Abstract
Parkinson’s disease is the most common neuro-degenerative movement disorder. α-Synuclein is a small synaptic protein that has been linked to familial Parkinson’s disease (PD) and is also the primary component of Lewy bodies, the hallmark neuropathology found in the brain of sporadic and familial PD patients. The function of α-synuclein is currently unknown, although it has been implicated in the regulation of synaptic vesicle localization or fusion. Recently, overexpression of α-synuclein was shown to cause cytoplasmic vesicle accumulation in a yeast model of α-synuclein toxicity, but the exact role α-synuclein played in mediating this vesicle aggregation is unclear. Here, we show that α-synuclein induces aggregation of many yeast Rab GTPase proteins, that α-synuclein aggregation is enhanced in yeast mutants that produce high levels of acidic phospholipids, and that α-synuclein colocalizes with yeast membranes that are enriched for phosphatidic acid. Significantly, we demonstrate that α-synuclein expression induces vulnerability to perturbations of Ypt6 and other proteins involved in retrograde endosome–Golgi transport, linking a specific trafficking defect to α-synuclein phospholipid binding. These data suggest new pathogenic mechanisms for α-synuclein neurotoxicity.
Keywords: Parkinson’s disease, Alpha-synuclein, Neurodegenerative disease, Vesicle trafficking, Rab GTPase, Yeast
Introduction
Parkinson’s disease (PD) is characterized clinically by bradykinesia, resting tremor, stooped posture, and rigidity (Galvin et al. 2001). Lewy bodies (LBs), the hallmark lesions found in the brain of patients with PD, are composed of α-synuclein amyloid fibrils (Spillantini et al. 1997), and α-synuclein can form similar fibrils in vitro in a time and concentration-dependent manner (Conway et al. 1998; Giasson et al. 1999; Wood et al. 1999). The identification of point mutations, i.e. A53T, A30P, and E46K, as well as duplications and triplications in the α-synuclein gene in early onset familial PD (Polymeropoulos et al. 1997; Kruger et al. 1998; Singleton et al. 2003; Chartier-Harlin et al. 2004; Zarranz et al. 2004) support a pivotal role for α-synuclein in the etiology and pathogenesis of PD. For example, mutations associated with early onset familial PD accelerate in vitro fibril formation (Conway et al. 1998; Li et al. 2002; Greenbaum et al. 2005), and transgenic mice overexpressing mutant A53T α-synuclein developed motoric phenotypes and LB-like inclusions (Giasson et al. 2002; Lee et al. 2002). These observations suggest that α-synuclein is directly involved with the pathogenesis of PD and strongly implicates fibril formation as a pathogenic mechanism.
α-Synuclein is a 140 amino acid protein of unknown function that localizes to synaptic vesicles in rat brain (Jakes et al. 1994; Iwai et al. 1995). The N terminus of α-synuclein contains six imperfect repeats of the KTKEGV motif which resembles the lipid binding domain of apolipoproteins (Segrest et al. 1992) and adopts an α-helical structure that facilitates membrane binding when in the presence of acidic phospholipids (Jensen et al. 1998; Perrin et al. 2000; Jao et al. 2004; Kubo et al. 2005; Kim et al. 2006; Stockl et al. 2008). Although α-synuclein knockout mice are viable with no abnormal brain morphology, these animals exhibit reduced levels of striatal dopamine and an attenuated response to amphetamine (Abeliovich et al. 2000; Yavich et al. 2004). Moreover, cultured hippocampal neurons from α-synuclein knockout mice have a decreased reserve pool of synaptic vesicles, as well as a decrease in the number of docked vesicles (Cabin et al. 2002). These data suggest that α-synuclein regulates synaptic vesicle trafficking at the synapse. Consistent with these observations, α-synuclein overexpression in PC12 cells results in an increase in docked vesicles at the synapse, further supporting the role of α-synuclein in vesicle tethering to the presynaptic plasma membrane (Larsen et al. 2006). In this regard, α-synuclein compensates for the loss of cysteine string protein-α (CSPα), a synaptic chaperone, and rescues deficits in SNARE complex assembly in CSPα knockout mice (Chandra et al. 2005) and antagonizes SNARE function in mammalian cells (Thayanidhi et al. 2010). The relationship between the ability of α-synuclein to regulate the synaptic vesicle cycle and the progression of human disease is currently unknown.
We previously reported a vesicle accumulation phenotype in Saccharomyces cerevisiae overexpressing human α-synuclein (Soper et al. 2008). This morphological phenotype is accompanied by cellular toxicity and disruption of Golgi organization and requires the integrity of both the α-synuclein N-terminal and central hydrophobic regions (Volles and Lansbury 2007; Soper et al. 2008). The accumulated vesicular structures contain membrane-associated α-synuclein and sequester the yeast Rab GTPase proteins Ypt1 and Sec4 (Gitler et al. 2008; Soper et al. 2008). Because of the suspected role of α-synuclein in regulation of the synaptic vesicle cycle, and the known involvement of Rab GTPases as regulators of vesicle tethering and fusion, we further characterized this vesicle aggregation phenotype. We now demonstrate that functional ablation of the Rab GTPase Ypt6, as well as of other endosomal Rab GTPases, cause a significant increase in vesicle aggregation and toxicity. Furthermore, we demonstrate that yeast cells expressing α-synuclein are sensitive to disruption of endosome–Golgi retrograde trafficking pathways and that this sensitivity is correlated with enhanced vesicle accumulation and toxicity. Finally, we demonstrate α-synuclein associates with phosphatidic acid-rich membranes in yeast, and yeast mutants that accumulate acidic phospholipids show a more severe vesicle clustering phenotype. Taken together, these findings are consistent with the idea that α-synuclein associates with Golgi-endosomal membranes in a phosphatidic acid-sensitive manner and that excessive membrane binding by α-synuclein interferes with specific vesicle trafficking pathways with resulting cell toxicity.
Materials and Methods
Yeast Strains and Media
The strains CTY182 (MATa, ura3-52, Δhis3-200, lys2-801am), CTY1-1A (MATa, ura3-52, Δhis3-200, lys2-801am, sec14-1ts), CTY159 (MATa, ura3-52, Δhis3-200, lys2-801am, sec14-1ts, kes1-1), and CTY160 (MATa, ura3-52, Δhis3-200, lys2-801am, sec14-1ts, cki1-1) were used for phospholipid studies. The BY4741 (MATa, his3Δ1, leu2Δ0, met15Δ0, ura3Δ0) and its associated deletion mutants (Brachmann et al. 1998) were used for all other experiments in this study. Transformation of yeast was performed using a standard lithium polyethylene glycol transformation procedure (Gietz et al. 1997). Yeast cultures were grown in selective minimal media, containing 2% glucose or 2% galactose, deficient in the required amino acids. For induction of gene expression, cells were cultured in glucose media overnight, and cells from this culture were taken to inoculate a second culture (starting A600=0.1) in galactose media, which was then grown at 30°C for 16 h.
Plasmids and Constructs
Integrative α-synuclein-EGFP constructs were generated as previously described (Soper et al. 2008). Briefly, integrative strains were generated by PCR amplification of the Gal1 promoter, α-syn-EGFP protein sequence, transcription terminator, and selection marker sequence out of the PYES2 vector. Primer sequences were chosen for directed integration into the yeast genome 500 bp upstream of the Gal1 promoter. N-terminal RFP-tagging vectors were generated by PCR cloning of the mcherry RFP (Shaner et al. 2004) sequence with ablation of the stop codon into the pRS 415 vector (ATCC) with either the Ypt1, alcohol dehydrogenase (ADH), or glyceraldehydes-3-phosphate dehydrogenase promoter sequences. Yeast Rab GTPases were PCR cloned and inserted in frame downstream of the RFP sequence. All vectors were sequenced and confirmed before use. The P423 TEF RFP-Spo2051–91 vector (Nakanishi et al. 2004) was kindly donated by Dr. Aaron Neiman (Department of Biochemistry and Cell Biology, Stony Brook University, Stony Brook, NY 11794, USA).
Phosphatidic Acid Quantification
Yeast cell cultures were incubated under the appropriate conditions in selective minimal media, pelleted, washed twice with ice-cold water, and the pellets containing 5×106 cells flash frozen in liquid nitrogen. Phospholipids were extracted from the cell pellets, prepared for analysis, and phosphatidic acid quantified by mass spectrometry as described (Ivanova et al. 2007). Data were normalized against an internal phosphatidic acid standard marked by its odd number acyl-chain carbon property (Ivanova et al. 2007).
Growth Assay
Growth was measured on solid media by making serial five-fold dilutions of log-phase cultures, starting with A600 =1.0. Samples of each dilution were spotted onto minimal media glucose and galactose plates, with appropriate amino acids for selection. Plates were incubated at 30°C for 2–4 days.
Immunoblot Analysis
Cell extracts were prepared as described previously (Kushnirov 2000). Briefly, cells were grown in galactose media to log-phase. Equal amounts of cells were pelleted by centrifugation, resuspended in one volume water and treated with one volume 0.2 M NaOH for 10 min. Cells were pelleted again, resuspended in SDS/PAGE buffer, and boiled for 5 min and protein samples were separated by SDS/PAGE. For immunoblotting, we used LB509, an anti-α-syn mouse mAb recognizing residues 115–122 (Jakes et al. 1999), SNL-1, an affinity-purified rabbit polyclonal α-synuclein antibody raised to amino acid residues 104–119 of α-syn (Giasson et al. 2000), monoclonal and polyclonal DsRed antibodies (Clontech), and 6C5, a monoclonal GAPDH antibody (Advanced ImmunoChemical Inc.).
Fluorescence Microscopy and Quantification
Cells were grown in galactose minimal media for 16 h and fixed in 4% paraformaldehyde in 40 mM phosphate buffer for 1 h at 30°C. Cells were then washed twice in 40 mM phosphate buffer and mounted on PDL coated dishes for microscopy. Images were captured on a Nikon TE-2000-E (Nikon, Japan) inverted epifluorescence microscope. Typically, the exposure time was between 100 and 2,000 ms, with the camera (CoolSnap-HQ, Photometrics, Tucson, AZ) operating at maximum gain. Images were typically captured at ×60 or ×100 magnification. All image acquisition and processing was done using Metamorph (Molecular Devices, Downingtown, PA). All images were scaled linearly in Metamorph and subsequently mounted in Adobe photoshop (Adobe, San Jose, CA) for display.
Quantification of accumulations was done by capturing images of cultures that had been grown in galactose media for 16 h. Twenty representative fields from four separate cultures were counted, with approximately 250 cells in each field, and cells were scored for presence of accumulations.
Protein Extraction, Immunoprecipitation, and Metabolic Labeling
Yeast cultures were grown in galactose media to log phase. Samples were washed once in 20 mM Hepes, pH 7.5, 20 mM NaF, 20 mM NaN3, and resuspended in RSB100 buffer (20 mM Tris, 2.5 mM MgCl2, 100 mM NaCl, 0.1 mM PMSF, pH 7.4, with protease inhibitors). Cells were lysed by vortexing for 10 min with acid-washed glass beads. Samples were centrifuged at 1,200×g for 4 min; the supernatant was centrifuged again at 1,200×g for 4 min, and then used for immunoprecipitation (IP).
For IP, 50 μL protein A/G beads were incubated with 10 μg of Syn211, an anti-α-synuclein mAb (Giasson et al. 2000), or T14, an anti-tau mAb, for 1 h at 4°C in 500 μL RSB100 buffer. Beads were washed twice in RSB100 buffer, 20 μg yeast cell extract was added to each IP reaction, and the IP sample was incubated for 1 h at 4°C. Beads were then washed five times, keeping the initial flow through, and resuspended in 50 μL SDS sample buffer for SDS–PAGE.
For metabolic labeling, yeast cultures were grown to log phase in galactose media, washed once with and resuspended in galactose media devoid of Uracil (Ura) and Methionine (Met); 0.5 mCi of [35S]-Met was added, and cultures were grown an additional 2 h at 30°C. Samples were then centrifuged and washed once in galactose media without Ura and Met, then washed in RSB100. Protein extraction and immunoprecipitation were then performed as described above, except 200 μg cell extract was used in each IP. SDS–PAGE was performed on a 5–20% tris-glycine gradient gel, fixed in 40% methanol, 10% acetic acid for 20 min. The gel was dried and exposed to a phosphoimager plate for 1 week.
Statistical Analyses
Comparison of sample means and calculation of P values were performed using GraphPad Prism 4.0 software.
Results
Expression of α-Synuclein Disrupts Localization of Rab GTPase Proteins in S. cerevisiae
Previous studies identified two Rab GTPase proteins, Sec4p and Ypt1p, that colocalize with α-synuclein in vesicle aggregates (Soper et al. 2008). To further evaluate if other Rab GTPases are also involved in α-synuclein-mediated vesicle aggregation, RFP-tagged Rab GTPase proteins were expressed under control of the constitutive ADH promoter in yeast coexpressing Syn-EGFP. The ADH promoter drove physiological levels of Ypt1p expression and the Ypt1 localized properly to endoplasmic reticulum (ER)-to-Golgi vesicles (Figure S1).
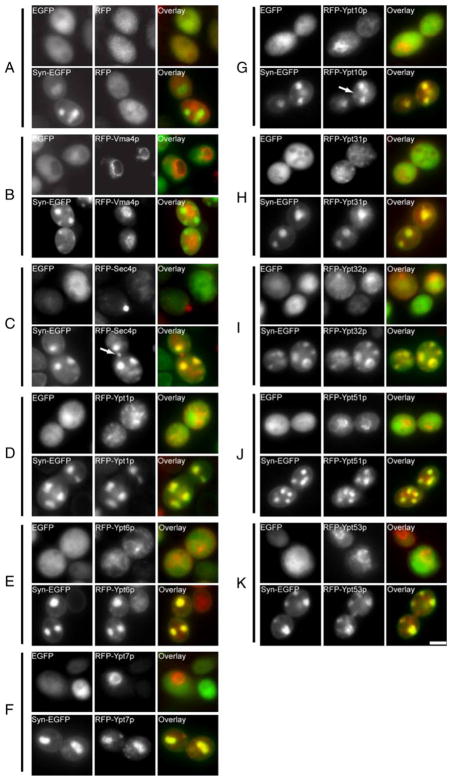
Surprisingly, all nine of the yeast Rab GTPase proteins that we examined colocalized with Syn-EGFP-labeled vesicle aggregates. This was judged to be a specific effect as RFP alone (Fig. 1a) and RFP-Vma4p (Fig. 1b), a subunit of the membrane domain of the vacuolar ATPase (Foury 1990), retained cytoplasmic and vacuole membrane labeling, respectively, in cells expressing EGFP or Syn-EGFP. As expected, RFP-Sec4p (Fig. 1c) localized to punctate secretory structures at the bud neck in EGFP cells (Guo et al. 1999) and colocalized with Syn-EGFP-positive puncta in Syn-EGFP cells, verifying that the RFP fusion protein behaves similarly to the HA-tagged protein. However, despite the mislocalization to vesicle aggregates, some RFP-Sec4p is still localized to the bud neck of Syn-EGFP expressing cells suggesting that it is localized correctly (Fig. 1c, arrow). Similar to previous reports, RFP-Ypt1p localized to punctate Golgi structures in EGFP cells, and colocalized with Syn-EGFP accumulations (Fig. 1d). We also observed colocalization between α-synuclein and Rab-GTPases involved in retrograde endosome–Golgi transport (Ypt6p, Fig. 1e) (Luo and Gallwitz 2003), endosome–vacuole transport and vacuolar inheritance (Ypt7p, Fig. 1f) (Wichmann et al. 1992; Schimmoller and Riezman 1993), the endocytic pathway (Ypt10p, Ypt51p, Ypt53p; Fig. 1g, j, k) (Singerkruger et al. 1994; Frei et al. 2006), and Golgi and post-Golgi trafficking (Ypt31p, Ypt32p; Fig. 1h, i) (Benli et al. 1996). Localization of RFP-Ypt10p to the vacuole membrane was still observed despite Ypt10p mislocalization, indicating that it may still be capable of performing its normal function in Syn-EGFP expressing cells (Fig. 1g, arrow). The proper localization of Ypt10p, as well as Vma4p, indicates that despite the mislocalization of many Rab-GTPase proteins onto large aggregates of cytoplasmic vesicles, protein trafficking to the vacuolar membrane was not obviously perturbed.
Fig. 1.
α-Synuclein disrupts localization of Rab GTPase proteins in S. cerevisiae. Fluorescence microscopy of yeast cells expressing RFP-tagged Rab GTPase proteins. RFP alone (a) is localized to the cytoplasm in yeast expressing EGFP and Syn-EGFP. RFP-Vma4p (b) labels the vacuole membrane in EGFP and Syn-EGFP cells. Rab GTPase proteins RFP-Sec4p (c), RFP-Ypt1p (d), RFP-Ypt6p (e), RFP-Ypt7p (f), RFP-Ypt10p (g), RFP-Ypt31p (h), RFP-Ypt32p (i), RFP-Ypt51p (j), and RFP-Ypt53p (k) all colocalize with Syn-EGFP accumulations. In cells expressing Syn-EGFP, localization of Ypt10p to the vacuole (arrow) can be seen in addition to colocalization with vesicle aggregations. Normal Sec4p labeling can also be seen in addition to colocalization with accumulations (arrow). Scale bar, 2 μM
Yeast Rab GTPases may be targeted to vesicle aggregates by direct interactions between α-synuclein and Rab GTPase proteins. To examine if there is a direct interaction between Rab GTPases and α-synuclein in yeast, we performed co-immunoprecipitation experiments with α-synuclein-EGFP and RFP-labeled Rab GTPases. Using Syn211, a C-terminal α-synuclein mAb, to immunoprecipitate α-synuclein-EGFP from yeast extracts, we were unable to detect a direct interaction between α-synuclein and RFP-Ypt6, RFP-Sec4 (Figure S2A), RFP-Ypt7, or RFP-Ypt31 (data not shown). Moreover, we were unable to identify unique proteins that specifically co-immunoprecipitate with α-synuclein from [35S]-Met radiolabeled yeast cells (Figure S2B). These data suggest that α-synuclein induces accumulation and mislocalization of many Rab GTPase proteins in yeast without a requirement for stable, direct physical interactions. Rather, α-synuclein may exert this effect by interfering with vesicular trafficking events.
Ypt6p Defects Enhance α-Synuclein-Induced Vesicle Aggregation
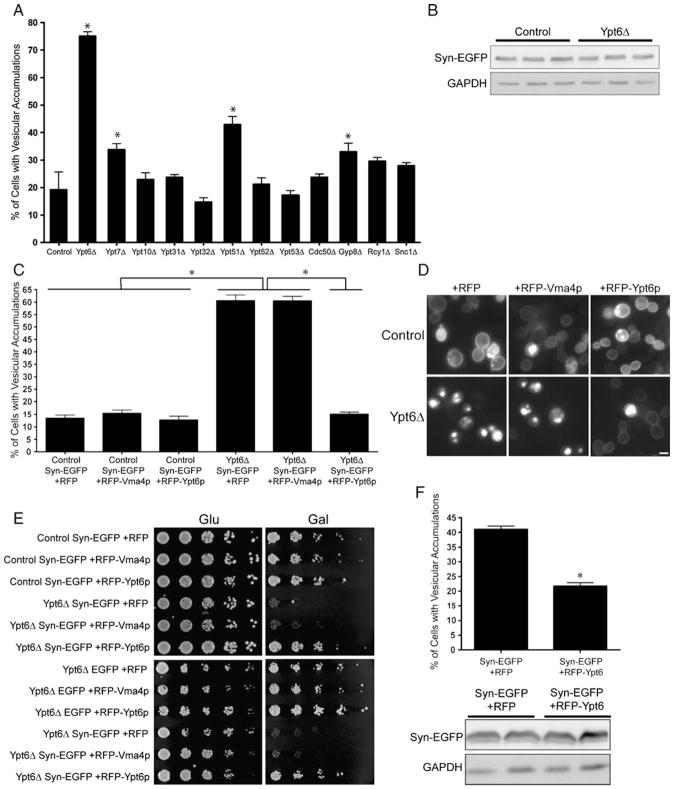
The mislocalization of Rab GTPases to vesicle aggregates may reflect either deranged cycling of Rabs on/off of vesicle membranes or may report a Rab requirement for clustering of these vesicles. To distinguish between these two possibilities, we examined vesicle aggregation in yeast mutants genetically ablated for particular Rab GTPase activities or for the activities of selected components of the vesicle trafficking machinery. Yeast knockout strains expressing Syn-EGFP were induced for 20 h, and the fraction of cells containing vesicle aggregations was quantified (Fig. 2a). Functional ablation of Ypt6p evoked a four-fold increase in the proportion of Syn-EGFP-expressing cells containing vesicle aggregations (19.22± 1.43% in control vs. 75.05±1.65% in ypt6Δ), while Ypt7p or Ypt51p inactivation supported a mild increase in vesicle aggregation (33.81±2.189% in ypt7Δ, 42.98±2.932% in ypt51Δ). Similarly, inactivation of Gyp8p, a Rab GTPase activating protein, resulted in a mild increase in vesicle aggregations. This effect may reflect its potent ability to accelerate Ypt6p GTP hydrolysis, although Ypt1p, Sec4p Ypt31p, and Ypt32p are also substrates for Gyp8p (De Antoni et al. 2002). Inactivation of the Ypt10p, Ypt31p, Ypt32p, Ypt52p, or Ypt53p RabGTPases had no effect on Syn-EGFP-induced vesicle aggregation. Similarly, deficiencies in Cdc50p (an endosomal protein involved in polarized growth; Misu et al. 2003), Rcy1p (a protein involved in plasma membrane protein recycling and early endocytosis; Wiederkehr et al. 2000), or Snc1p, a v-SNARE involved in secretory vesicle fusion with the plasma membrane; Protopopov et al. 1993), had no effect on vesicle aggregation in Syn-EGFP-expressing cells (Fig. 2a).
Fig. 2.
Deletion of Ypt6 increases α-synuclein-induced vesicle aggregations and toxicity in S. cerevisiae. (a) Quantification of vesicle aggregations in yeast knockout strains expressing Syn-EGFP. Ypt6 knockout yeasts have a significant increase in Syn-EGFP vesicle aggregations. Error bars indicate SEM *p<0.001. (b) Western blot of extracts from control and Ypt6 knockout yeast expressing Syn-EGFP. Increased vesicle aggregation was not due to a change in Syn-EGFP expression level. (c) Quantification of vesicle aggregations in yeast cells coexpressing Syn-EGFP and RFP, RFP-Vma4p, or RFP-Ypt6p. Expression of RFP-Ypt6p in Ypt6 knockout yeast restores vesicle aggregation levels back to that of control. (d) Fluorescence microscopy of yeast coexpressing Syn-EGFP and RFP, RFP-Vma4p, or RFP-Ypt6p. (e) Deletion of Ypt6 impairs growth of yeast expressing Syn-EGFP, but not EGFP alone. This effect can be rescued by expression of RFP-Ypt6p, but not RFP or RFP-Vma4p. (f) Expression of RFP-Ypt6p reduces vesicle aggregation in yeast grown in 5% DMSO. (g) Western blot of extracts from cells expressing Syn-EGFP and RFP or RFP-Ypt6p. Scale bar, (d) 2 μM
Western blot analyses (Fig. 2b) indicated that the increase in vesicle aggregation in ypt6Δ mutants was not the trivial consequence of increased α-synuclein-EGFP expression. Rather, the increased vesicle aggregation may be the indirect consequence of increased vesicle content in the cytoplasm of these deletion mutants. Consistent with this possibility, yeast deficient in Ypt6p (Tsukada and Gallwitz 1996) and Ypt51p (Singerkruger et al. 1994) do exhibit increased numbers of cytoplasmic vesicles. However, Snc1p defects also result in an increased load of cytoplasmic vesicles (Grote et al. 2000); yet, no effect on α-synuclein-induced vesicle aggregation was observed in Snc1p-deficient yeast. We were unable to assess the effect of Sec4p and Ypt1p, as these proteins perform essential housekeeping functions and knockout of these genes are lethal. However, it is unlikely that the secretory vesicle fusion step plays a vital role in vesicle aggregation, as snc1Δ yeast did not show a significant increase in vesicle aggregation. Figure S3 presents a schematic of Rab GTPases that we identified to be involved in α-synuclein-induced vesicle aggregation.
To further confirm the role Ypt6 played in α-synuclein-induced vesicle aggregation, we asked if RFP-Ypt6p expression in ypt6Δ cells could rescue the enhanced vesicle aggregation phenotype (Fig. 2c, d). As expected, ectopic RFP-Ypt6p expression-reduced vesicle aggregation down to the level exhibited by control cells (13.50±1.25% in control Syn-EGFP+RFP, 60.59±2.29% in ypt6Δ Syn-EGFP+RFP, 15.07±0.86% in ypt6Δ Syn-EGFP+RFP-Ypt6p). Expression of the negative controls, RFP and RFP-Vma4p, failed to exert rescue. Moreover, Ypt6p-deficient cells are exquisitely sensitive to Syn-EGFP expression, and this sensitivity was also remedied by RFP-Ypt6p production (Fig. 2e). To examine if Ypt6p expression in otherwise wild-type yeast expressing Syn-EGFP reduces vesicle aggregation, yeast cells were cultured in 5% DMSO to elevate basal levels of vesicle aggregation. Under these conditions, expression of RFP-Ypt6p, but not RFP alone, lowered the fraction of cells exhibiting vesicle aggregations from 41.04±1.12% to 21.74±1.15% (Fig. 2f). This effect was not due to reduced Syn-EGFP expression levels (Fig. 2g).
Taken together, these data implicate Ypt6p as a significant regulator of α-synuclein-induced formation of vesicle aggregations. Not only does Ypt6 dysfunction enhance vesicle aggregation and sensitize cells to Syn-EGFP but also ectopic expression of Ypt6p protects cells against α-synuclein-induced vesicle accumulation.
Disruption of Endosome-Golgi Retrograde Transport Increases Formation of α-Synuclein-Induced Vesicle Aggregations and Toxicity
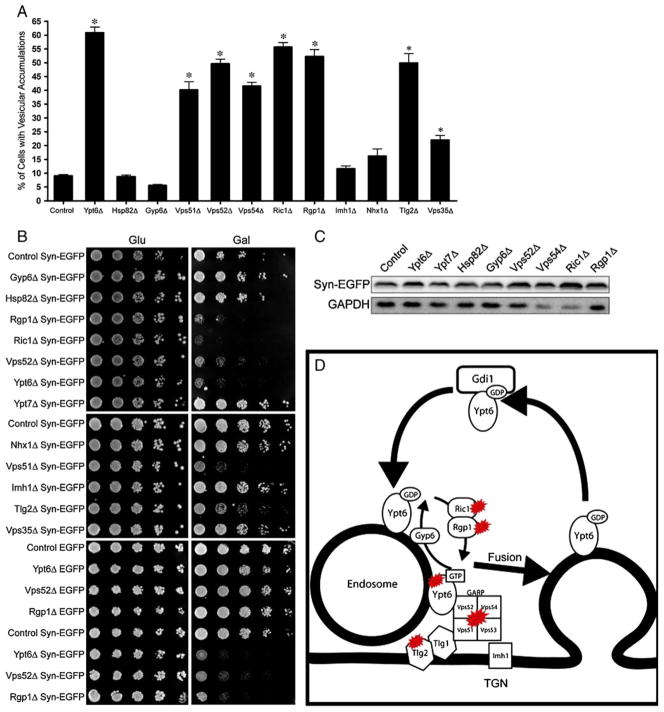
Ypt6p exchanges bound GDP for GTP in a reaction catalyzed by a heterodimer of two proteins, Ric1p and Rgp1p (Siniossoglou et al. 2000). As shown in Fig. 3a and d, enhanced Syn-EGFP-dependent vesicle aggregation was observed in both ric1Δ and rgp1Δ mutants (9.04±0.48% in control vs 55.71±1.60% in ric1Δ and 52.25±2.56% in rgp1Δ). Functional ablation of Gyp6p, a GTPase activating protein (GAP) for Ypt6p (Strom et al. 1993), exerted a modest diminution in the efficiency of Syn-EGFP-induced vesicle aggregation. The weakness of the effect may reflect the involvement of other GAPs in promoting GTP hydrolysis by Ypt6p (Albert and Gallwitz 1999; Vollmer et al. 1999; De Antoni et al. 2002).
Fig. 3.
Ypt6 regulates α-synuclein induced vesicle aggregation through endosome–Golgi retrograde transport. (a) Quantification of vesicle aggregations in yeast knockout strains. Deletion of the GTP exchange factor for Ypt6 (Ric1/Rgp1), deletion of components of the Golgi-associated retrograde protein complex (Vps51/52/Vps54), and deletions of Tlg2p and Vps35p result in increased vesicle aggregation. Error bars indicate SEM *P<0.001. (b) Knockout strains that have increased vesicle aggregation cause increased toxicity in cells expressing Syn-EGFP (right Gal panels), but not EGFP alone (bottom panels). (c) Western blot on extracts used for quantification. Variations in vesicle aggregation are not due to differences in Syn-EGFP expression level. (d) Schematic depicting Ypt6 cycling and endosome–Golgi fusion. Knockouts of Ypt6p, Ric1p, Rgp1p, Vps51p, Vps52p, Vps53p, Vps54p, and Tlg2p result in a large increase in α-syn-induced vesicle aggregation and toxicity. Ric1p/Rgp1p are guanine nucleotide exchange factors for Ypt6p. Vps51p/Vps52p/Vps53p/Vps54p compose the Golgi associated retrograde protein complex (GARP), and Tlg2p is a t-SNARE involved in endosome–Golgi fusion
Ypt6p regulates fusion of endocytic vesicles with late Golgi compartments through its functional interaction with the Golgi-associated retrograde protein (GARP) complex. The GARP complex is composed of the Vps51p, Vps52p, Vps53p, and Vps54p subunits (Siniossoglou and Pelham 2001), and dual inactivation of Vps51p and Ypt6p is incompatible with cell viability (Tong et al. 2004). We therefore examined vesicle aggregation in GARP-deficient cells producing Syn-EGFP. Indeed, large increases in formation of vesicle aggregates were scored in the GARP mutants (40.18±2.98% for vps51Δ, 49.62±1.70% for vps52Δ, vps53Δ data not shown, and 41.5 6±1.29% for vps54Δ; Fig. 3a). The enhanced vesicle aggregation phenotypes were accompanied by enhanced sensitivity of these mutants to Syn-EGFP expression (Fig. 3b). Again, we did not observe an increase in Syn-EGFP expression level (Fig. 3c).
Several other known Ypt6p interactors were examined, including Imh1p, a Golgi-associated coiled-coil protein containing a GRIP Golgi localization domain that is synthetically lethal with Ypt6p deletions (Munro and Nichols 1999), andNhx1p, an endosomal Na+/H+ exchanger whose dysfunction results in inefficient vesicular trafficking from the prevacuolar compartment (Bowers et al. 2000) and, interestingly, alleviates the temperature sensitive growth deficits of ypt6Δ mutants. Ablation of either Imh1p or Nhx1p did not exert a significant effect on vesicle aggregation in, or growth of, yeast expressing α-synuclein (Fig. 3a, b). Functional ablation of Vps35p, a component of the retromer protein complex required for cargo retrieval from endosomes to the Golgi (Nothwehr et al. 2000), resulted in only a mild increase in vesicle aggregation (9.04±0.48% in control, 22.03±1.62% in vps35Δ) without an increase in cell sensitivity to Syn-EGFP expression (Fig. 3a, b). These modest effects were recorded even though vps35Δ is synthetically lethal with ypt6Δ (Luo and Gallwitz 2003), Therefore, some (Ric1p, Rgp1p, Vps51p), but not all (Imh1p, Vps35p), components required for cell viability in the face of Ypt6p dysfunction are also sensitized to α-synuclein expression. Likewise, several deficiencies that are tolerated by Ypt6p-deficient yeast (i.e., Vps52, Vps54) are highly sensitized to the presence of α-synuclein. These data suggest that α-synuclein does not have a direct inhibitory effect on Ypt6p, but instead affects Ypt6p-regulated fusion process.
Fusion of endosomes to late Golgi compartments requires an interaction between the Ypt6/GARP protein complex and the essential t-SNARE Tlg1p (Siniossoglou and Pelham 2002). Tlg2p, a related Golgi t-SNARE, forms a SNARE complex with Tlg1p (Coe et al. 1999) and is also involved in endosome–Golgi fusion (Abeliovich et al. 1999). Tlg2p itself is not essential for yeast viability, however. Syn-EGFP expression in tlg2Δ yeast resulted in increased vesicle aggregation (9.04±0.48% in control vs 49.86±3.48% in tlg2Δ, Fig. 3a). The Tlg2p-deficient mutants were also sensitized to Syn-EGFP expression (Fig. 3b). Finally, affinity capture mass spectrometry experiments report Ypt6p interacts with Hsp82p (Zhao et al. 2005), the yeast homolog of mammalian Hsp90. This interaction is irrelevant to the robustness of Syn-EGFP-dependent vesicle aggregation, however (Fig. 3a).
In summary, we have identified several genes involved in the regulation of endosome–Golgi fusion that are sensitized to the presence of α-synuclein and have an inhibitory effect on α-synuclein vesicular aggregation. These genes include Ypt6p and its regulators, Ric1p and Rgp1p; components of the GARP, Vps51p, Vps52p, Vps53p, and Vps54p, and the t-SNARE Tlg2p (Fig. 3d).
α-Synuclein Interacts with Acidic Phospholipids to Promote Formation of Vesicle Aggregations
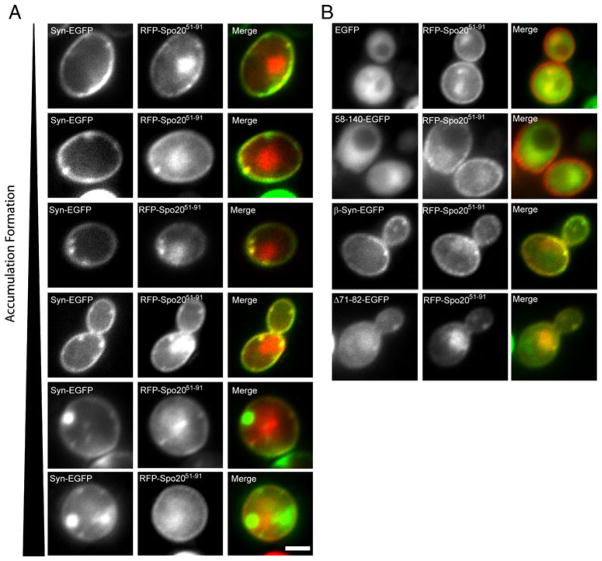
Previously, we have reported that the formation of α-synuclein-associated vesicle aggregations nucleates at the plasma membrane and requires the α-synuclein N-terminal region (Soper et al. 2008). Because this region binds acidic phospholipids (Davidson et al. 1998; Rhoades et al. 2006; Stockl et al. 2008), we compared the localization profiles of α-synuclein-EGFP and RFP-Spo2051–91. The RFP chimera harbors the phosphatidic acid-binding region of the Spo20p t-SNARE (Nakanishi et al. 2004). Indeed, α-synuclein-EGFP and RFP-Spo2051–91 strongly colocalize at the plasma membrane in yeast (Fig. 4a). By contrast, EGFP is diffusely distributed throughout the cytoplasm (Fig. 4b). Deletion of the first 57 amino acids of the α-synuclein-EGFP chimera, which removes the first four α-synuclein lipid-binding repeats, also produces a protein which is diffusely distributed throughout the cytoplasm.
Fig. 4.
Colocalization of α-Synuclein with the phosphatidic acid marker Spo2051–91 in S. cerevisiae. (a) Fluorescence microscopy of cells expressing α-synuclein-EGFP and RFP-Spo2051–91. α-Synuclein-EGFP colocalizes with RFP-Spo2051–91 at the plasma membrane and in early accumulations. As the accumulations mature to large cytoplasmic accumulations (lower panels), they no longer colocalize with RFP-Spo2051–91. (b) Fluorescence microscopy of cells expressing EGFP, 58–140-EGFP, β-synuclein-EGFP, or Δ71–82-α-syn-EGFP and RFP-Spo2051–91. EGFP and 58–140-EGFP do localize to the cytoplasm and do not colocalize with RFP-Spo2051–91. β-Synuclein-EGFP and Δ71–82-α-syn-EGFP colocalize with RFP-Spo2051–91 at the plasma membrane, but do not form large cytoplasmic accumulations. Scale Bar, (a) 1 μM
β-Synuclein-EGFP, which contains an N terminus which resembles that of α-synuclein, colocalizes with RFP-Spo2051–91 at the plasma membrane but does not support formation of the large cytoplasmic vesicle aggregations seen induced by α-synuclein expression, most likely because β-synuclein lacks the hydrophobic NAC domain. Consistent with this scenario, the Δ71–82-α-synuclein-EGFP (for which the hydrophobic NAC domain is also deleted) exhibits a localization profile similar to that of β-synuclein. These data confirm our previous report that the N terminus of α-synuclein is required for lipid binding in the yeast system. Not surprisingly, this N-terminal region is also required for colocalization with RFP-Spo2051–91.
Small α-syn-EGFP-enriched domains that form at the plasma membrane colocalize with RFP-Spo2051–91, suggesting that α-synuclein binding to phosphatidic acid nucleates the early stages of aggregation. Surprisingly, the larger cytoplasmic vesicle aggregations at later time points are no longer populated with a detectable pool of RFP-Spo2051–91 (Fig. 4a), suggesting that these vesicle aggregates are either depleted of phosphatidic acid or these become enriched in other phospholipids during maturation.
To further examine the role of phosphatidic acid in the formation of α-synuclein-induced vesicle aggregates, the effects of α-synuclein expression were monitored in several sec14ts mutants that exhibit increased levels of phosphatidic acid. Sec14p is the major yeast phosphatidylinosito/phosphatidylcholine transfer protein, and it plays an essential role in coordinating lipid metabolism with TGN/endosomal membrane dynamics (Salama et al. 1990; Bankaitis et al. 1990; Cleves et al. 1991; Mousley et al. 2008). The normally essential Sec14p requirement for cell viability is relieved by inactivation of several genes, including the structural genes for yeast choline kinase (Cleves et al. 1991), and the oxysterol binding protein Kes1p (Fang et al. 1996). These cki1 and kes1 mutations require the production of a phospholipase D-dependent phosphatidic acid pool for manifestation of “bypass Sec14” phenotypes (Xie et al. 1998). If phosphatidic acid plays an early role in α-synuclein accumulation in yeast, α-synuclein aggregation phenotypes should be enhanced in these mutants due to expanded phosphatidic acid pools.
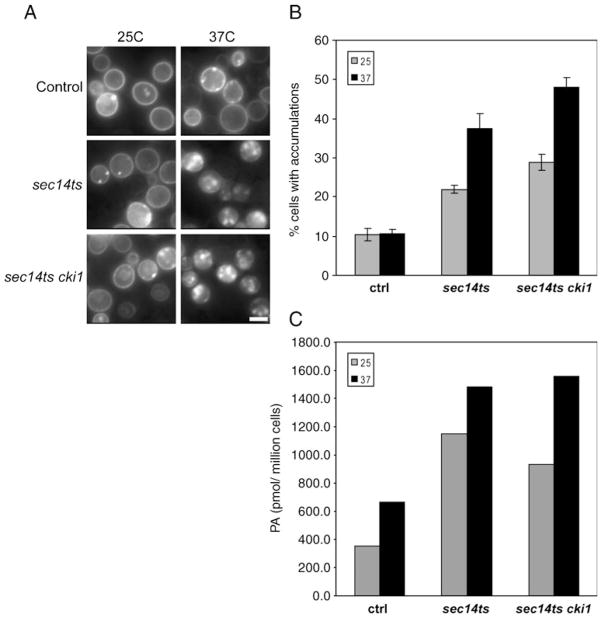
We examined α-synuclein-EGFP aggregation in isogenic sec14ts, sec14ts cki1, and sec14ts kes1 mutants both at the sec14ts permissive (25°C) and restrictive (37°C) temperatures. Indeed, α-synuclein-EGFP aggregation was markedly increased in all sec14ts mutants at both restrictive and permissive temperatures (Fig. 5a). Quantitative analyses revealed enhanced α-synuclein-EGFP aggregation in sec14ts at 25°C (Fig. 5b, 10.3±1.6% in controls; 21.9±0.9% in sec14ts), and a further two-fold enhancement at 37°C. The kes1 and cki1 “bypass Sec14” mutations failed to alleviate the aggregation phenotype. Rather, the phenotype was even more robust in the “bypass sec14” mutants at both 25°C (34.9±1.9% in sec14ts kes1 and 28.9±2.0% in sec14ts cki1) and 37°C (40.8±0 1.7% in sec14ts kes1 and 47.9±2.5% in sec14ts cki1). These data suggest that enhanced α-synuclein-EGFP aggregation in sec14ts mutants is not a simple consequence of vesicle trafficking defects as the “bypass Sec14” mutations rescue trafficking defects without alleviating the aggregation phenotypes. Lipidomic analyses confirmed previous demonstrations that sec14ts and sec14ts cki1 yeast exhibit elevated bulk cellular phosphatidic acid levels (Xie et al. 1998) and that phosphatidic acid levels were proportionally increased to the robustness of α-synuclein aggregation in these mutants at 25°C and 37°C (Fig. 5c).
Fig. 5.
Sec14ts and sec14ts bypass mutants increase α-synuclein accumulation in S. cerevisiae. (a) Fluorescence images of control, sec14ts, sec14ts kes1, and sec14ts cki1 cells expressing α-synuclein-EGFP at 25°C and 37°C. (b) Quantification of accumulation formation in control, sec14ts, sec14ts kes1, and sec14ts cki1 cells. Accumulation did not increase in control cells when shifted from 25°C to 37°C. Accumulation levels significantly increased in sec14ts, sec14ts kes1, and sec14ts cki1 strains when shifted from 25°C to 37°C. Sec14ts kes1 and sec14ts cki1 had higher baseline levels of accumulation than sec14ts and control cells. (c) Mass spec analysis of phosphatidic acid (PA) in pooled samples of control, sec14ts, and sec14ts cki1 cells. Sec14ts and sec14ts cki cells had higher levels of PA at 25°C. Shifting cells to 37°C increased PA levels in all strains. Scale Bar, (a) 2 μM
These data suggest that α-synuclein interacts with phosphatidic acid rich membranes and that this interaction plays a pivotal role in the disruption of intracellular vesicle trafficking and development of α-synuclein vesicular aggregations.
Discussion
In this study, we have further characterized the formation of α-synuclein-induced vesicle aggregations in S. cerevisiae. Here, we have shown that nine of the eleven yeast Rab GTPases colocalize with these vesicle aggregations, which is surprising considering their known normal localization to many distinct vesicular structures. These data are supported by similar findings in a recent study where multiple Rab GTPases were found to colocalize with α-synuclein in yeast (Gitler et al. 2008). Colocalization of these Rab GTPase proteins with α-syn in vesicle aggregations suggests that they may be sequestering the Rab GTPases away from their normal target membranes, which could result in wide-spread deficiencies in inter-organelle trafficking. The mechanism by which α-synuclein sequesters Rab GTPases to vesicle aggregations is currently unknown. We were unable to identify a direct physical interaction between α-synuclein and Rab GTPases, although an interaction between A30P α-synuclein and Rab3a, Rab5, and Rab8 has been observed in transgenic mice (Dalfo et al. 2004). Given that Rab GTPases cycle on and off membranes (Soldati et al. 1993), their colocalization with vesicle aggregations may simply be a result of mistargeting to these vesicles and not a direct interaction with α-synuclein.
We found that deletion of three Rab GTPases, Ypt6p, Ypt7p, and Ypt51p, resulted in an increase in vesicle aggregations. All three of these Rab GTPases are involved with the endocytic pathway (Wichmann et al. 1992; Singerkruger et al. 1994; Luo and Gallwitz 2003), suggesting that the vesicles found in these accumulations originate from the endocytotic pathway. Deletion of Ypt6 in particular resulted in a dramatic increase in vesicle aggregations and toxicity, which was able to be rescued by expression of RFP-Ypt6p, but not RFP alone or RFP-Vma4. Furthermore, we demonstrated the ability of overexpressed Ypt6p to reduce vesicle aggregation in wild type yeast cells.
To further examine this phenomenon, we expressed α-synuclein in yeast with deletions of known Ypt6 effector proteins. Deletion of either Ric1p or Rgp1p, GTP exchange factors for Ypt6p, results in an increase in vesicle aggregation and toxicity, similar to the effect seen in Ypt6Δ cells. Therefore, functional Ypt6p inhibits the formation or promotes the removal of α-synuclein-induced vesicle aggregations. Ypt6p regulates retrograde endosome–Golgi fusion through an interaction with the GARP complex, composed of Vps51p, Vps52p, Vps53p, and Vps54p (Siniossoglou and Pelham 2002). Deletion of any of these GARP components results in an increase in vesicle aggregation and toxicity. Interestingly, although Vps51p is the only GARP component reported to be synthetically lethal with Ypt6p; Vps51p, Vps52p, and Vps54p caused synthetic lethality with α-synuclein. These data imply that α-synuclein is not directly inhibiting Ypt6p, as α-synuclein causes synthetic lethality with different genes than does Ypt6p knockout strain. Our findings suggest that α-synuclein interferes with an endosome–Golgi trafficking event that is downstream from, but dependent on, Ypt6p-mediated tethering of endosomes to the Golgi.
Fusion of endosomes with the Golgi requires association of the GARP complex with an essential Golgi t-SNARE, Tlg1p (Siniossoglou and Pelham 2001; Siniossoglou and Pelham 2002) and another non-essential t-SNARE, Tlg2p (Abeliovich et al. 1999). Deletion of Tlg2p is not toxic to yeast cells; however, it causes toxicity and massive vesicle aggregation in yeast expressing α-synuclein. These data suggest that α-synuclein directly interferes with endosome–Golgi trafficking. α-Synuclein may play a role in the formation of SNARE complexes, as has been observed in mouse models (Chandra et al. 2005), or antagonize SNARE function, as has been observed in mammalian cells (Thayanidhi et al. 2010).
We hypothesize that one function of α-synuclein is to regulate vesicular fusion, through an unknown mechanism. Overexpression of α-synuclein may stall vesicular fusion, and in yeast this specifically causes massive accumulation of vesicles and toxicity associated with disruption of endosome–Golgi trafficking. These data are consistent with observations of increased synaptic vesicle pools in PC12 cells overexpression α-synuclein (Larsen et al. 2006), and the observation that α-synuclein knockout mice have a reduction in both docked and reserve synaptic vesicle pools in hippocampal neurons (Chandra et al. 2004). α-Synuclein has also been shown to interfere with exocytosis and synaptic vesicle recycling in mammalian neurons (Scott et al. 2010; Nemani et al. 2010), which may be the result of a similar toxic mechanism.
While we observed a specific defect in endosome–Golgi retrograde trafficking in yeast expressing α-synuclein, we have not ruled out that α-synuclein may cause defects in vesicle fusion at multiple transport steps. Indeed, it has been reported that expression of α-synuclein causes deficits in endocytosis in yeast (Outeiro and Lindquist 2003), deficits in ER–Golgi trafficking (Cooper et al. 2006) and that yeast expressing α-synuclein are sensitive to disruption of vesicular trafficking (Willingham et al. 2003; Zabrocki et al. 2008). These data are further supported by the recent observation that disruption of endocytosis in α-synuclein expressing worms results in severe growth impairments and motor dysfunction (Kuwahara et al. 2008). These observations support disruption of endocytosis as a possible mechanism for α-synuclein toxicity.
Cooper et al. have reported that α-synuclein induces carboxypeptidase Y (CPY) and alkaline phosphatase (ALP) glycosylation defects and that these defects and toxicity can be rescued by overexpression of the Rab GTPase, Ypt1p. These observations are consistent with reported phenotypes in yeast harboring a temperature-sensitive Ypt6p mutation. It has been shown that temperature-sensitive Ypt6p mutants have deficits in CPY and ALP trafficking, possibly through an inhibition of retrograde transport of glycotransferases and that these cells can be rescued by overexpression of Ypt1p (Luo and Gallwitz 2003). A specific sensitivity to disruption of Ypt1p through overexpression of a dominant-negative form of the protein in the presence of α-synuclein has been previously reported, indicating that α-synuclein may affect ER–Golgi trafficking (Cooper et al. 2006). Gitler et al. have reported α-synuclein-induced deficit in vesicle fusion in a cell-free ER–Golgi transport assay (Gitler et al. 2008), whereas in this study, we have demonstrated that α-synuclein may interfere with a fusion event at the endosome–Golgi step. Given α-synuclein’s synaptic localization in mammals, it is reasonable to assume that α-synuclein can regulate vesicle fusion at the synapse. The surprising trafficking phenotypes observed in yeast may be due to the conservation of basic SNARE-mediated vesicle fusion machinery between yeast and mammals (Kloepper et al. 2007).
In this study, we have further examined the relationship between α-synuclein membrane localization and accumulation. Significantly, we have found that α-synuclein colocalizes with the phosphatidic acid marker Spo2051–91 at the plasma membrane and in early accumulations. Yeast strains that produce increased pools of phosphatidic acid show increased accumulations. These data may also explain the strong membrane localization and vesicle aggregation phenotype in yeast, as yeast plasma membrane is particularly enriched for acidic phospholipids compared to other organelle membranes (Zinser and Daum 1995). These observations suggest that the known membrane binding properties of α-synuclein may be important in the early stages of its toxic function in yeast, and that disruption of α-synuclein phospholipid binding may be a possible therapeutic mechanism for the treatment of Parkinson’s disease
It is provocative to speculate on the relationship between α-synuclein-induced vesicle aggregation and neurodegeneration in PD. α-Synuclein fibrillization has long been studied as a potential pathogenic mechanism, and vesicle content has been observed in Lewy bodies and pale bodies in PD (Duffy and Tennyson 1965; Forno and Norville 1976; Watanabe et al. 1977; Hayashida et al. 1993; Soper et al. 2008). It is possible that accumulation of vesicles may serve as a “seed” to induce formation of α-synuclein fibrils and subsequent inclusion formation, due to the high concentration of α-synuclein in these accumulations. Alternatively, disruption of trafficking events by α-synuclein could directly cause toxicity in neurons, particularly in the affected dopaminergic neurons in PD patients. Accumulation of dopamine-containing vesicles may result in accumulation of potentially toxic dopamine metabolites, resulting in selective degeneration of dopaminergic neurons (Xu et al. 2002). Further characterization of the role α-synuclein plays in vesicle tethering and fusion, as well as the functional consequences of α-synuclein interaction with acidic phospholipids, may provide important insights into both the normal function and abnormal pathogenic function of α-synuclein and could reveal new therapeutic targets for the treatment of Parkinson’s disease.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health (1-P50-NS053488-01A2) and the Picower Foundation. V.M.-Y.L. is the John H. Ware III professor in Alzheimer’s disease research.
Abbreviations
- PD
Parkinson’s disease
- LB
Lewy body
- ADH
Alcohol dehydrogenase
- ER
Endoplasmic reticulum
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s12031-010-9455-5) contains supplementary material, which is available to authorized users.
Contributor Information
James H. Soper, Email: soper@mail.med.upenn.edu, Center for Neurodegenerative Disease Research, Department of Pathology and Laboratory Medicine, University of Pennsylvania, Philadelphia, PA 19104, USA
Victoria Kehm, Center for Neurodegenerative Disease Research, Department of Pathology and Laboratory Medicine, University of Pennsylvania, Philadelphia, PA 19104, USA.
Christopher G. Burd, Department of Cell and Developmental Biology, University of Pennsylvania, Philadelphia, PA 19104, USA
Vytas A. Bankaitis, Department of Cell and Developmental Biology, Lineberger Comprehensive Cancer Center, University of North Carolina School of Medicine, Chapel Hill, NC 27599-7090, USA
Virginia M.-Y. Lee, Email: vmylee@upenn.edu, Center for Neurodegenerative Disease Research, Department of Pathology and Laboratory Medicine, University of Pennsylvania, Philadelphia, PA 19104, USA. Maloney 3, HUP, 3600, Spruce Street, Philadelphia, PA 19104-4283, USA
References
- Abeliovich H, Darsow T, Emr SD. Cytoplasm to vacuole trafficking of aminopeptidase I requires a t-SNARE-Sec1p complex composed of Tlg2p and Vps45p. EMBO J. 1999;18:6005–6016. doi: 10.1093/emboj/18.21.6005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JMG, Armanini M, Ryan A, Hynes M, Phillips H, Sulzer D, Rosenthal A. Mice lacking α-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–252. doi: 10.1016/s0896-6273(00)80886-7. [DOI] [PubMed] [Google Scholar]
- Albert S, Gallwitz D. Two new members of a family of Ypt/Rab GTPase activating proteins—promiscuity of substrate recognition. J Biol Chem. 1999;274:33186–33189. doi: 10.1074/jbc.274.47.33186. [DOI] [PubMed] [Google Scholar]
- Bankaitis VA, Aitken JR, Cleves AE, Dowhan W. An essential role for a phospholipid transfer protein in yeast Golgi function. Nature. 1990;347:561–562. doi: 10.1038/347561a0. [DOI] [PubMed] [Google Scholar]
- Benli M, Doring F, Robinson DG, Yang XP, Gallwitz D. Two GTPase isoforms, ypt31p and ypt32p, are essential for Golgi function in yeast. EMBO J. 1996;15:6460–6475. [PMC free article] [PubMed] [Google Scholar]
- Bowers K, Levi BP, Patel FI, Stevens TH. The sodium/proton exchanger Nhx1p is required for endosomal protein trafficking in the yeast Saccharomyces cerevisiae. Mol Biol Cell. 2000;11:4277–4294. doi: 10.1091/mbc.11.12.4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brachmann CB, Davies A, Cost GJ, Caputo E, Li JC, Hieter P, Boeke JD. Designer deletion strains derived from Saccharomyces cerevisiae S288C: a useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast. 1998;14:115–132. doi: 10.1002/(SICI)1097-0061(19980130)14:2<115::AID-YEA204>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Cabin DE, Shimazu K, Murphy D, Cole NB, Gottschalk W, McIlwain KL, Orrison B, Chen A, Ellis CE, Paylor R, Lu B, Nussbaum RL. Synaptic vesicle depletion correlates with attenuated synaptic responses to prolonged repetitive stimulation in mice lacking α-synuclein. J Neurosci. 2002;22:8797–8807. doi: 10.1523/JNEUROSCI.22-20-08797.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra S, Fornai F, Kwon HB, Yazdani U, Atasoy D, Liu XR, Hammer RE, Battaglia G, German DC, Castillo PE, Sudhof TC. Double-knockout mice for alpha- and beta-synucleins: effect on synaptic functions. Proc Natl Acad Sci USA. 2004;101:14966–14971. doi: 10.1073/pnas.0406283101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra S, Gallardo G, Fernandez-Chacon R, Schluter OM, Sudhof TC. α-synuclein cooperates with CSP alpha in preventing neurodegeneration. Cell. 2005;123:383–396. doi: 10.1016/j.cell.2005.09.028. [DOI] [PubMed] [Google Scholar]
- Chartier-Harlin MC, Kachergus J, Roumier C, Mouroux V, Douay X, Lincoln S, Levecque C, Larvor L, Andrieux J, Hulihan M, Waucquier N, Defebvre L, Amouyel P, Farrer M, Destee A. α-synuclein locus duplication as a cause of familial Parkinson’s disease. Lancet. 2004;364:1167–1169. doi: 10.1016/S0140-6736(04)17103-1. [DOI] [PubMed] [Google Scholar]
- Cleves AE, Mcgee TP, Whitters EA, Champion KM, Aitken JR, Dowhan W, Goebl M, Bankaitis VA. Mutations in the Cdp choline pathway for phospholipid biosynthesis bypass the requirement for an essential phospholipid transfer protein. Cell. 1991;64:789–800. doi: 10.1016/0092-8674(91)90508-v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe JGS, Lim ACB, Xu J, Hong WJ. A role for Tlg1p in the transport of proteins within the Golgi apparatus of Saccharomyces cerevisiae. Mol Biol Cell. 1999;10:2407–2423. doi: 10.1091/mbc.10.7.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway KA, Harper JD, Lansbury PT. Accelerated in vitro fibril formation by a mutant α-synuclein linked to early-onset Parkinson disease. Nat Med. 1998;4:1318–1320. doi: 10.1038/3311. [DOI] [PubMed] [Google Scholar]
- Cooper AA, Gitler AD, Cashikar A, Haynes CM, Hill KJ, Bhullar B, Liu KN, Xu KX, Strathearn KE, Liu F, Cao SS, Caldwell KA, Caldwell GA, Marsischky G, Kolodner RD, LaBaer J, Rochet JC, Bonini NM, Lindquist S. α-synuclein blocks ER–Golgi traffic and Rab1 rescues neuron loss in Parkinson’s models. Science. 2006;313:324–328. doi: 10.1126/science.1129462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalfo E, Gomez-Isla T, Rosa JL, Bodelon MN, Tejedor MC, Barrachina M, Ambrosio S, Ferrer I. Abnormal α-synuclein interactions with rab proteins in α-synuclein A30P transgenic mice. J Neuropathol Exp Neurol. 2004;63:302–313. doi: 10.1093/jnen/63.4.302. [DOI] [PubMed] [Google Scholar]
- Davidson WS, Jonas A, Clayton DF, George JM. Stabilization of α-synuclein secondary structure upon binding to synthetic membranes. J Biol Chem. 1998;273:9443–9449. doi: 10.1074/jbc.273.16.9443. [DOI] [PubMed] [Google Scholar]
- De Antoni A, Schmitzova J, Trepte HH, Gallwitz D, Albert S. Significance of GTP hydrolysis in Ypt1p-regulated endoplasmic reticulum to Golgi transport revealed by the analysis of two novel Ypt1-GAPs. J Biol Chem. 2002;277:41023–41031. doi: 10.1074/jbc.M205783200. [DOI] [PubMed] [Google Scholar]
- Duffy PE, Tennyson VM. Phase and electron microscopic observations of Lewy bodies and Melanin granules in substantia Nigra and Locus Caeruleus in Parkinsons Disease. J Neuropathol Exp Neurol. 1965;24:398. [Google Scholar]
- Fang M, Kearns BG, Gedvilaite A, Kagiwada S, Kearns M, Fung MKY, Bankaitis VA. Kes1p shares homology with human oxysterol binding protein and participates in a novel regulatory pathway for yeast Golgi-derived transport vesicle biogenesis. EMBO J. 1996;15:6447–6459. [PMC free article] [PubMed] [Google Scholar]
- Forno LS, Norville RL. Ultrastructure of Lewy bodies in stellate ganglion. Acta Neuropathol. 1976;34:183–197. doi: 10.1007/BF00688674. [DOI] [PubMed] [Google Scholar]
- Foury F. The 31-Kda polypeptide is an essential subunit of the vacuolar atpase in Saccharomyces cerevisiae. J Biol Chem. 1990;265:18554–18560. [PubMed] [Google Scholar]
- Frei SB, Rahl PB, Nussbaum M, Briggs BJ, Calero M, Janeczko S, Regan AD, Chen CZ, Barral Y, Whittaker GR, Coins RN. Bioinformatic and comparative functional insights into localization of Rab proteins reveals the uncharacterized GTPases Ypt10p and Ypt11p. Mol Cell Biol. 2006;26:7299–7317. doi: 10.1128/MCB.02405-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvin JE, Lee VMY, Trojanowski JQ. Synucleinopathies—Clinical and pathological implications. Arch Neurol. 2001;58:186–190. doi: 10.1001/archneur.58.2.186. [DOI] [PubMed] [Google Scholar]
- Giasson BI, Uryu K, Trojanowski JQ, Lee VMY. Mutant and wild type human α-synucleins assemble into elongated filaments with distinct morphologies in vitro. J Biol Chem. 1999;274:7619–7622. doi: 10.1074/jbc.274.12.7619. [DOI] [PubMed] [Google Scholar]
- Giasson BI, Jakes R, Goedert M, Duda JE, Leight S, Trojanowski JQ, Lee VMY. A panel of epitope-specific antibodies detects protein domains distributed throughout human α-synuclein in Lewy bodies of Parkinson’s disease. J Neurosci Res. 2000;59:528–533. doi: 10.1002/(SICI)1097-4547(20000215)59:4<528::AID-JNR8>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Giasson BI, Duda JE, Quinn SM, Zhang B, Trojanowski JQ, Lee VMY. Neuronal α-synucleinopathy with severe movement disorder in mice expressing A53T human α-synuclein. Neuron. 2002;34:521–533. doi: 10.1016/s0896-6273(02)00682-7. [DOI] [PubMed] [Google Scholar]
- Gietz RD, TriggsRaine B, Robbins A, Graham KC, Woods RA. Identification of proteins that interact with a protein of interest: applications of the yeast two-hybrid system. Mol Cell Biochem. 1997;172:67–79. [PubMed] [Google Scholar]
- Gitler AD, Bevis BJ, Shorter J, Strathearn KE, Hamamichi S, Su LJ, Caldwell KA, Caldwell GA, Rochet JC, McCaffery JM, Barlowe C, Lindquist S. The Parkinson’s disease protein α-synuclein disrupts cellular Rab homeostasis. Proc Natl Acad Sci USA. 2008;105:145–150. doi: 10.1073/pnas.0710685105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenbaum EA, Graves CL, Mishizen-Eberz AJ, Lupoli MA, Lynch DR, Englander SW, Axelsen PH, Giasson BI. The E46K mutation in α-synuclein increases amyloid fibril formation. J Biol Chem. 2005;280:7800–7807. doi: 10.1074/jbc.M411638200. [DOI] [PubMed] [Google Scholar]
- Grote E, Vlacich G, Pypaert M, Novick PJ. A snc1 endocytosis mutant: phenotypic analysis and suppression by overproduction of dihydrosphingosine phosphate lyase. Mol Biol Cell. 2000;11:4051–4065. doi: 10.1091/mbc.11.12.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Roth D, Walch-Solimena C, Novick P. The exocyst is an effector for Sec4p, targeting secretory vesicles to sites of exocytosis. EMBO J. 1999;18:1071–1080. doi: 10.1093/emboj/18.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashida K, Oyanagi S, Mizutani Y, Yokochi M. An early cytoplasmic change before Lewy body maturation—an ultrastructural-study of the Substantia-Nigra from an autopsy case of Juvenile Parkinsonism. Acta Neuropathol. 1993;85:445–448. doi: 10.1007/BF00334457. [DOI] [PubMed] [Google Scholar]
- Ivanova PT, Milne SB, Byrne MO, Xiang Y, Brown HA. Lipidomics and Bioactive Lipids: Mass-Spectrometry-Based Lipid Analysis. Vol. 432. 2007. Glycerophospholipid identification and quantitation by electro-spray ionization mass spectrometry; pp. 21–57. [DOI] [PubMed] [Google Scholar]
- Iwai A, Masliah E, Yoshimoto M, Ge NF, Flanagan L, deSilva HAR, Kittel A, Saitoh T. The precursor protein of non-a-beta component of Alzheimers-disease amyloid is a presynaptic protein of the central-nervous-system. Neuron. 1995;14:467–475. doi: 10.1016/0896-6273(95)90302-x. [DOI] [PubMed] [Google Scholar]
- Jakes R, Spillantini MG, Goedert M. Identification of 2 Distinct Synucleins from Human Brain. FEBS Lett. 1994;345:27–32. doi: 10.1016/0014-5793(94)00395-5. [DOI] [PubMed] [Google Scholar]
- Jakes R, Crowther RA, Lee VMY, Trojanowski JQ, Iwatsubo T, Goedert M. Epitope mapping of LB509, a monoclonal antibody directed against human α-synuclein. Neurosci Lett. 1999;269:13–16. doi: 10.1016/s0304-3940(99)00411-5. [DOI] [PubMed] [Google Scholar]
- Jao CC, Der-Sarkissian A, Chen J, Langen R. Structure of membrane-bound α-synuclein studied by site-directed spin labeling. Proc Natl Acad Sci USA. 2004;101:8331–8336. doi: 10.1073/pnas.0400553101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen PH, Nielsen MS, Jakes R, Dotti G, Goedert M. Binding of α-synuclein to brain vesicles is abolished by familial Parkinson’s disease mutation. J Biol Chem. 1998;273:26292–26294. doi: 10.1074/jbc.273.41.26292. [DOI] [PubMed] [Google Scholar]
- Kim YS, Laurine E, Woods W, Lee SJ. A novel mechanism of interaction between α-synuclein and biological membranes. J Mol Biol. 2006;360:386–397. doi: 10.1016/j.jmb.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Kloepper TH, Kienle CN, Fasshauer D. An elaborate classification of SNARE proteins sheds light on the conservation of the eukaryotic endomembrane system. Mol Biol Cell. 2007;18:3463–3471. doi: 10.1091/mbc.E07-03-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S, Przuntek H, Epplen JT, Schols L, Riess O. Ala30Pro mutation in the gene encoding α-synuclein in Parkinson’s disease. Nat Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- Kubo S, Nemani VM, Chalkley RJ, Anthony MD, Hattori N, Mizuno Y, Edwards RH, Fortin DL. A combinatorial code for the interaction of α-synuclein with membranes. J Biol Chem. 2005;280:31664–31672. doi: 10.1074/jbc.M504894200. [DOI] [PubMed] [Google Scholar]
- Kushnirov VV. Rapid and reliable protein extraction from yeast. Yeast. 2000;16:857–860. doi: 10.1002/1097-0061(20000630)16:9<857::AID-YEA561>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Kuwahara T, Koyama A, Koyama S, Yoshina S, Ren CH, Kato T, Mitani S, Iwatsubo T. A systematic RNAi screen reveals involvement of endocytic pathway in neuronal dysfunction in α-synuclein transgenic C. elegans. Hum Mol Genet. 2008;17:2997–3009. doi: 10.1093/hmg/ddn198. [DOI] [PubMed] [Google Scholar]
- Larsen KE, Schmitz Y, Troyer MD, Mosharov E, Dietrich P, Quazi AZ, Savalle M, Nemani V, Chaudhry FA, Edwards RH, Stefanis L, Sulzer D. α-synuclein overexpression in PC12 and chromaffin cells impairs catecholamine release by interfering with a late step in exocytosis. J Neurosci. 2006;26:11915–11922. doi: 10.1523/JNEUROSCI.3821-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MK, Stirling W, Xu YQ, Xu XY, Qui D, Mandir AS, Dawson TM, Copeland NG, Jenkins NA, Price DL. Human α-synuclein-harboring familial Parkinson’s disease-linked Ala-53 ->Thr mutation causes neurodegenerative disease with α-synuclein aggregation in transgenic mice. Proc Natl Acad Sci USA. 2002;99:8968–8973. doi: 10.1073/pnas.132197599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Uversky VN, Fink AL. Conformational behavior of human α-synuclein is modulated by familial Parkinson’s disease point mutations A30P and A53T. Neurotoxicology. 2002;23:553–567. doi: 10.1016/s0161-813x(02)00066-9. [DOI] [PubMed] [Google Scholar]
- Luo ZL, Gallwitz D. Biochemical and genetic evidence for the involvement of yeast Ypt6-GTPase in protein retrieval to different Golgi compartments. J Biol Chem. 2003;278:791–799. doi: 10.1074/jbc.M209120200. [DOI] [PubMed] [Google Scholar]
- Misu K, Fujimura-Kamada K, Ueda T, Nakano A, Katoh H, Tanaka K. Cdc50p, a conserved endosomal membrane protein, controls polarized growth in Saccharomyces cerevisiae. Mol Biol Cell. 2003;14:730–747. doi: 10.1091/mbc.E02-06-0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousley CJ, Tyeryar K, Ile KE, Schaaf G, Brost RL, Boone C, Guan XL, Wenk MR, Bankaitis VA. Trans-golgi network and endosome dynamics connect ceramide homeostasis with regulation of the unfolded protein response and TOR signaling in yeast. Mol Biol Cell. 2008;19:4785–4803. doi: 10.1091/mbc.E08-04-0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S, Nichols BJ. The GRIP domain—a novel Golgi-targeting domain found in several coiled-coil proteins. Curr Biol. 1999;9:377–380. doi: 10.1016/s0960-9822(99)80166-3. [DOI] [PubMed] [Google Scholar]
- Nakanishi H, los Santos P, Neiman AM. Positive and negative regulation of a SNARE protein by control of intracellular localization. Mol Biol Cell. 2004;15:1802–1815. doi: 10.1091/mbc.E03-11-0798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemani VM, Lu W, Berge V, Nakamura K, Onoa B, Lee MK, Chaudhry FA, Nicoll RA, Edwards RH. Increased expression of alpha-Synuclein reduces neurotransmitter release by inhibiting synaptic vesicle reclustering after endocytosis. Neuron. 2010;65:66–79. doi: 10.1016/j.neuron.2009.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nothwehr SF, Ha SA, Bruinsma P. Sorting of yeast membrane proteins into an endosome-to-Golgi pathway involves direct interaction of their cytosolic domains with Vps35p. J Cell Biol. 2000;151:297–309. doi: 10.1083/jcb.151.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outeiro TF, Lindquist S. Yeast cells provide insight into α-synuclein biology and pathobiology. Science. 2003;302:1772–1775. doi: 10.1126/science.1090439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin RJ, Woods WS, Clayton DF, George JM. Interaction of human α-synuclein and Parkinson’s disease variants with phospholipids—Structural analysis using site-directed mutagenesis. J Biol Chem. 2000;275:34393–34398. doi: 10.1074/jbc.M004851200. [DOI] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, DiIorio G, Golbe LI, Nussbaum RL. Mutation in the α-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- Protopopov V, Govindan B, Novick P, Gerst JE. Homologs of the synaptobrevin Vamp family of synaptic vesicle proteins function on the late secretory pathway in Saccharomyces cerevisiae. Cell. 1993;74:855–861. doi: 10.1016/0092-8674(93)90465-3. [DOI] [PubMed] [Google Scholar]
- Rhoades E, Ramlall TF, Webb WW, Eliezer D. Quantification of α-synuclein binding to lipid vesicles using fluorescence correlation spectroscopy. Biophys J. 2006;90:4692–4700. doi: 10.1529/biophysj.105.079251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salama SR, Cleves AE, Malehorn DE, Whitters EA, Bankaitis VA. Cloning and characterization of Kluyveromyces-Lactis Sec14, A gene whose product stimulates Golgi secretory function in Saccharomyces cerevisiae. J Bacteriol. 1990;172:4510–4521. doi: 10.1128/jb.172.8.4510-4521.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmoller F, Riezman H. Involvement of Ypt7P, a small Gtpase, in traffic from late endosome to the vacuole in yeast. J Cell Sci. 1993;106:823–830. doi: 10.1242/jcs.106.3.823. [DOI] [PubMed] [Google Scholar]
- Scott DA, Tabarean I, Tang Y, Cartier A, Masliah E, Roy S. A pathologic cascade leading to synaptic dysfunction in α-synuclein-induced neurodegeneration. J Neurosci. 2010;30:8083–8095. doi: 10.1523/JNEUROSCI.1091-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segrest JP, Jones MK, Deloof H, Brouillette CG, Venkatachalapathi YV, Anantharamaiah GM. The Amphipathic helix in the exchangeable apolipoproteins—a review of secondary structure and function. J Lipid Res. 1992;33:141–166. [PubMed] [Google Scholar]
- Shaner NC, Campbell RE, Steinbach PA, Giepmans BNG, Palmer AE, Tsien RY. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat Biotechnol. 2004;22:1567–1572. doi: 10.1038/nbt1037. [DOI] [PubMed] [Google Scholar]
- Singerkruger B, Stenmark H, Dusterhoft A, Philippsen P, Yoo JS, Gallwitz D, Zerial M. Role of 3 Rab5-Like Gtpases, Ypt51P, Ypt52P, and Ypt53P, in the endocytic and vacuolar protein sorting pathways of yeast. J Cell Biol. 1994;125:283–298. doi: 10.1083/jcb.125.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J, Hardy J, Gwinn-Hardy K. α-synuclein locus triplication causes Parkinson’s disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- Siniossoglou S, Pelham HRB. An effector of Ypt6p binds the SNARE Tlg1p and mediates selective fusion of vesicles with late Golgi membranes. EMBO J. 2001;20:5991–5998. doi: 10.1093/emboj/20.21.5991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siniossoglou S, Pelham HRB. Vps51p links the VFT complex to the SNARE Tlg1p. J Biol Chem. 2002;277:48318–48324. doi: 10.1074/jbc.M209428200. [DOI] [PubMed] [Google Scholar]
- Siniossoglou S, Peak-Chew SY, Pelham HRB. Ric1p and Rgp1p form a complex that catalyses nucleotide exchange on Ypt6p. EMBO J. 2000;19:4885–4894. doi: 10.1093/emboj/19.18.4885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldati T, Riederer MA, Pfeffer SR. Rab Gdi—a solubilizing and recycling factor for Rab9-protein. Mol Biol Cell. 1993;4:425–434. doi: 10.1091/mbc.4.4.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soper JH, Roy S, Stieber A, Lee E, Wilson RB, Trojanowski JQ, Burd CG, Lee VMY. α-synuclein-induced aggregation of cytoplasmic vesicles in Saccharomyces cerevisiae. Mol Biol Cell. 2008;19:1093–1103. doi: 10.1091/mbc.E07-08-0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillantini MG, Schmidt ML, Lee VMY, Trojanowski JQ, Jakes R, Goedert M. α-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- Stockl M, Fischer P, Wanker E, Herrmann A. alpha-Synuclein selectively binds to anionic phospholipids embedded in liquid-disordered domains. J Mol Biol. 2008;375:1394–1404. doi: 10.1016/j.jmb.2007.11.051. [DOI] [PubMed] [Google Scholar]
- Strom M, Vollmer P, Tan TJ, Gallwitz D. A Yeast Gtpase-activating protein that interacts specifically with a member of the Ypt/Rab family. Nature. 1993;361:736–739. doi: 10.1038/361736a0. [DOI] [PubMed] [Google Scholar]
- Thayanidhi N, Helm JR, Nycz DC, Bentley M, Liang Y, Hay JC. {alpha}-Synuclein delays endoplasmic reticulum (ER)-to-Golgi transport in Mammalian cells by antagonizing ER/Golgi SNAREs. Mol Biol Cell. 2010;21:1850–1863. doi: 10.1091/mbc.E09-09-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong AHY, Lesage G, Bader GD, Ding HM, Xu H, Xin XF, Young J, Berriz GF, Brost RL, Chang M, Chen YQ, Cheng X, Chua G, Friesen H, Goldberg DS, Haynes J, Humphries C, He G, Hussein S, Ke LZ, Krogan N, Li ZJ, Levinson JN, Lu H, Menard P, Munyana C, Parsons AB, Ryan O, Tonikian R, Roberts T, Sdicu AM, Shapiro J, Sheikh B, Suter B, Wong SL, Zhang LV, Zhu HW, Burd CG, Munro S, Sander C, Rine J, Greenblatt J, Peter M, Bretscher A, Bell G, Roth FP, Brown GW, Andrews B, Bussey H, Boone C. Global mapping of the yeast genetic interaction network. Science. 2004;303:808–813. doi: 10.1126/science.1091317. [DOI] [PubMed] [Google Scholar]
- Tsukada M, Gallwitz D. Isolation and characterization of SYS genes from yeast, multicopy suppressors of the functional loss of the transport GTPase Ypt6p. J Cell Sci. 1996;109:2471–2481. doi: 10.1242/jcs.109.10.2471. [DOI] [PubMed] [Google Scholar]
- Volles MJ, Lansbury PT. Relationships between the sequence of α-synuclein and its membrane affinity, fibrillization propensity, and yeast toxicity. J Mol Biol. 2007;366:1510–1522. doi: 10.1016/j.jmb.2006.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmer P, Will E, Scheglmann D, Strom M, Gallwitz D. Primary structure and biochemical characterization of yeast GTPase-activating proteins with substrate preference for the transport GTPase Ypt7p. Eur J Biochem. 1999;260:284–290. doi: 10.1046/j.1432-1327.1999.00192.x. [DOI] [PubMed] [Google Scholar]
- Watanabe I, Vachal E, Tomita T. Dense core vesicles around Lewy body in incidental Parkinsons-disease—electron-microscopic study. Acta Neuropathol. 1977;39:173–175. doi: 10.1007/BF00703325. [DOI] [PubMed] [Google Scholar]
- Wichmann H, Hengst L, Gallwitz D. Endocytosis in yeast—evidence for the involvement of a small Gtp-binding protein (Ypt7P) Cell. 1992;71:1131–1142. doi: 10.1016/s0092-8674(05)80062-5. [DOI] [PubMed] [Google Scholar]
- Wiederkehr A, Avaro S, Prescianotto-Baschong C, Haguenauer-Tsapis R, Riezman H. The F-box protein Rcy1p is involved in endocytic membrane traffic and recycling out of an early endosome in Saccharomyces cerevisiae. J Cell Biol. 2000;149:397–410. doi: 10.1083/jcb.149.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willingham S, Outeiro TF, Devit MJ, Lindquist SL, Muchowski PJ. Yeast genes that enhance the toxicity of a mutant huntingtin fragment or α-synuclein. Science. 2003;302:1769–1772. doi: 10.1126/science.1090389. [DOI] [PubMed] [Google Scholar]
- Wood SJ, Wypych J, Steavenson S, Louis JC, Citron M, Biere AL. α-synuclein fibrillogenesis is nucleation-dependent—Implications for the pathogenesis of Parkinson’s disease. J Biol Chem. 1999;274:19509–19512. doi: 10.1074/jbc.274.28.19509. [DOI] [PubMed] [Google Scholar]
- Xie ZG, Fang M, Rivas MP, Faulkner AJ, Sternweis PC, Engebrecht J, Bankaitis VA. Phospholipase D activity is required for suppression of yeast phosphatidylinositol transfer protein defects. Proc Natl Acad Sci USA. 1998;95:12346–12351. doi: 10.1073/pnas.95.21.12346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Kao SY, Lee FJS, Song WH, Jin LW, Yankner BA. Dopamine-dependent neurotoxicity of α-synuclein: a mechanism for selective neurodegeneration in Parkinson disease. Nat Med. 2002;8:600–606. doi: 10.1038/nm0602-600. [DOI] [PubMed] [Google Scholar]
- Yavich L, Tanila H, Vepsalainen S, Jakala P. Role of α-synuclein in presynaptic dopamine recruitment. J Neurosci. 2004;24:11165–11170. doi: 10.1523/JNEUROSCI.2559-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabrocki P, Bastiaens I, Delay C, Bammens T, Ghillebert R, Pellens K, De Virgilio C, Van Leuven F, Winderickx J. Phosphorylation, lipid raft interaction and traffic of [alpha]-synuclein in a yeast model for Parkinson. Biochim Biophys Acta. 2008;1783(10):1767–1780. doi: 10.1016/j.bbamcr.2008.06.010. [DOI] [PubMed] [Google Scholar]
- Zarranz JJ, Alegre J, Gomez-Esteban JC, Lezcano E, Ros R, Ampuero I, Vidal L, Hoenicka J, Rodriguez O, Atares B, Llorens V, Tortosa EG, del Ser T, Munoz DG, de Yebenes JG. The new mutation, E46K, of α-synuclein causes Parkinson and Lewy body dementia. Ann Neurol. 2004;55:164–173. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- Zhao R, Davey M, Hsu YC, Kaplanek P, Tong A, Parsons AB, Krogan N, Cagney G, Mai D, Greenblatt J, Boone C, Emili A, Houry WA. Navigating the chaperone network: an integrative map of physical, genetic, and chemical-genetic interactions mediated by the yeast Hsp90 chaperone system. FEBS J. 2005;272:349. doi: 10.1016/j.cell.2004.12.024. [DOI] [PubMed] [Google Scholar]
- Zinser E, Daum G. Isolation and biochemical-characterization of organelles from the yeast, Saccharomyces cerevisiae. Yeast. 1995;11:493–536. doi: 10.1002/yea.320110602. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.