Abstract
Background
Epidemic keratoconjunctivitis (EKC) is a highly contagious infection of the ocular surface. 316 cases were diagnosed in Germany in the first 8 months of 2010, corresponding to a 300% increase above the typical figures for recent years. This outbreak motivates us to present the current recommendations concerning EKC.
Methods
Selective literature review.
Results
EKC is an adenoviral infection that typically starts with a unilateral foreign body sensation and then develops, within a few hours or days, into bilateral keratoconjunctivitis with marked chemosis, epiphora, and photophobia. Visual impairment can persist for months because of subepithelial corneal infiltrates (nummuli) and irregular astigmatism. Randomized clinical trials have not shown any clear benefit in the acute phase from any of a variety of treatments, including steroids, calcineurin inhibitors, virostatic drugs and disinfecting agents. In the chronic phase, cyclosporin A eye drops can accelerate the regression of subepithelial infiltrates. Hygienic measures, including conscientious hand and surface disinfection, can lessen the spread of the disease.
Conclusion
The first priority in the treatment of patients with definite or suspected EKC is the rigorous application of hygienic measures in medical facilities, particularly because there is still no effective drug treatment for this disease. No virostatic agent has yet been demonstrated to influence its course, either subjectively or objectively.
Epidemic keratoconjunctivitis (EKC) is a highly contagious infectious disease that is notifiable in Germany and mainly involves the surface of the eye. It is caused by adenoviruses that are highly resistant to environmental influences and are transmitted from person to person by way of infectious secretions, mainly as tear fluid. In the Western world, transmission often occurs in places where large numbers of people gather together, such as schools, homes for the elderly, and factories, as well as in health-care institutions such as hospitals and doctors’ offices (including ophthalmologists’ offices) (1). Epidemic outbreaks are common all over the world (1) and may necessitate the temporary closure of hospital wards and doctors’ offices. EKC occurs around the world in all age groups and at all times of year (2). In Asia, however, keratoconjunctivitis is endemic and mainly affects children (1).
Persons with EKC suffer for three to six weeks from an intense foreign body sensation, pain, reduced visual acuity, and often a general feeling of being unwell. EKC is sometimes followed by the development of corneal opacities, called nummuli, which may persist for months (1, 3, 4).
Epidemiology
316 cases of EKC were reported across Germany in the first eight months of 2010, more than three times as many as had been reported over the same period in each of the preceding two years (2). The outbreak was most pronounced in the northern part of the country, with the highest incidences in the federal states of Mecklenburg–West Pomerania (4.6 cases per 100 000 inhabitants), Hamburg (2.37 per 100 000 inhabitants), and Saxony-Anhalt (1.39 per 100 000 inhabitants). The figures for these three states were well above the average German incidence figures for 2001–2004 (0.2 to 0.8 cases per 100 000 inhabitants) (5). According to §7 of the German Infectious Disease Protection Law (Infektionssschutzgesetz), the direct demonstration of adenoviruses in conjunctival swab material is notifiable; furthermore, §6 (3) of this Law requires that a cluster of nosocomial infections that seems likely to represent an epidemic outbreak must be reported as such, without naming the individual patients affected. The reported numbers, however, are probably no more than the tip of the iceberg, as only infections with demonstrated adenovirus in a conjunctival swab find their way into the registry of the Robert Koch Institute (Germany’s central authority for epidemic control, comparable to the Centers for Disease Control in the United States). The statistics take no account of EKC cases diagnosed on clinical grounds alone, which they often are, as the clinical appearance of the disease is quite characteristic. (The German states of Thuringia and Saxony-Anhalt are exceptional in that the mere clinical suspicion of EKC is notifiable in these states.) The real number of cases can only be guessed at but is probably far higher than the official statistics suggest.
EKC bears no relation to sex, ethnic origin, social status, or nutritional state (1), as the current data have demonstrated once again (2). EKC reportedly accounts for 6% to 60% of all cases of infectious conjunctivitis (6, 7); it has been found that 8% of patients coming to the emergency department of an eye clinic had EKC (8). EKC is thus the most common viral disease of the eye and causes major economic losses by keeping patients away from work (3).
Adenoviruses
Epidemic keratoconjunctivitis is caused by adenoviruses. The family Adenoviridae comprises more than 130 different serotypes and includes viruses that can infect human beings, other mammals, birds, reptiles, and amphibians. This broad spectrum of hosts seems to imply that the adenoviruses are descended from a common precursor virus that existed 350 to 400 million years ago. The 54 types of adenovirus now known to be pathogenic in man are classified in seven groups, which are labeled A through G.
Adenoviruses are double-stranded DNA viruses roughly 80 to 110 nm in size. They are surrounded by an icosahedral capsid bearing group- and type-specific antigens; they have no outer lipid bilayer. They are highly resistant to environmental influences and can survive contact with many of the usual commercially available types of disinfectant. They remain infectious for weeks when kept at room temperature (9) and thus have a high aptitude for causing nosocomial infections (10, 11).
Adenoviruses are found all over the world and are transmitted through droplets and smears of infected bodily fluids that enter the human body through the nose, throat, and conjunctiva. The viral incubation time is 2 to 12 days. The disease is probably contagious even before symptoms arise, and it certainly remains so as long as the virus can still be demonstrated in bodily fluids; this period (for tear fluid) usually lasts two to three weeks from the date of transmission of the virus.
The disease can be transmitted on the hands as well as on objects such as tissues and handkerchiefs, doorknobs, etc. Nosocomial EKC contracted in eye clinics and doctors’ offices is usually due to contaminated instruments (e.g., tonometers) and eyedrops (12, 13).
Adenoviruses cause a wide variety of diseases—not just ocular infections, but also respiratory and gastrointestinal ones. Individual serotypes typically cause specific types of disease; thus, EKC is usually due to serotypes 8, 19, and 37, follicular conjunctivits to serotypes 3, 4 and 7, and pharyngeal-conjunctival fever to serotypes 3, 7, and (rarely) 14. Respiratory infections such as pneumonia, tonsillitis, and pharyngitis are caused by serotypes 1–5, 7, 14, and 21, while serotypes 1, 2, 5, 31, 40 und 41 cause gastroenteritis. Serotypes 1, 2, and 5 can produce sepsis-like manifestations, particularly in severely immunocompromised patients (9, 13).
Clinical features
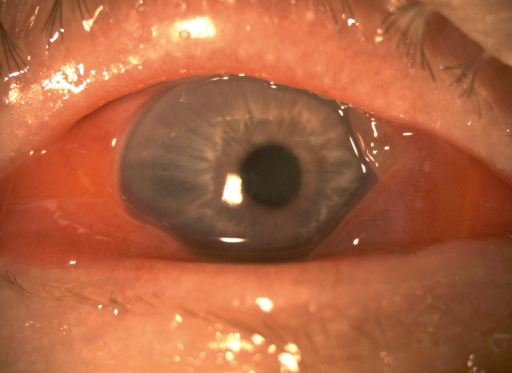
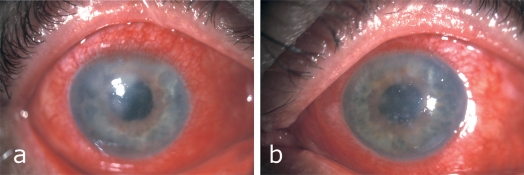
Typically, patients initially complain of an increasingly severe foreign body sensation in one eye, starting from the nasal corner of the eye and spreading laterally (1). This is followed by marked lid swelling, tearing (epiphora), itching, photophobia, and blurred vision (Figure 1). Similar, but usually much milder, manifestations commonly appear in the other eye in two to seven days. The severity of EKC ranges from subclinical conjunctivitis to very severe disease with bacterial superinfection (Figure 2) and with marked systemic symptoms such as generalized weakness and pain in the limbs. The involvement of the second eye is often so mild as to go unnoticed (1, 3, 4). Pre-auricular lymphadenopathy is also characteristic.
Figure 1.
A man with an acute EKC infection. Massive chemosis with swelling of the lid and caruncle, as well as epiphora
Figure 2.
A man with severe conjunctival infection and bilateral corneal infiltration by a bacterial superinfection with endotheliitis and anterior uveitis.
a) right eye
b) left eye
Split-lamp microscopy reveals conjunctival redness and swelling, sometimes with pseudomembrane formation. Swelling of the plica and caruncle is seen to a greater or lesser degree of severity in all patients and points to the diagnosis of EKC. Edematous lid swelling and the associated inflammatory ptosis are usually seen only in the primarily affected eye. The corneal component of the disease can arise from the fourth day onward but it can also be entirely lacking (1). If the cornea is affected, the first sign is usually the development of small epithelial punctatae that tend to enlarge and then remain visible, once the acute phase is over, as individual or confluent lesions called nummuli. These consist of immune complexes deposited beneath the epithelium in the anterior third of the corneal stroma. Large nummuli can substantially impair visual acuity (1, 3).
The acute phase heals in three to six weeks. Nummuli can persist beyond this time, continuing to impair visual acuity (but only in the primarily affected eye, in most cases) by scattering light, causing irregular astigmatism, and giving rise to photophobia. Nummuli that impair visual acuity usually regress in few weeks but can persist for years in rare cases (1, 3, 4, 14). For some patients, the aftermath of EKC is marked by dryness of the eyes that can persist for a long time and require treatment (3).
Diagnostic evaluation
EKC can usually be diagnosed from its typical clinical features, which were described above; the differential diagnosis includes other types of conjunctivitis (Table 1) as well as all other causes of “red eye” (uveitis, [epi-] scleritis, trauma, glaucoma, etc.).
Table 1. The differential diagnosis of EKC: other types of conjunctivitis.
| Diagnosis | Symptoms (compared to EKC) | Physical findings(compared to EKC) | Helpful aids to differential diagnosis |
| Bacterial conjunctivitis | Less severe foreign body sensation | Usually unilateral, mucopurulent secretion, less chemosis | Conjunctival swab |
| Allergic conjunctivitis | Itching (!), tearing, foreign body sensation | Papillary conjunctival edema ranging to so-called cobblestones | History (often, known allergy) |
| Toxic conjunctivitis | Irritation, burning, foreign body sensation | Diffuse conjunctival hyperemia or pallor, follicular changes | History (e.g., chronic use of vasoconstrictive eyedrops) |
| Other types of viral conjunctivitis (e.g., due to herpes virus) | Similar | Conjunctival hyperemia, follicular swelling, usually unilateral | Lids (Molluscum contagiosum lesions?)History (immune status) |
Laboratory confirmation of the diagnosis can help physicians initiate suitable hygienic measures rapidly and determine the epidemiological significance of the infection. The available tests are antigen detection, nucleic acid detection, electron microscopy, and cell culture tests. The latter two techniques can only be performed in special laboratories. Commercially available rapid tests for the detection of the adenovirus antigen are less sensitive and less specific than tests for nucleic acid detection, yet they are useful because they can be performed easily and rapidly in clinical practice. One test of this type, sold by the Rapid Pathogen Screening company of Sarasota, Florida, and approved by the United States Food and Drug Administration (FDA), has been found in various studies to have a sensitivity of 9% to 88% and a specificity of 91% to 100% (15). In summary, nucleic acid detection with the aid of nucleic acid amplification techniques, such as the polymerase chain reaction (PCR), is the diagnostic test of choice for EKC because of its high sensitivity, high specificity, and rapidity (7, 9).
Treatment
There is no currently available causally directed treatment that is effective against EKC (Table 2). Steroids are frequently given in the acute phase, yet clinical trials have shown that these have no more than a weak beneficial effect on the disease manifestations while markedly prolonging the duration of illness, increasing the likelihood of recurrence, and giving rise to steroid-associated side effects (16, 17). Virustatic agents such as trifluridine, vidarabine, and ganciclovir have been found to be only mildly effective or entirely ineffective against adenoviral diseases, whether in vitro, in vivo in animal models, or in human clinical trials (18– 21). Ganciclovir lowered the adenoviral burden to a statistically insignificant extent in an animal model (22) and thus might potentially lower the risk of keratitis, or of the spread of disease to the other eye. In Germany, ganciclovir is available as a gel for ophthalmic use (approved for the treatment of herpetic keratitis).
Table 2. Potential drug treatments for EKC that have been tested to date.
| Class of drug | Substance(s) | Mechanism of action | Studies cited | Treatment recommendation |
| Steroids | Dexamethasone, prednisolone | Immune suppression, anti-inflammatory effect | Trauzettel-Klosinski et al. 1980 (RCT) | Only when accompanied by uveitis or severe pseudomembrane formation |
| Romanowski et al. 1996 (AE,IV) | ||||
| Ward et al. 1993 (RCT) | ||||
| Virustatic agents | Trifluridine, vidarabine, methisazone, ganciclovir, cidofovir | Inhibition of viral replication | Ward et al. 1993 (RCT) | None; ganciclovir can be given to lessen the viral burden |
| Little et al. 1968 (RA) | ||||
| Hutter et al. 1990 (RCT) | ||||
| Waring et al. 1976 (RCT) | ||||
| Trousdale et al. 1994 (RCT) | ||||
| Hillenkamp et al. 2001 (RCT) | ||||
| Hillenkamp et al. 2002 (RCT) | ||||
| Gordon et al. 1994 (CR) | ||||
| Gordon et al. 1996 (IV, AE) | ||||
| Interferon | Interferon | Immune modulation | Hutter et al. 1990 (RCT) | None; can be given prophylactically in an epidemic |
| Romano et al. 1984 (CR) | ||||
| Adams et al. 1984 (RCT) | ||||
| Wilhelmus et al. 1987 (RCT) | ||||
| Reilly et al. 1986 (RCT) | ||||
| Rossa et al. 1991 (RA) | ||||
| Antiseptic agents | Povidone-iodine, N-chlorotaurine | Disinfection, microbicidal effect | Monnerat et al. 2006 (IV) | Potentially useful, but large-scale trials are lacking |
| Clement et al. 2010 (AE) | ||||
| Hutter et al. 1990 (RCT) | ||||
| Nagl et al. 1998 (IV) | ||||
| Teuchner et al. 2005 (RCT) | ||||
| Immune supressant | Cyclosporine A | Immune suppression | Levinger et al. 2010 (RA) | Can be used to treat chronic nummuli |
| Reinhard et al. 2000 (RA) | ||||
| Hillenkamp et al. 2002 (RCT) |
IV, in vitro; AE, animal experiments; CR, case report(s); RA, (retrospective) analysis of multiple cases / case series; RCT, randomized clinical trial
In a clinical trial published in 2001, Hillenkamp et al. (23) tested the causally directed treatment of EKC with the broad-spectrum virustatic agend cidofovir, which had already yielded promising results in a McEwen/New Zealand rabbit model (24) and in a single case report concerning one patient (25). In this trial, the administration of cidofovir 1% eyedrops with or without cyclosporine A eyedrops was found to lower the frequency of severe corneal opacification in comparison to the control group, but there was no overall benefit on the course of the illness. The substance had major side effects, including a local toxic effect on the conjunctiva and the neighboring lid tissue and the development of pseudomembranes and lacrimal duct stenoses, which had already been observed in the animal model (24). A lower, less toxic dose (0.2%) was clinically ineffective (e1).
Another once-promising therapeutic approach was the topical administration of interferon. Although initial case reports were positive (e2), human interferon was found to be ineffective in randomized clinical trials (20, e3– e5). A study by Rossa and Sundmacher nevertheless showed that interferon eyedrops might prevent infection in exposed persons (e6).
On the other hand, non-causally-directed treatment with the antimicrobial product povidone-iodine was found to eliminate adenoviruses very effectively in vitro (e7); in an animal model, a combination of povidone-iodine and dexamethasone markedly lowered the viral concentration and improved the manifestations of the disease (e8). Similar findings were obtained in a small-scale clinical trial (20): the local application of povidone-iodine in eyedrop or gel form was well tolerated and led to a slightly shorter duration of illness and to a somewhat lower frequency of nummuli. Thus, despite the absence of controlled studies, povidone-iodine seems to be a potential, albeit non-specific, treatment option (e9).
Yet another type of treatment that has been tested is the local administration of N-chlorotaurine (NCT), a broad-spectrum endogenous antimicrobial substance obtained from the supernatant of stimulated granulocytes (e10). After successful in vitro testing (e11), this subtance was evaluated in a small-scale, double-blind, phase II clinical trial in Austria (e12). A 1% dose was well tolerated, but statistically significant improvement (both subjective and objective) was seen only in the subgroup of patients with severe disease. The substance did not prevent nummuli from arising.
Beyond the treatment of the acute manifestations of EKC, the treatment of persistent nummuli remains a major problem. Histologically, these lesions seem to consist of lymphocytes, histiocytes, and antigen-presenting Langerhans cells (e13, e14). Local treatment with steroid eyedrops nearly always succeeds in the short term, but often leads to recurrence once it is stopped and to steroid dependence with prolonged virus persistence, in addition to the usual steroid complications, such as intraocular hypertension and cataract formation (4, 16, e15). Better results were obtained with the use of calcineurin inhibitors such as local cyclosporine A to treat nummuli. In two case series with long-term follow-up, symptoms and vision improved (by about two lines) in two-thirds of the patients treated (14, e16). Neither of these two series, however, had a control arm to determine the spontaneous course of nummuli without treatment. Nummuli can also be surgically removed with an excimer laser; this has been shown to improve visual acuity, yet it is fraught with the considerable disadvantage of a surgical procedure, the danger of reactivating the nummuli, and the risk of a hyperopic refractive change (e17, e18). Excimer treatment is mainly reserved for chronically scarred nummuli that respond inadequately, or not at all, to three to six months of immunosuppressive treatment.
In summary, there is still no effective, causally directed treatment for EKC. The treatment remains purely symptomatic, with artificial tear drops and (in some cases) antibiotics to prevent or treat superinfection (e10). Disinfection with povidone-iodine, or administration of ganciclovir, to reduce the viral load is not an adequate treatment. Chronic subepithelial infiltrates that do not resolve spontaneously over time can be treated locally with cyclosporine A eyedrops; late fibrosis can be treated with an excimer laser. Steroids are now considered an obsolete form of treatment, except when the disease is combined with uveitis, or when it has led to marked pseudomembrane formation (4, 20).
Hygienic measures in clinical practice
As there is neither an effective treatment for EKC nor a vaccine against it, hygienic measures are of paramount importance in preventing the spread of infection.
Hands and contaminated objects are the main transmitters of adenoviruses; thus, rigorous disinfection of hands and surfaces is the most important preventive measure. As mentioned above, adenoviruses are resistant to many types of disinfectant, and therefore only so-called virucidal disinfectants should be used. In ophthalmological practice, the use of disposable tonometer heads and single-patient (disposable) eyedrop dispensers is recommended. Hospitalized patients with EKC should be isolated, and outpatients with EKC should be treated separately from other patients at the end of the day. Ophthalmologists should always wear gloves when examining patients and should disinfect the hands, instruments, and surfaces thereafter (e9).
Medical personnel who have EKC are considered infectious and should not be allowed to work during the symptomatic phase (10, 13). Patients in the acute phase of the illness should be extensively informed about what they can expect to happen in the coming days and weeks, so that they will be less likely to return for follow-up before the acute phase is over. This will help prevent the spread of infection to other patients visiting the ophthalmologist’s practice.
Overview
Half a century after the discovery by Jawetz that adenoviruses are the cause of EKC (e19), this disease remains an unsolved medical and hygienic problem. The high degree of resistance of adenovirus particles to environmental influences should lead us to expect further outbreaks of EKC in the future. Hygienic measures must be rigorously established in doctors’ offices and hospitals; meanwhile, the search must also continue for an effective virustatic agent that can treat the cause of this disease to help the affected patients and prevent the spread of infection to those around them.
Key Messages.
Epidemic keratoconjunctivitis is a highly contagious infectious disease that mainly involves the surface of the eye.
In 2010, 316 cases of EKC were reported in Germany; this was three times more than in previous years.
There is no effective, causally directed treatment.
Hygienic measures in doctors’ offices and hospitals are of paramount importance.
Footnotes
Conflict of Interest Statement
The authors declare that no conflict of interest exists.
References
- 1.Ford E, Nelson KE, Warren D. Epidemiology of epidemic keratoconjunctivitis. Epidemiol Rev. 1987;9:244–261. doi: 10.1093/oxfordjournals.epirev.a036304. [DOI] [PubMed] [Google Scholar]
- 2.Adloch C, et al. Auswertung der bundesweit übermittelten Meldedaten zu Adenoviruskonjunktivitiden. Epidemiologisches Bulletin 37/ 2010 [Google Scholar]
- 3.Sundmacher R, Hillenkamp J, Reinhard T. Prospects for therapy and prevention of adenovirus keratoconjunctivitis. Ophthalmologe. 2001;98:991–1007. doi: 10.1007/s003470170052. [DOI] [PubMed] [Google Scholar]
- 4.Bohringer D, Birnbaum F, Reinhard T. Cyclosporin A eyedrops for keratitis nummularis after adenovirus keratoconjunctivitis. Ophthalmologe. 2008;105:592–594. doi: 10.1007/s00347-008-1731-1. [DOI] [PubMed] [Google Scholar]
- 5.Schrauder A, Altmann D, Laude G, et al. Epidemic conjunctivitis in Germany, 2004. Euro Surveill. 2006;11:185–187. [PubMed] [Google Scholar]
- 6.Singh K. Epidemic kerato-conjunctivitis in Malaya. Med J Malaya. 162(17):4–11. [PubMed] [Google Scholar]
- 7.Sambursky RP, Fram N, Cohen EJ. The prevalence of adenoviral conjunctivitis at the Wills Eye Hospital Emergency Room. Optometry. 2007;78:236–239. doi: 10.1016/j.optm.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 8.Tullo AB. Clinical and epidemiological features of adenovirus keratoconjunctivitis. Trans Ophthalmol Soc UK. 1980;100:263–267. [PubMed] [Google Scholar]
- 9.Doerr HW. 2nd edition. Stuttgart: Thieme; 2010. Medizinische Virologie: Grundlagen, Diagnostik, Prävention und Therapie viraler Erkrankungen; 639ff pp. [Google Scholar]
- 10.Chaberny IE, Schnitzler P, Geiss HK, Wendt C. An outbreak of epidemic keratoconjunctivtis in a pediatric unit due to adenovirus type 8. Infect Control Hosp Epidemiol. 2003;24:514–519. doi: 10.1086/502247. [DOI] [PubMed] [Google Scholar]
- 11.Frickmann H. Wenn Zeitersparnis zum Risiko wird. Nosokomialer Ausbruch von Keratoconjunctivitis epidemica. Management & Krankenhaus. 2010;3 [Google Scholar]
- 12.Robert Koch-Institut. Keratoconjunctivitis epidemica: Erkennung und Verhütung. Bundesgesundheitsblatt. 1999;42:284–286. [Google Scholar]
- 13.Robert Koch-Institut. Merkblätter für Ärzte: Keratokonjunktivitis epidemica und andere Konjunktivitiden durch Adenoviren. www.rki.de. 2010.
- 14.Reinhard T, Godehardt E, Pfahl HG, Sundmacher R. Local cyclosporin A in nummuli after keratoconjunctivitis epidemica. A pilot study. Ophthalmologe. 2000;97:764–768. doi: 10.1007/s003470070025. [DOI] [PubMed] [Google Scholar]
- 15.Siamak NM, Kowalski RP, Thompson PP, Romanowski EG, Shanks RM, Gordon YJ. RPS Adeno Detector. Ophthalmology. 2009;116:591–591. doi: 10.1016/j.ophtha.2008.10.025. [DOI] [PubMed] [Google Scholar]
- 16.Trauzettel-Klosinski S, Sundmacher R, Wigand R. The effects of topical steroids in epidemic kerato-conjunctivitis. Klin Monbl Augenheilkd. 1980;176:899–906. doi: 10.1055/s-2008-1057579. [DOI] [PubMed] [Google Scholar]
- 17.Romanowski EG, Roba LA, Wiley L, Araullo-Cruz T, Gordon YJ. The effects of corticosteroids of adenoviral replication. Arch Ophthalmol. 1996;114:581–585. doi: 10.1001/archopht.1996.01100130573014. [DOI] [PubMed] [Google Scholar]
- 18.Ward JB, Siojo LG, Waller SG. A prospective, masked clinical trial of trifluridine, dexamethasone, and artificial tears in the treatment of epidemic keratoconjunctivitis. Cornea. 1993;12:216–221. doi: 10.1097/00003226-199305000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Little JM, Lorenzetti DW, Brown DC, Schweem HH, Jones BR, Kaufman HE. Studies of adenovirus type 3 infection treated with methisazone and trifluorothymidine. Proc Soc Exp Biol Med. 1968;127:1028–1032. doi: 10.3181/00379727-127-32862. [DOI] [PubMed] [Google Scholar]
- 20.Hutter H. Epidemic keratoconjunctivitis: treatment results during an epidemic. Klin Monbl Augenheilkd. 1990;197:214–217. doi: 10.1055/s-2008-1046272. [DOI] [PubMed] [Google Scholar]
- 21.Waring G. Use of vidarabine in epidemic keratoconjunctivitis due to adenovirus types 3, 7, 8, and 19. Am J Ophthalmol. 1976;82:781–785. doi: 10.1016/0002-9394(76)90017-9. [DOI] [PubMed] [Google Scholar]
- 22.Trousdale MD, Goldschmidt PL, Nóbrega R. Activity of Ganciclovir against human Adenovirus Type-5 infection in cell culture and cotton rat eyes. Cornea. 1994;13:435–439. doi: 10.1097/00003226-199409000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Hillenkamp J, Reinhard T, Ross RS, et al. The effects of cidofovir 1% with and without cyclosporin a 1% as a topical treatment of acute adenoviral keratoconjunctivitis: a controlled clinical pilot study. Ophthalmology. 2002;109:845–850. doi: 10.1016/s0161-6420(02)00992-2. [DOI] [PubMed] [Google Scholar]
- 24.Gordon YJ, Romanowski EG, Araullo-Cruz T. Topical HPMPC inhibits adenovirus type 5 in the New Zealand rabbit ocular replication model. Invest Ophthalmol Vis Sci. 1994;35:4135–4143. [PubMed] [Google Scholar]
- 25.Gordon YJ, Naesens L, DeClercq E, Maudgal PC, Veckeneer M. Treatment of adenoviral conjunctivitis with topical cidofovir. Cornea. 1996;15 doi: 10.1097/00003226-199609000-00018. [DOI] [PubMed] [Google Scholar]
- e1.Hillenkamp J, Reinhard T, Ross RS, et al. Topical treatment of acute adenoviral keratoconjunctivitis with 02% cidofovir and 1% cyclosporine: a controlled clinical pilot study. Arch Ophthalmol. 2001;119(10):1487–1491. doi: 10.1001/archopht.119.10.1487. [DOI] [PubMed] [Google Scholar]
- e2.Romano A, Ladizensky E, Guarari-Rotman D, Revel M. Clinical effect of human-fibroblast-derived (beta) interferon in treatment of adeno-virus epidemic keratoconjunctivitis and its complication. Metab Pediatr Syst Ophthalmol. 1983;7:31–36. [PubMed] [Google Scholar]
- e3.Adams CP, Jr, Cohen EJ, Albrecht J, Laibson PR. Interferon treatment of adenoviral conjunctivitis. Am J Ophthalmol. 1984;98:429–432. doi: 10.1016/0002-9394(84)90125-9. [DOI] [PubMed] [Google Scholar]
- e4.Wilhelmus KR, Dunkel EC, Herson J. Topical human fibroblast interferon for acute adenoviral conjunctivitis. Graefes Arch Clin Exp Ophthalmol. 1987;225:461–464. doi: 10.1007/BF02334177. [DOI] [PubMed] [Google Scholar]
- e5.Reilly S, Dhillon BJ, Nkanza KM, et al. Adenovirus type 8 keratoconjunctivitis—an outbreak and its treatment with topical human fibroblast interferon. J Hyg (Lond) 1986;96:557–575. doi: 10.1017/s0022172400066365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e6.Rossa V, Sundmacher R. Local prevention with interferon of „epidemic“ conjunctivitis caused by a currently unidentifiable virus. Klin Monbl Augenheilkd. 1991;199:192–194. doi: 10.1055/s-2008-1046070. [DOI] [PubMed] [Google Scholar]
- e7.Monnerat N, Bossart W, Thiel MA. Povidone-iodine for treatment of adenoviral conjunctivitis: an in vitro study. Klin Monbl Augenheilkd. 2006;223:349–352. doi: 10.1055/s-2006-926633. [DOI] [PubMed] [Google Scholar]
- e8.Clement C, Capriotti JA, Kumar M, et al. Clinical and antiviral efficacy of an ophthalmic formulation of dexamethasone povidone-Iodine in a rabbit model of adenoviral keratoconjunctivitis. Invest Ophthalmol Vis Sci. 2011;52(1):339–344. doi: 10.1167/iovs.10-5944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e9.Pleyer U. Viral ocular infections: Topical treatment and prevention Developments in Ophthalmology. In: Behrens-Baumann W, Kramer A, editors. Antiseptic prophylaxis and therapy of ocular infections. Basel: Karger; 2001. pp. 281–296. [DOI] [PubMed] [Google Scholar]
- e10.Kinchington PR, Romanowski EG, Jerold Gordon Y. Prospects for adenovirus antivirals. J Antimicrob Chemother. 2005;55:424–429. doi: 10.1093/jac/dki057. [DOI] [PubMed] [Google Scholar]
- e11.Nagl M, Larcher C, Gottardi W. Activity of N-chlorotaurine against herpes simplex and adenoviruses. Antiviral Res. 1998;38:25–30. doi: 10.1016/s0166-3542(98)00005-9. [DOI] [PubMed] [Google Scholar]
- e12.Teuchner B, Nagl M, Schidlbauer A, et al. Tolerability and efficacy of N-chlorotaurine in epidemic keratoconjunctivitis—a double-blind, randomized, phase-2 clinical trial. J Ocul Pharmacol Ther. 2005;21:157–165. doi: 10.1089/jop.2005.21.157. [DOI] [PubMed] [Google Scholar]
- e13.Lund OE, Stefani FH. Corneal histology after epidemic keratoconjunctivitis. Arch Ophthalmol. 1978;96:2085–2088. doi: 10.1001/archopht.1978.03910060465016. [DOI] [PubMed] [Google Scholar]
- e14.Günther R. Pathologisch-anatomischer Befund einer Hornhaut bei Keratitis epidemica. Klin Monatsbl. 1937 [Google Scholar]
- e15.Sundmacher R, Engelskirchen U. Recurrent and persistent nummuli after epidemic keratoconjunctivitis. Klin Monbl Augenheilkd. 1991;198:550–554. doi: 10.1055/s-2008-1046030. [DOI] [PubMed] [Google Scholar]
- e16.Levinger E, Slomovic A, Sansanayudh W, Bahar I, Slomovic AR. Topical treatment with 1% cyclosporine for subepithelial infiltrates secondary to adenoviral keratoconjunctivitis. Cornea. 2010;29:638–640. doi: 10.1097/ICO.0b013e3181c33034. [DOI] [PubMed] [Google Scholar]
- e17.Quentin CD, Tondrow M, Vogel M. Phototherapeutic keratectomy (PTK) after epidemic keratoconjunctivitis. Ophthalmologe. 1999;96:92–96. doi: 10.1007/s003470050381. [DOI] [PubMed] [Google Scholar]
- e18.Forster W, Grewe S, Busse H. Clinical use of the excimer laser in treatment of surface corneal opacities–therapeutic strategy and case reports. Klin Monbl Augenheilkd. 1993;202:126–129. doi: 10.1055/s-2008-1045570. [DOI] [PubMed] [Google Scholar]
- e19.Jawetz E, Kimura S, Nicholas AN, Thygeson P, Hanna L. New type of APC virus from epidemic keratoconjunctivitis. Science. 1955;122:1190–1191. doi: 10.1126/science.122.3181.1190-a. [DOI] [PubMed] [Google Scholar]