible RNAi and IPTG-inducible expression of the tagged tRNAMet within the same cell line.
Quantification of tRNA import in double-inducible RNAi cell lines. Normalization of loading differences was essential when comparing total and mitochondrial RNA fractions between differentially treated double-inducible RNAi cell lines. The steady-state levels of total and mitochondrial tRNAs were chosen as standards, as they are not expected to change during the early time points after Tet induction, when the import phenotypes were measured. The steady-state levels of total and mitochondrial tRNAs were quantified by densitometric scanning of the ethidium bromide-stained gel, whereby the untreated samples (−Tet and −IPTG) were set to 100%.
Moreover, it was evident that in some cell lines a small fraction of the tagged tRNA was expressed even in the absence of IPTG. This can confound the analysis as it does not represent newly synthesized tRNA. To account for this, the levels of the constitutively expressed tagged tRNA in the uninduced sample (−IPTG and −Tet) were quantified using a Phosphorimager. After normalizations of the loading differences as described above, the obtained signal was subtracted from the signals for the corresponding fractions of the treated samples (+IPTG and −Tet; +IPTG and +Tet).
Finally, in some induced RNAi cell lines (+Tet and +IPTG), less tagged tRNA was transcribed compared with the controls (−Tet and +IPTG). Thus, after correction for loading differences, we determined the ratio of the newly synthesized tRNA between the cytosolic and mitochondrial fractions, which remains constant, independent of how much of the tRNA is expressed (supplementary Fig S1B,C online). The obtained ratio in the uninduced RNAi cell line (−Tet and +IPTG) was then set to 100% and compared with that in the induced RNAi cell line (+Tet and +IPTG).
Inserts for RNAi stem–loop constructs. RNAi cell lines targeting Nbp35, eEF1a and Tim17 have been described previously (Bouzaidi-Tiali et al, 2007; Gentle et al, 2007; Bruske et al, 2009). For the remaining RNAi cell lines, we used the following inserts: a 495 (3–498) bp fragment of the F1α gene (Tb927.7.7420); a 496 (451–947) bp fragment of the F1β gene (Tb927.3.1380); a 452 (1,523–1,975) bp fragment of the mHsp70 gene (Tb927.6.3800); and a 473 (247–720) bp fragment of the Sam50 gene (Tb927.3.4380).
Miscellaneous. ATP production assays (Schneider et al, 2007), digitonin fractionation, RNA isolation, northern blot analysis (Bouzaidi-Tiali et al, 2007) and in vitro protein-import assays (Hauser et al, 1996) were done as described. In vitro translated precursor of yeast alcohol dehydrogenase III was used as an import substrate.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Acknowledgments
We thank B. Wickstead, K. Gull, C. Clayton and P. Englund for plasmids and antibodies. This work was supported by grant 3100A0_121937 from the Swiss National Foundation.
Footnotes
The authors declare that they have no conflict of interest.
References
- Bochud-Allemann N, Schneider A (2002) Mitochondrial substrate level phosphorylation is essential for growth of procyclic Trypanosoma brucei. J Biol Chem 277: 32849–32854 [DOI] [PubMed] [Google Scholar]
- Bouzaidi-Tiali N, Aeby E, Charrière F, Pusnik M, Schneider A (2007) Elongation factor 1a mediates the specificity of mitochondrial tRNA import in T. brucei. EMBO J 26: 4302–4312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruske EI, Sendfeld F, Schneider A (2009) Thiolated tRNAs of Trypanosoma brucei are imported into mitochondria and dethiolated after import. J Biol Chem 284: 36491–36499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crausaz-Esseiva A, Marechal-Drouard L, Cosset A, Schneider A (2004) The T-stem determines the cytosolic or mitochondrial localization of trypanosomal methionyl-tRNAs. Mol Biol Cell 15: 2750–2757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristodero M, Seebeck T, Schneider A (2010) Mitochondrial translation is essential in bloodstream forms of Trypanosoma brucei. Mol Microbiol 78: 757–769 [DOI] [PubMed] [Google Scholar]
- Delage L, Dietrich A, Cosset A, Maréchal-Drouard L (2003) In vitro import of a nuclearly encoded tRNA into mitochondria of Solanum tuberosum. Mol Cell Biol 23: 4000–4012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentle IE et al. (2007) Conserved motifs reveal details of ancestry and structure in the small TIM chaperones of the mitochondrial intermembrane space. Mol Biol Evol 24: 1149–1160 [DOI] [PubMed] [Google Scholar]
- Goswami S, Dhar G, Mukherjee S, Mahata B, Chatterjee S, Home P, Adhya S (2006) A bifunctional tRNA import receptor from Leishmania mitochondria. Proc Natl Acad Sci USA 103: 8354–8359 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Hauser R, Pypaert M, Häusler T, Horn EK, Schneider A (1996) In vitro import of proteins into mitochondria of Trypanosoma brucei and Leishmania tarentolae. J Cell Sci 109: 517–523 [DOI] [PubMed] [Google Scholar]
- Lefebvre-Legendre L, Vaillier J, Benabdelhak H, Velours J, Slonimski PP, di Rago JP (2001) Identification of a nuclear gene (FMC1) required for the assembly/stability of yeast mitochondrial F(1)-ATPase in heat stress conditions. J Biol Chem 276: 6789–6796 [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Basu S, Home P, Dhar G, Adhya S (2007) Necessary and sufficient factors for the import of transfer RNA into the kinetoplast mitochondrion. EMBO Rep 8: 589–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris Z, Rubio MA, Lukes J, Alfonzo JD (2009) Mitochondrial tRNA import in Trypanosoma brucei is independent of thiolation and the Rieske protein. RNA 15: 1398–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusnik M, Charrière F, Mäser P, Waller RF, Dagley MJ, Lithgow T, Schneider A (2009) The single mitochondrial porin of Trypanosoma brucei is the main metabolite transporter in the outer mitochondrial membrane. Mol Biol Evol 26: 671–680 [DOI] [PubMed] [Google Scholar]
- Salinas T, Duchene AM, Delage L, Nilsson S, Glaser E, Zaepfel M, Marechal-Drouard L (2006) The voltage-dependent anion channel, a major component of the tRNA import machinery in plant mitochondria. Proc Natl Acad Sci USA 103: 18362–18367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas T, Duchêne AM, Maréchal-Drouard L (2008) Recent advances in tRNA mitochondrial import. Trends Biochem Sci 33: 320–329 [DOI] [PubMed] [Google Scholar]
- Schekman R (2010) Editorial expression of concern: a bifunctional tRNA import receptor from Leishmania mitochondria. Proc Natl Acad Sci USA 107: 9476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider A, Bouzaidi-Tiali N, Chanez A-L, Bulliard L (2007) ATP production in isolated mitochondria of procyclic Trypanosoma brucei. Methods Mol Biol 372: 379–387 [DOI] [PubMed] [Google Scholar]
- Schneider A, Bursać D, Lithgow T (2008) The direct route: a simplified pathway for protein import into the mitochondrion of trypanosomes. Trends Cell Biol 18: 12–18 [DOI] [PubMed] [Google Scholar]
- Sharma S, Singha UK, Chaudhuri M (2010) Role of Tob55 on mitochondrial protein biogenesis in Trypanosoma brucei. Mol Biochem Parasitol 174: 89–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson AG, Roger AJ (2004) The real ‘kingdoms’ of eukaryotes. Curr Biol 14: R693–R696 [DOI] [PubMed] [Google Scholar]
- Singha UK, Peprah E, Williams S, Walker R, Saha L, Chaudhuri M (2008) Characterization of the mitochondrial inner membrane protein translocator Tim17 from Trypanosoma brucei. Mol Biochem Parasitol 159: 30–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarassov I, Kamenski P, Kolesnikova O, Karicheva O, Martin RP, Krasheninnikov IA, Entelis N (2007) Import of nuclear DNA-encoded RNAs into mitochondria and mitochondrial translation. Cell Cycle 6: 2473–2477 [DOI] [PubMed] [Google Scholar]
- Yermovsky-Kammerer AE, Hajduk S (1999) In vitro import of a nuclearly encoded tRNA into the mitochondrion of Trypanosoma brucei. Mol Cell Biol 19: 6253–6259 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.