Abstract
Behaviour is a consequence of computation in neural circuits composed of massive synaptic connections among sensory neurons and interneurons. The cyclic AMP response element-binding protein (CREB) responsible for learning and memory is expressed in almost all neurons. Nevertheless, we find that the Caenorhabditis elegans CREB orthologue, CRH-1, is only required in the single bilateral thermosensory neuron AFD, for a memory-related behaviour. Restoration of CRH-1 in AFD of CREB-depleted crh-1 mutants rescues its thermotactic defect, whereas restorations in other neurons do not. In calcium-imaging analyses, the AFD neurons of CREB-depleted crh-1 mutants exhibit an abnormal response to temperature increase. We present a new platform for analysing the mechanism of behavioural memory at single-cellular resolution within the neural circuit.
Keywords: thermotaxis, behaviour, CREB, memory
Introduction
Animals remember environmental information and thereby alter their behavioural strategies. Memory-based behaviour is hardwired into neural circuits that comprise many synaptic connections between various types of neuron. The currently held view is that strengthening of synaptic connections underlies behavioural memory, the mechanism of which involves cyclic AMP response element-binding protein (CREB; Kandel, 2001; Greer & Greenberg, 2008). CREB functions as a transcription factor that is expressed in almost all neurons, and has a central role in modification of the strengthening of synaptic connections by regulating several downstream genes in accordance with environmental changes (Carlezon et al, 2005). Thus, the mechanism for behavioural memory has been extensively described in terms of regulation of synaptic strength. By contrast, little is known about the molecular mechanism underlying the establishment of behavioural memory at the single-cellular level, partly because of the difficulty in dissecting the spatiotemporally diverse role of CREB in individual neurons within differentiated and intricate neural networks.
Caenorhabditis elegans thermotaxis (TTX) is a simple model system in which to explore temperature-dependent behavioural memory (Giles et al, 2006; Mori et al, 2007). When C. elegans are cultivated at a point between 15 and 25°C for a few hours with a food source, and then placed on a thermal gradient, most of the animals migrate to the cultivation temperature (Hedgecock & Russell, 1975; Mori & Ohshima, 1995; Mohri et al, 2005). The neural circuit underlying TTX has been identified by a series of laser ablation and genetic experiments (Fig 1A; Mori & Ohshima, 1995; Kuhara et al, 2008; Biron et al, 2008). In the circuit, the single type of bilateral AFD neuron is the best-characterized thermosensory neuron in terms of its behavioural and molecular characteristics (Kimura et al, 2004; Biron et al, 2006; Mori et al, 2007).
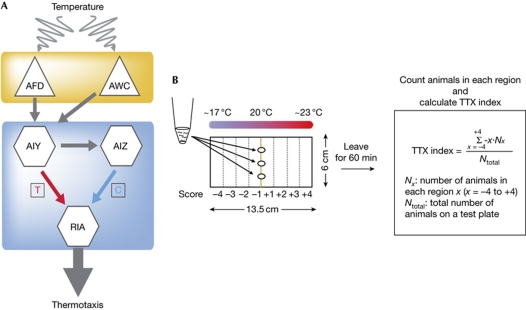
Figure 1.
Diagram of the neural circuit of thermotaxis. (A) In thermotactic neural circuit, temperature is sensed by AFD and AWC thermosensory neurons, both of which synapse onto the AIY interneuron. The activated AIY–RIA accelerates the movement towards a temperature higher than the cultivation temperature (thermophilic drive; T), and the activated AIZ–RIA accelerates the movement towards a temperature lower than the cultivation temperature (cryophilic drive, C). (B) For population assay (Ito et al, 2006), a linear temperature gradient was established along the agar surface of 13.5 cm in the TTX plate (13.5 × 6 cm). The steepness of the temperature gradient was maintained at 0.44°C/cm. Animals were collected and placed at the surface of agar at 20°C and left for 60 min. The TTX plate was divided into eight regions with score −4 to +4, and the TTX index was calculated from the formula shown in the right panel. TTX, thermotaxis.
Recent physiological studies have indicated that the AFD thermosensory neurons perform many complex functions (Clark et al, 2006). Calcium-imaging experiments have shown that when temperature was raised from 15 to 25°C in a step-like or oscillatory manner, the AFD neurons respond to warming that is above a threshold set by their cultivation temperature (Kimura et al, 2004; Biron et al, 2006; Mori et al, 2007). The amount of calcium transient in the sensory ending of AFD is further observable in a cultivation-temperature-dependent manner even after the dendritic process was severed to disconnect the sensory ending from the cell body using a femtosecond laser microbeam (Clark et al, 2006). These observations suggest the possibility that the AFD neurons alone remember the cultivation temperature (Mori et al, 2007). Nevertheless, there is no molecular evidence about the establishment of the temperature memory at the single-cellular level within a neural circuit.
In this study, we reveal that the C. elegans CREB orthologue, CRH-1, functions only in AFD for thermotactic behaviour. CRH-1 loss-of-function mutants exhibited abnormal athermotactic behaviour. Although CRH-1 is broadly expressed (Kimura et al, 2002), expression of CRH-1 only in AFD of crh-1 mutants recovered its behavioural defect, whereas expressions in other neurons that comprise the thermotactic neural circuit and in the ASH polymodal neuron did not rescue the behavioural defect of crh-1(tz2) mutants. Furthermore, expression of the dominant-negative form of CRH-1 (CRH-1DN) in AFD affects the acquisition rate of the ability to migrate towards the new cultivation temperature. In calcium-imaging analysis, AFD of crh-1 mutants responded abnormally to temperature increase. These results suggest that AFD holds the CREB-dependent temperature memory within a neural circuit.
Results
Thermotaxis defect of CREB-depleted crh-1 mutants
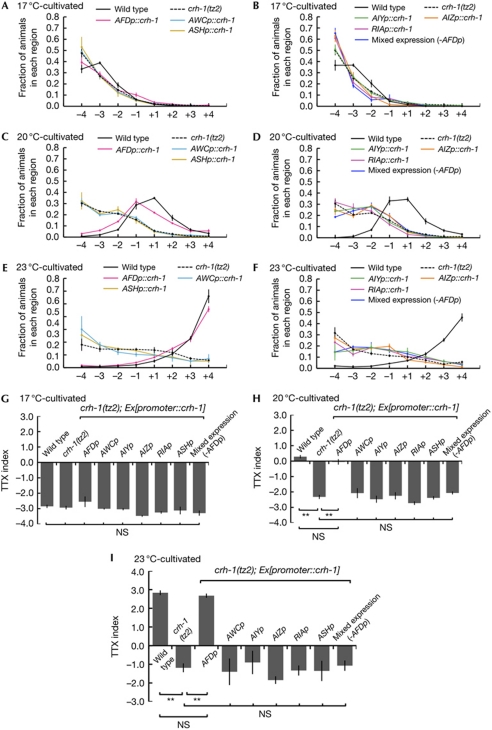
To investigate the role of CRH-1 in TTX, we used the YT3 crh-1(tz2) strain (Kimura et al, 2002) that carries a deletion of 38 amino acids at the carboxy-terminus of the bZIP region of the crh-1 gene. In the quantitative population TTX assay (Fig 1B), 17°C-cultivated crh-1(tz2) mutants migrated to colder regions; this behaviour is similar to 17°C-cultivated wild-type animals (Fig 2A,G). By contrast, 23°C-cultivated crh-1(tz2) mutants showed the dispersed distribution from +4 region on the thermal gradient and had a lower TTX index value, compared with 23°C-cultivated wild-type animals (Figs 1B and 2E,I). When cultivated at 20°C, crh-1(tz2) mutants also migrated towards colder regions than wild-type animals (Fig 2C,H). Thus, crh-1(tz2) mutants tended to migrate to colder regions after cultivation at 23°C or 20°C, in contrast to wild-type animals, suggesting that their thermotactic behavioural phenotype is abnormal.
Figure 2.
Quantitative population thermotaxis assays for crh-1(tz2) mutants. (A–I) Cell-specific rescue experiment for behavioural defect of crh-1(tz2) mutants by population assay. Animals cultivated at 17°C (A,B,G), 20°C (C,D,H) or 23°C (E,F,I) were placed on the surface of agar at 20°C and left for 60 min. Distributions (A–F) and TTX indices (G–I) are shown for wild-type animals, crh-1(tz2) mutants and crh-1(tz2) mutants expressing crh-1 cDNA in sensory neurons (A,C,E), interneurons (B,D,F) and AWC, AIY, AIZ and RIA neurons (B,D,F). The crh-1 cDNA is expressed in each indicated neuron at a concentration of 2 ng/μl. A minimum of two lines were assayed for each transgenic strain. Assays for each transgenic strain were carried out at least three times. The statistically significant differences of thermotactic behaviour were determined by Bonferroni's post hoc test for multiple comparisons. Double asterisk indicates P<0.01. Error bar indicates s.e.m. cDNA, complementary DNA; NS, not significant; TTX, thermotaxis.
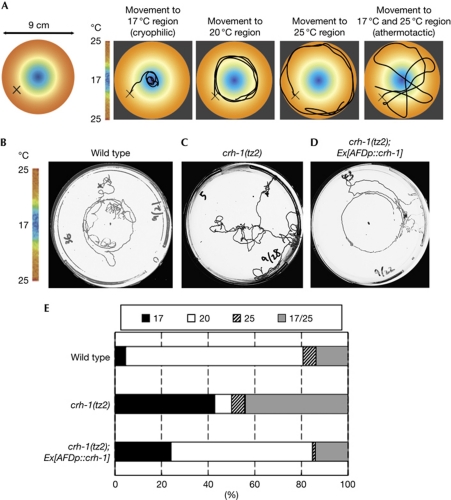
The behavioural defect of crh-1(tz2) mutants resembles that of AFD-ablated animals, as revealed previously by the qualitative single-worm assay (Mori & Ohshima, 1995). In the qualitative assay, wild-type animals migrated to the cultivation temperature and then showed isothermal-tracking behaviour, moving isothermally on a radial thermal gradient (Fig 3A,B; Hedgecock & Russell, 1975; Mori & Ohshima, 1995; Mohri et al, 2005). By contrast, the 20°C-cultivated AFD-ablated animals had two abnormal behavioural phenotypes: cryophilic movement is to migrate to colder regions than wild-type animals and athermotactic movement is random movement on the thermal gradient (Fig 3A). To assess isothermal-tracking behaviour, we performed the qualitative single-worm assay. Whereas 20°C-cultivated wild-type animals moved isothermally around the 20°C region (Fig 3B,E), crh-1(tz2) mutants exhibited the cryophilic and/or athermotactic movements (Fig 3C,E). Thus, the behavioural phenotype of crh-1(tz2) mutants observed in quantitative and qualitative assays suggest that CRH-1 function is important for AFD.
Figure 3.
Qualitative single-worm assays for crh-1 mutants. (A–D) Single-animal TTX assays of wild-type animals (B), crh-1(tz2) mutants (C) and crh-1(tz2) mutants expressing crh-1 complementary DNA (2 ng/μl) (D) in AFD neurons, using a radial temperature gradient. The L4 larvae were cultivated with a food source at 20°C for 8–12 h. A single animal was placed on X-indication on the assay plate (9 cm Petri dish). A 45-ml glass vial (Wheaton) containing frozen acetic acid was used, in which adult animals were allowed to move freely for 60 min on the assay plate. TTX of individual animals was evaluated using four phenotypic categories, as described in A. Animals that moved to the blue area, yellow area or orange area were classified as 17, 20 or 25, respectively. Animals that moved randomly, or to both 17 and 25°C regions, were classified as 17/25. (E) The spectra of TTX phenotypes of wild-type animals, crh-1(tz2) mutants and crh-1(tz2) mutants expressing crh-1 in AFD. Each animal was cultivated at 20°C before TTX assays. Representative tracks of each animal and the phenotypic categories are described in A. For each assay, at least 60 animals were individually assayed on a radial thermal gradient. TTX, thermotaxis.
Identification of the site of action of CRH-1
Given the behavioural abnormality of crh-1(tz2) mutants, we attempted to identify the cells in which CRH-1 is required for TTX. The SAGE database search indicated that CRH-1 is expressed in AFD (http://elegans.bcgsc.bc.ca/). Thus, we initially constructed the crh-1(tz2) mutants expressing crh-1 complementary DNA specifically in AFD under gcy-8 promoter, and performed population TTX assay (Fig 2; supplementary Fig S1 online). Strikingly, after cultivation at each temperature, the crh-1(tz2) mutants expressing crh-1 in AFD only showed normal thermotactic migration, similar to wild-type animals (Fig 2A,C,E,G–I). Thus, expression of CRH-1 in AFD restores the behavioural defect of crh-1(tz2) mutants. Furthermore, the qualitative single-worm assay also indicated that the crh-1(tz2) mutants expressing crh-1 in AFD moved isothermally around the 20°C region as observed in wild-type animals (Fig 3D,E), suggesting that expression of crh-1 in AFD also recovers isothermal-tracking behaviour. We expressed crh-1 complementary DNA in each neuron that comprises the thermotactic neural circuit or the ASH polymodal neuron (Fig 1A; Roayaie et al, 1998). In contrast to the clear behavioural restoration by AFD expression, expressions of crh-1 in AWC, AIY, AIZ, RIA or ASH neurons (Fig 1A) could not rescue the behavioural defect of crh-1(tz2) mutants (Fig 2). We therefore suggest that CRH-1 is required in the thermosensory neuron AFD, but not in other neurons, for TTX.
Effect of expression of CRH-1DN in AFD
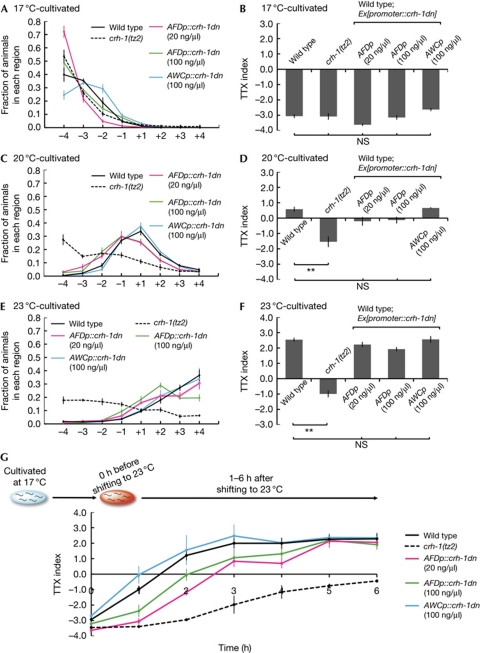
We constructed the wild-type animals expressing CRH-1DN, which carries a point mutation in its phosphorylation site Ser 29, to specifically inhibit endogenous CRH-1 function (supplementary Fig S1B online). When cultivated at 17°C, 20°C and 23°C, the wild-type animals expressing CRH-1DN in AFD or AWC neurons at the injected concentration of 100 ng/μl had normal thermotactic behaviour (Fig 4A–F). We thus performed the time-course assay by shifting the cultivation temperature from 17 to 23°C (Fig 4G). As shown previously, the time-course assay enabled us to estimate the ability to acquire temperature memory (Gomez et al, 2001; Biron et al, 2006). Wild-type animals expressing CRH-1DN in AFD neurons migrated towards cultivation temperatures (17°C or 23°C) before and at 5 h after the temperature change; however, they migrated to the colder region in contrast to wild-type animals up to 4 h after the temperature change (Fig 4G). In particular, at 3 and 4 h after the temperature change, wild-type animals almost completed acquisition of the ability to migrate towards the new cultivation temperature, whereas animals expressing CRH-1DN in AFD neurons did not. These results indicate that although wild-type animals expressing CRH-1DN in AFD neurons could acquire the ability to migrate towards the cultivation temperature at 5 h after the temperature change, this acquisition was slower than in wild-type animals, as previously observed in other memory-impaired mutants, such as ncs-1 and dgk-3 mutants (Gomez et al, 2001; Biron et al, 2006). By contrast, wild-type animals expressing CRH-1DN in AWC neurons showed thermotactic behaviour similar to wild-type animals after the temperature change, although wild-type animals expressing CRH-1DN in AFD neuron—even at the diluted injected concentration (20 ng/μl)—also acquired this ability more slowly (Fig 4G). These results suggest that endogenous CRH-1 functions in AFD neurons, and that it is important for the acquisition rate of the ability to migrate to the new cultivation temperature.
Figure 4.
Effect of expression of dominant-negative form of CRH-1 in AFD neurons. Population TTX assays of animals cultivated at 17°C (A,B), 20°C (C,D) or 23°C (E,F) from birth. CRH-1DN is expressed in AFD neurons at the injected concentration of 20 or 100 ng/μl, and in AWC neurons at the injected concentration of 100 ng/μl. The statistically significant differences in thermotactic behaviour were determined by Bonferroni's post hoc test for multiple comparisons. n=3. Error bar indicates s.e.m. (G) Time-course assay of wild-type animals and crh-1(tz2) mutants and wild-type animals expressing CRH-1DN in AFD or AWC, by using a quantitative population TTX assay. Cultivation temperature was shifted from 17 to 23°C. The population TTX assay was performed just before shifting the cultivation temperature (0 h) and at every 1 h after shifting the temperature. Assays were performed three times. Double asterisk indicates P<0.01. Error bar indicates s.e.m. CRH-1DN, dominant-negative form of CRH-1; NS, not significant; TTX, thermotaxis.
Requirement of CRH-1 for calcium transient of AFD
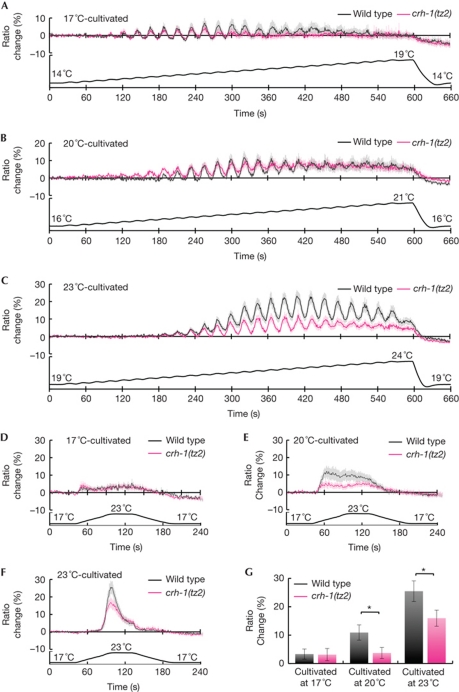
To physiologically examine the possibility that CRH-1 functions in AFD neurons, we performed calcium imaging by directly monitoring stimulus-evoked calcium-concentration change in AFD neurons of 23°C-cultivated wild-type animals and crh-1(tz2) mutants with a genetically encoded calcium indicator cameleon (supplementary Fig S2 online). To investigate the responsive temperature range of AFD neurons in 17°C-, 20°C- and 23°C-cultivated crh-1(tz2) mutants, temperature was moderately raised from 14.5, 17.1 and 20.3°C, respectively, in an oscillatory manner (Biron et al, 2006; Clark et al, 2006). When cultivated at 17°C, 20°C and 23°C, the responsive temperature ranges of AFD neurons in crh-1(tz2) mutants were similar to those in wild-type animals (Fig 5A–C). However, the ratio changes of AFD neurons in 23°C-cultivated crh-1(tz2) mutants are lower than those in 23°C-cultivated wild-type animals (Fig 5C). Thus, we also observed the magnitude of the response of AFD neurons in crh-1(tz2) mutants to the transient up-and-down temperature changes, as reported previously (Fig 5D–G; Kimura et al, 2004; Kuhara et al, 2008; Chatzigeorgiou et al, 2010). The magnitude of calcium-concentration changes of AFD neurons in 17°C-cultivated crh-1(tz2) mutants is similar to that in 17°C-cultivated wild-type animals (Fig 5D,G), and this result is consistent with behavioural observation (Fig 2A,G) in which there is no difference between wild-type animals and crh-1(tz2) mutants. By contrast, when cultivated at 20°C and 23°C, the magnitude of calcium-concentration changes of AFD neurons in crh-1(tz2) mutants is significantly lower than that in wild-type animals (Fig 5E–G). Given that the calcium-transient level correlates with neural activity (Kerr et al, 2000; Suzuki et al, 2003; Kuhara et al, 2008), the attenuation of calcium-concentration changes reflects the abnormal neural activity, which might cause the behavioural defect of crh-1(tz2) mutants. Together with the results from calcium imaging, this suggest that CRH-1 regulates the function of AFD neurons by affecting the magnitude of its calcium-concentration change, although the responsive temperature range does not seem to be defective in crh-1(tz2) mutants. The impairment in this calcium-concentration change of AFD neurons, which probably reflects abnormal neuronal activity, might cause the abnormal TTX.
Figure 5.
Calcium imaging of AFD neurons in crh-1(tz2) mutants. Calcium imaging of AFD neurons of 17°C- (A), 20°C- (B) or 23°C-cultivated (C) wild-type animals and crh-1(tz2) mutants during oscillatory warming. Temperature stimuli are shown as a black line at the bottom. The average YFP/CFP fluorescence ratio changes of the cameleon protein in AFD neurons were measured for wild-type animals (black) and crh-1(tz2) mutants (red). n⩾10. Error bar indicates s.e.m. Calcium imaging of AFD neurons of 17°C- (D), 20°C- (E) or 23°C-cultivated (F) wild-type animals and crh-1(tz2) mutants during the transient up-and-down temperature change. Temperature stimuli are shown as a black line at the bottom. The ratio changes of the cameleon protein in AFD neurons were measured for wild-type animals (black) and crh-1(tz2) mutants (red). The maximum ratio change of AFD was indicated as a bar graph in G, and the statistically significant differences between wild-type animals and crh-1(tz2) mutants were determined by t-tests. Single asterisk indicates P<0.05. n⩾13 animals. Error bar indicates s.e.m. CFP, cyan fluorescent protein; YFP, yellow fluorescent protein.
Discussion
Previous studies in diverse model organisms revealed that CREB is broadly expressed in the nervous system and that its biological function is a characteristic of memory and learning (Kandel, 2001; Greer & Greenberg, 2008). We now provide evidence that restoration of C. elegans CREB orthologue in one neuron recovers the CREB-dependent behavioural defect. C. elegans is an ideal model organism in which to define molecular mechanisms, because of the ease with which genetic manipulations and cell-specific experiments can be conducted (Giles et al, 2006). Our findings therefore represent a promising resource with which to investigate CREB-dependent behavioural memory at the single-cell level within the intact circuit.
Decades of research on the mechanisms of memory have led to the prevailing view that memories are stored through modification of synaptic strength (Benito & Barco, 2010). Several recent studies, however, have highlighted the new role of CREB and revealed that memories can also be stored by non-synaptic processes, which are expressed as changes in intrinsic neuronal excitability (Benito & Barco, 2010). Our calcium-imaging analysis indicates that calcium transient in AFD neurons is attenuated in crh-1(tz2) mutants (Fig 5). As calcium transient correlates with neural activity (Kerr et al, 2000; Suzuki et al, 2003; Kuhara et al, 2008), our calcium-imaging analysis suggests that CRH-1 might regulate the neural activity of AFD neurons. Furthermore, although our behavioural analyses using CRH-1DN support the notion that expression of CRH-1 in AFD neurons contributes to memory acquisition, CRH-1 might also regulate the thermosensation of AFD neurons. Therefore, the relationship between thermosensation and memory should be examined through the analysis of the role of CRH-1 in AFD neurons.
There are several potential avenues through which to investigate the way in which the behavioural memory of AFD neurons is established by CRH-1. Calcium-imaging analyses raise the possibility that CRH-1 regulates ion channels involved in calcium transient of AFD neurons through their transcriptions or indirectly through downstream genes, in accordance with the change of cultivation temperature. Earlier studies indicated that the two cyclic nucleotide-gated cation channels, TAX-2 and TAX-4, have an essential role in thermotactic behaviour (Coburn & Bargmann, 1996; Komatsu et al, 1996). Furthermore, the temperature-evoked calcium transient of AFD neurons requires TAX-4 channel (Kimura et al, 2004; Ramot et al, 2008). Thus, one possibility is that CRH-1 could regulate the TAX-2 or TAX-4 channels through direct or indirect processes, and such a regulatory mechanism might underlie the memory formation in AFD neurons.
It might be interesting to investigate the CREB-dependent expression changes by using microarray analysis, because unknown genes downstream of CRH-1 might function as regulators for calcium concentration. Genetic analysis and calcium-imaging analysis of these genes in the context of AFD regulation could provide mechanistic insights into behavioural memory formation. Overall, our findings present an opportunity to determine the molecular basis for behavioural memory at the single-cellular resolution within intact neural circuits.
Methods
For DNA construction, behavioural assay and calcium-imaging procedures, see supplementary information online and references.
Strain preparation. All C. elegans strains used in this study were maintained and handled using standard methods (Brenner, 1974). We used the wild-type N2 Bristol strain and YT17 crh-1(tz2) III strain. Germline transformations were performed as described (Mello et al, 1991). For cell-specific rescue experiments, coinjection mixes consisted of pKDK66 ges-1p∷nls–gfp (nls, nuclear localizaton signal; gfp, green fluorescent protein; 50 ng/μl) and experimental DNA at the concentration of 2 ng/μl. For expression of CRH-1DN in AFD or AWC neurons of wild-type animals, pNYU20 gcy8p∷crh-1dn (20 or 100 ng/μl) and pNYU21 ceh-36p∷crh-1dn (100 ng/μl) were coinjected with pKDK66 (50 ng/μl).
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Acknowledgments
We thank A. Fire for sharing pPD plasmids; Caenorhabditis Genetic Center, C. elegans Knockout Consortium for strains. I.M. is a Scholar of the Institute for Advanced Research in Nagoya University, Japan. SAGE data were obtained from the Genome BC C. elegans Gene Expression Consortium (http://elegans.bcgsc.bc.ca/). These data were produced at the Michael Smith Genome Sciences Centre with funding from Genome Canada. This work was supported by CREST, the Japan Science and Technology Agency and in part by a Grant-in-Aid for Scientific Research from MEXT, Japan (to I.M.).
Author contributions: Y.N., T.S. and I.M. designed the research; Y.N., T.S. and M.N. conducted the experiments and analysed the data; and Y.N., T.S. and I.M. wrote the paper.
Footnotes
The authors declare that they have no conflict of interest.
References
- Benito E, Barco A (2010) CREB's control of intrinsic and synaptic plasticity: implications for CREB-dependent memory models. Trends Neurosci 33: 230–240 [DOI] [PubMed] [Google Scholar]
- Biron D, Shibuya M, Gabel C, Wasserman SM, Clark DA, Brown A, Sengupta P, Samuel AD (2006) A diacylglycerol kinase modulates long-term thermotactic behavioral plasticity in C. elegans. Nat Neurosci 9: 1499–1505 [DOI] [PubMed] [Google Scholar]
- Biron D, Wasserman S, Thomas JH, Samuel AD, Sengupta P (2008) An olfactory neuron responds stochastically to temperature and modulates Caenorhabditis elegans thermotactic behavior. Proc Natl Acad Sci USA 105: 11002–11007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S (1974) The genetics of Caenorhabditis elegans. Genetics 77: 71–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA Jr, Duman RS, Nestler EJ (2005) The many faces of CREB. Trends Neurosci 28: 436–445 [DOI] [PubMed] [Google Scholar]
- Chatzigeorgiou M et al. (2010) Specific roles for DEG/ENaC and TRP channels in touch and thermosensation in C. elegans nociceptors. Nat Neurosci 13: 861–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DA, Biron D, Sengupta P, Samuel AD (2006) The AFD sensory neurons encode multiple functions underlying thermotactic behavior in Caenorhabditis elegans. J Neurosci 26: 7444–7451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coburn CM, Bargmann CI (1996) A putative cyclic nucleotide-gated channel is required for sensory development and function in C. elegans. Neuron 17: 695–706 [DOI] [PubMed] [Google Scholar]
- Giles AC, Rose JK, Rankin CH (2006) Investigations of learning and memory in Caenorhabditis elegans. Int Rev Neurobiol 69: 37–71 [DOI] [PubMed] [Google Scholar]
- Gomez M et al. (2001) Ca2+ signaling via the neuronal calcium sensor-1 regulates associative learning and memory in C. elegans. Neuron 30: 241–248 [DOI] [PubMed] [Google Scholar]
- Greer PL, Greenberg ME (2008) From synapse to nucleus: calcium-dependent gene transcription in the control of synapse development and function. Neuron 59: 846–860 [DOI] [PubMed] [Google Scholar]
- Hedgecock EM, Russell RL (1975) Normal and mutant thermotaxis in the nematode Caenorhabditis elegans. Proc Natl Acad Sci USA 72: 4061–4065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H, Inada H, Mori I (2006) Quantitative analysis of thermotaxis in the nematode Caenorhabditis elegans. J Neurosci Methods 154: 45–52 [DOI] [PubMed] [Google Scholar]
- Kandel ER (2001) The molecular biology of memory storage: a dialogue between genes and synapses. Science 294: 1030–1038 [DOI] [PubMed] [Google Scholar]
- Kerr R et al. (2000) Optical imaging of calcium transients in neurons and pharyngeal muscle of C.elegans. Neuron 26: 583–594 [DOI] [PubMed] [Google Scholar]
- Kimura KD, Miyawaki A, Matsumoto K, Mori I (2004) The C. elegans thermosensory neuron AFD responds to warming. Curr Biol 14: 1291–1295 [DOI] [PubMed] [Google Scholar]
- Kimura Y et al. (2002) A CaMK cascade activates CRE-mediated transcription in neurons of Caenorhabditis elegans. EMBO Rep 3: 962–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu H, Mori I, Rhee JS, Akaike N, Ohshima Y (1996) Mutations in a cyclic nucleotide-gated channel lead to abnormal thermosensation and chemosensation in C. elegans. Neuron 17: 707–718 [DOI] [PubMed] [Google Scholar]
- Kuhara A, Okumura M, Kimata T, Tanizawa Y, Takano R, Kimura KD, Inada H, Matsumoto K, Mori I (2008) Temperature sensing by an olfactory neuron in a circuit controlling behavior of C. elegans. Science 320: 803–807 [DOI] [PubMed] [Google Scholar]
- Mello CC, Kramer JM, Stinchcomb D, Ambros V (1991) Effifient gene transfer in C. elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J 10: 3959–3970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohri A, Kodama E, Kimura KD, Koike M, Mizuno T, Mori I (2005) Genetic control of temperature preference in the nematode Caenorhabditis elegans. Genetics 169: 1437–1450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori I, Ohshima Y (1995) Neural regulation of thermotaxis in Caenorhabditis elegans. Nature 376: 344–348 [DOI] [PubMed] [Google Scholar]
- Mori I, Sasakura H, Kuhara A (2007) Worm thermotaxis: a model system for analyzing thermosensation and neural plasticity. Curr Opin Neurobiol 17: 712–719 [DOI] [PubMed] [Google Scholar]
- Ramot D, MacInnis BL, Goodman MB (2008) Bidirectional temperature-sensing by a single thermosensory neuron in C. elegans. Nat Neurosci 11: 908–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roayaie K, Crump JG, Sagasti A, Bargmann CI (1998) The G alpha protein ODR-3 mediates olfactory and nociceptive function and controls cilium morphogenesis in C. elegans olfactory neurons. Neuron 20: 55–67 [DOI] [PubMed] [Google Scholar]
- Suzuki H et al. (2003) In vivo imaging of C. elegans mechanosensory neurons demonstrates a specific role for the MEC-4 channel in the process of gentle touch sensation. Neuron 39: 1005–1017 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.